Abstract
The cerebellum is a brain structure involved in the coordination, control and learning of movements, and elucidation of its function is an important issue. Japanese scholars have made seminal contributions in this field of neuroscience. Electrophysiological studies of the cerebellum have a long history in Japan since the pioneering works by Ito and Sasaki. Elucidation of the basic circuit diagram of the cerebellum in the 1960s was followed by the construction of cerebellar network theories and finding of their neural correlates in the 1970s. A theoretically predicted synaptic plasticity, long-term depression (LTD) at parallel fibre to Purkinje cell synapse, was demonstrated experimentally in 1982 by Ito and co-workers. Since then, Japanese neuroscientists from various disciplines participated in this field and have made major contributions to elucidate molecular mechanisms underlying LTD. An important pathway for LTD induction is type-1 metabotropic glutamate receptor (mGluR1) and its downstream signal transduction in Purkinje cells. Sugiyama and co-workers demonstrated the presence of mGluRs and Nakanishi and his pupils identified the molecular structures and functions of the mGluR family. Moreover, the authors contributed to the discovery and elucidation of several novel functions of mGluR1 in cerebellar Purkinje cells. mGluR1 turned out to be crucial for the release of endocannabinoid from Purkinje cells and the resultant retrograde suppression of transmitter release. It was also found that mGluR1 and its downstream signal transduction in Purkinje cells are indispensable for the elimination of redundant synapses during post-natal cerebellar development. This article overviews the seminal works by Japanese neuroscientists, focusing on mGluR1 signalling in cerebellar Purkinje cells.
Keywords: metabotropic glutamate receptor, long-term depression, endocannabinoid, synapse elimination, Purkinje cell, cerebellum
1. Introduction
The cerebellum is a brain structure involved in the coordination, control and learning of movements, and elucidation of its function is an important issue in neuroscience. Japan has a long history of electrophysiological study of the cerebellum particularly since the pioneering work by two pupils of Sir John Eccles, Masao Ito and Kazuo Sasaki. After return from Eccles' laboratory in Canberra, Australia, Masao Ito discovered that the Purkinje cell, the principal cell type solely constituting the output of the cerebellar cortex, is an inhibitory neuron (Ito & Yoshida 1964, 1966; Ito et al. 1964, 1966) and uses γ-aminobutyric acid (GABA) as its neurotransmitter (Obata et al. 1967). Since it was believed in the early 1960s that all principal neurons were excitatory and only local interneurons were inhibitory, Ito's discovery that the principal neuron of the cerebellar cortex is inhibitory was a great surprise. By using a microelectrode technique, Sasaki studied the neuronal circuit of the cerebellar cortex very intensively with Eccles, Llinas and Strata in Canberra. They clarified the synaptic actions of four types of interneurons in the cerebellar cortex (i.e. granule, basket, stellate and Golgi cells (Eccles et al. 1966b,c,d,e,f, 1967b)), as well as those of mossy and climbing fibres, the two major inputs to the cerebellar cortex (Eccles et al. 1966a,d, 1967c; Sasaki & Strata 1967). By the end of the 1960s, the basic neuronal circuit of the cerebellum was dissected in detail both electrophysiologically and morphologically, as documented in the monographs (Eccles et al. 1967a; Palay & Chan-Palay 1974; Ito 1984). Purkinje cells in the cerebellar cortex receive two distinct excitatory inputs, namely parallel and climbing fibres. Parallel fibres are the axons of granule cells in the cerebellar cortex and form synapses on the spines of the Purkinje cell's dendrites. Synaptic inputs from individual parallel fibres are weak, but the number of parallel fibres innervating a single Purkinje cell counts as many as 100 000–200 000. Granule cells are driven by the excitatory inputs from mossy fibres originating from various precerebellar nuclei such as the pontine nuclei. On the other hand, climbing fibres originate from the inferior olive in the contralateral medulla and form contacts directly on Purkinje cells. In contrast to parallel fibres, only one climbing fibre innervates a single Purkinje cell in the adult cerebellum but each climbing fibre makes strong synaptic contacts on the Purkinje cell's proximal dendrites. Purkinje cells in turn form inhibitory synaptic contacts on neurons in the deep cerebellar and vestibular nuclei. After the dissection of the basic neuronal network of the cerebellum, the main interest of researchers shifted to uncovering the elaborate neuronal mechanisms of the cerebellum and defining its roles in the entire nervous system function. Ito proceeded in the former direction and discovered long-term depression (LTD) as detailed below, while Sasaki advanced in the latter direction and studied the roles of the cerebro-cerebellar communication loop.
2. Long-term depression
(a) Discovery of LTD
Marr proposed in his epoch-making network theory of the cerebellar cortex that conjunctive activation of certain parallel and climbing fibre synapses on the same Purkinje cell leads to long-term potentiation of these parallel fibre synapses (Marr 1969). Later, Albus suggested that depression rather than potentiation is expected to occur following conjunctive parallel and climbing fibre activation, in view of the stable operation of the system (Albus 1971). Ito and co-workers found that in the flocculus, an evolutionally old part of the cerebellum, mossy fibres arising from vestibular organs and climbing fibres conveying visual information from the retina converge on Purkinje cells (Fukuda et al. 1972; Ito 1972; Maekawa & Simpson 1973). Inspired by the theoretical works by Marr (1969) and Albus (1971), Ito proposed a hypothesis that the cerebellar flocculus adaptively controls the gain of the vestibulo-ocular reflex depending on the retinal error signals conveyed by the climbing fibre inputs to the flocculus (Ito 1970, 1972, 1982). This ‘flocculus hypothesis’ is based on plasticity assumption, i.e. the strength of parallel fibre to Purkinje cell synapse is modified when these synapses are activated conjunctively with climbing fibre synaptic inputs. It has been shown that, during the adaptation of vestibulo-ocular reflex, Purkinje cell firing frequency in the flocculus undergoes changes in the manner predicted by Albus' hypothesis (Ghelarducci et al. 1975; Dufosse et al. 1978). As well, Gilbert and Thach showed that, in monkeys adapting to compensate for a sudden change in arm load, Purkinje cells changed their firings in the manner predicted by Albus' hypothesis (Gilbert & Thach 1977). Despite these lines of circumstantial evidence, the direct demonstration of the plasticity at parallel fibre to Purkinje cell synapse did not occur until the demonstration of LTD by Ito et al. (1982). In decerebrate rabbits, they showed that the responses of flocculus Purkinje cells to vestibular nerve stimulation undergoes LTD after the vestibular nerve stimulation is applied conjunctively with the stimulation of the contralateral inferior olive at 4 Hz for 25 s (Ito et al. 1982). Moreover, it turned out that LTD can be induced by the repeated stimulation of parallel fibres in conjunction with climbing fibres in decerebrate rabbits (Ito & Kano 1982; Ekerot & Kano 1985, 1989). These observations confirmed the validity of the Marr–Albus–Ito's synaptic plasticity assumption. Subsequently, Sakurai demonstrated LTD in cerebellar slice preparations from rat (Sakurai 1987) and that LTD requires an elevation of intracellular Ca2+ level in the Purkinje cell (Sakurai 1990). Hirano demonstrated LTD in cultured Purkinje cells (Hirano 1990). The demonstration of LTD in such reduced preparations paved the way for the researches seeking for molecular mechanisms of LTD in the 1990s.
Because parallel fibres were thought to use glutamate as their neurotransmitter, Kano and Kato explored the subtype of glutamate receptors involved in LTD (Kano & Kato 1987). In the middle of the 1980s, it was thought that glutamate receptors were classified into at least three subtypes: N-methyl-d-aspartate (NMDA), quisqualate, and kainate receptors (Foster & Fagg 1988). The clear concept of metabotropic glutamate receptor was not yet established at that time while it had already been shown that glutamate stimulation can enhance inositol phosphate turnover in nervous tissues (Sladeczek et al. 1985; Nicoletti et al. 1986). In addition, many subtype-specific antagonists that are widely used today were not developed. Therefore, we examined which of the glutamate agonists available at that time could induce LTD of parallel fibre to Purkinje cell synaptic transmission after an agonist was iontophoretically applied to parallel fibre synapses in conjunction with climbing fibre stimulation. The order of potency to induce LTD turned out to be quisqualate>glutamate⋙kainite and aspartate (Kano & Kato 1987). This order of preference did not match that of any glutamate receptor subtype known at that time (Foster & Fagg 1988). Therefore, this result indicated that LTD induction requires a novel subtype of glutamate receptor that is highly sensitive to quisqualate.
(b) Identification of metabotropic glutamate receptor
Sugiyama et al. (1987) demonstrated the presence of metabotropic glutamate receptor that is coupled to G protein, which activates inositol phospholipid metabolism and mobilizes Ca2+ from internal stores. When injected with mRNA from rat brain, Xenopus oocytes expressed a novel type of glutamate receptor that activated Ca2+-dependent Cl–currents due to inositol trisphosphate (IP3)-induced Ca2+ release from internal stores. Masu et al. (1991) reported the cloning and characterization of cDNA for type-1 metabotropic glutamate receptor (mGluR1) that is coupled to IP3/Ca2+ signal transduction. Importantly, mGluR1 is richly expressed in cerebellar Purkinje cells and has an agonist preference of the order of quisqualate>glutamate⋙kainite (Masu et al. 1991; Aramori & Nakanishi 1992). Therefore, mGluR1 appeared to correspond to the ‘quisqualate receptor’ demonstrated to be involved in cerebellar LTD (Kano & Kato 1987). After the identification of mGluR1, seven other members of mGluRs have been identified, and they are classified into three groups (Conn & Pin 1997). Group I consists of mGluR1 and mGluR5 and is coupled to the G protein Gq family (Gq and G11) that mediates IP3-induced Ca2+ mobilization and the activation of protein kinase C (PKC). Group II consists of mGluR2 and mGluR3 and is coupled to the Gi/o protein. Group III consists of mGluR4, mGluR6, mGluR7 and mGluR8 and is also coupled to the Gi/o protein.
(c) Requirement of mGluR1 in LTD induction
In the meantime, Linden et al. (1991) showed pharmacologically that quisqualate-preferring glutamate receptors were involved in the LTD of the glutamate responsiveness of cultured cerebellar Purkinje cells. Crepel et al. (1991) also reported that an mGluR agonist induced LTD-like persistent reduction of parallel fibre to Purkinje cell synaptic transmission in cerebellar slice preparation. Shigemoto et al. (1994) raised antibodies against mGluR1, which functionally block mGluR1-mediated Ca2+ mobilization. They showed that these antibodies blocked LTD of the glutamate responsiveness of cultured cerebellar Purkinje cells. Meanwhile, Atsu Aiba produced an mGluR1 knockout mouse in Susumu Tonegawa's laboratory. In collaboration with Kano, Aiba observed that LTD is deficient in the mGluR1 knockout mice, yet the morphology of the cerebellum and the basic properties of excitatory synaptic transmission were apparently normal (Aiba et al. 1994). In addition, Aiba et al. (1994) observed that the mGluR1 knockout mice were ataxic, displayed motor discoordination, and were impaired in delay eyeblink conditioning, a paradigm known to require intact cerebellum and thought to represent learning of an elementary movement (discrete motor learning). Therefore, the mGluR1 knockout mouse is the first mouse model whose phenotypes support the notion that LTD at parallel fibre to Purkinje cell synapse is a cellular basis of cerebellum-dependent discrete motor learning. Conquet et al. (1994) also reported in the same year that cerebellar LTD was deficient in mice lacking mGluR1. After he returned to Japan, Aiba and co-workers created a mouse strain in which mGluR1 is expressed only in cerebellar Purkinje cells (Ichise et al. 2000). They introduced rat mGluR1α (full-length mGluR1 splice variant) into the mGluR1 knockout mouse by using a Purkinje cell-specific promoter, L7. This mGluR1-rescue mouse exhibits apparently normal motor coordination, although the mGluR1 expression level is approximately 1/40 of that of the wild-type (Ichise et al. 2000). Importantly, both cerebellar LTD (Ichise et al. 2000) and delay eyeblink conditioning (Kishimoto et al. 2002) are restored in the mGluR1-rescue mouse, indicating that mGluR1 in Purkinje cell is responsible for cerebellar LTD and discrete motor learning.
Then, how is mGluR1 activated by synaptic activity? Immunoelectron microscopic examinations demonstrate that mGluR1 is localized in the perisynaptic annuli of the Purkinje cell dendritic spines facing parallel fibre synaptic terminals (Baude et al. 1993; Nusser et al. 1994; Petralia et al. 1998; Lopez-Bendito et al. 2001). Therefore, glutamate released from parallel fibre synaptic terminals is expected to activate mGluR1 at the spine annuli. Consistent with this expectation, strong repetitive stimulation of parallel fibres was shown to evoke an mGluR1-mediated excitatory postsynaptic potential (EPSP) in the Purkinje cells (Batchelor et al. 1994; Batchelor & Garthwaite 1997). In 1998, two groups independently demonstrated that a brief train of parallel fibre stimulation induces mGluR1-mediated local Ca2+ release from the internal stores that is confined to the dendritic region receiving activated parallel fibre inputs (Finch & Augustine 1998; Takechi et al. 1998). These results clearly indicate that mGluR1 can be activated by parallel fibre activity at Purkinje cell dendritic spines. Therefore, mGluR1 is thought to be activated at parallel fibre synapses during conjunctive activation of parallel and climbing fibre inputs for inducing LTD.
(d) Modulation of mGluR1 signalling may influence LTD induction
Glutamate is thought to be the primary activator of mGluRs in vivo. However, it was clarified that mGluR1 activity could be modulated by extracellular factors other than glutamate. Kubo et al. (1998) found an unexpected phenomenon that mGluRs can be activated by extracellular Ca2+ in the absence of glutamate. Tabata et al. (2002) have demonstrated that extracellular Ca2+ significantly broadens the dynamic range of mGluR1-mediated responses to its agonist in cultured Purkinje cells. Hirono et al. (2001b) reported that the mGluR1-mediated responses of Purkinje cells in cerebellar slices were enhanced by the activation of type-B γ-aminobutyric acid receptor (GABABR). This effect was mediated by the Gi/o protein (Hirono et al. 2001b). GABABR is localized perisynaptically at excitatory parallel fibre synapses on Purkinje cell dendritic spines where mGluR1 is concentrated (Kulik et al. 2002). In addition to the Gi/o protein-mediated enhancement, Tabata et al. (2004) have shown that GABABR activation enhances the mGluR1-mediated responses of cultured Purkinje cells through the Gi/o protein-independent direct interaction between GABABR and mGluR1. Surprisingly, this GABABR–mGluR1 interaction occurs in the absence of GABA but is caused by extracellular Ca2+ (Tabata et al. 2004), indicating that GABABR can act as Ca2+-dependent cofactor of mGluR1 signalling in the Purkinje cells. These data suggest that LTD induction may be influenced by the GABABR activity through Gi/o protein-dependent and/or -independent pathway. In support of this hypothesis, Tabata et al. (2004) showed that blockade of GABABR reduced the magnitude of LTD in cultured Purkinje cells. Furthermore, more direct evidence has been recently provided by Kamikubo et al. (2007) that in cultured Purkinje cells, GABABR activation enhanced LTD in a Gi/o protein-dependent manner.
Besides the interplay with GABABR, mGluR1 has been reported to interact with Gi/o protein-coupled A1-subtype adenosine receptor (A1R; Tabata et al. 2007). Tabata et al. reported that the agonists for A1R induced continuous depression of an mGluR1-coupled inward current. This inhibitory effect from A1R to mGluR1 was independent of Gi/o protein, suggesting a direct interaction between the two receptors. This A1R–mGluR1 interaction might influence the induction of LTD (Tabata et al. 2007).
Jin et al. (2007) recently reported that mGluR1-mediated slow excitatory postsynaptic current (EPSC) and its coincident Ca2+ transient after a brief burst of parallel fibres were selectively and persistently depressed by repeated climbing fibre-evoked depolarization of Purkinje cells. This ‘LTD’ of mGluR1 responses (LTD(mGluR1)) blocked subsequent induction of LTD of AMPA receptor-mediated EPSC at parallel fibre-Purkinje cell synapses (Jin et al. 2007). Thus, LTD(mGluR1) may have a metaplastic function, blocking induction of AMPA receptor LTD.
(e) Signalling molecules downstream of mGluR1 required for LTD induction
The mGluR1 downstream signalling cascade involved in LTD induction was studied by using mutant mice lacking the candidate molecules. mGluR1 is coupled to the G protein Gq family (Gq and G11), which activates the β isoforms of phospholipase C (PLC), produces diacylglycerol (DG) and IP3, and leads to DG-mediated activation of PKC and IP3-mediated Ca2+ release from internal stores (Conn & Pin 1997). Immunohistochemical studies indicate that Gαq and Gα11 are concentrated in the perisynaptic region of the Purkinje cell dendritic spines where mGluR1 is localized (Tanaka et al. 2000). Hartmann et al. (2004) have demonstrated that a Gαq-knockout mouse exhibits clear ataxia, deficient mGluR1-mediated Ca2+ mobilization, deficient LTD and impaired motor learning. By contrast, a Gα11 knockout mouse has no apparent ataxia and normal mGluR1-mediated Ca2+ mobilization but exhibits mild impairment of LTD and mild motor deficit (Hartmann et al. 2004). These results can be attributed to the fact that the expression level of Gαq is more than 10-fold higher than that of Gα11 in the Purkinje cells (Hartmann et al. 2004) despite their overlapping subcellular localization (Tanaka et al. 2000). These results unequivocally indicate that both Gαq and Gα11 mediate mGluR1 signalling and LTD induction within Purkinje cells.
In situ hybridization and immunohistochemical studies indicate that Purkinje cells in the rostral cerebellum mainly express PLCβ4, whereas those in the caudal cerebellum mainly have PLCβ3 (Kano et al. 1998; Nakamura et al. 2004). Miyata et al. (2001) analysed the PLCβ4 knockout mice and found that both mGluR1-mediated Ca2+ mobilization and LTD were deficient in the rostral cerebellum, whereas these responses were intact in the caudal cerebellum. Hirono et al. (2001a) confirmed the observation by Miyata et al. by analysing the other strain of PLCβ4 knockout mice. Importantly, Miyata et al. (2001) showed that delay eyeblink conditioning was severely impaired in the PLCβ4 knockout mice. Since lobule simplex or lobule HVI of Larsell, a part of the cerebellar cortex with rich PLCβ4 expression in the Purkinje cells, is crucial for delay eyeblink conditioning (Hesslow & Yeo 1998), these results indicate that PLCβ4 is crucial for LTD and discrete motor learning in the rostral cerebellum.
Several pharmacological data in the 1990s suggest that PKC is involved in LTD induction (Crepel & Jaillard 1990; Linden & Connor 1991; Hartell 1994; Freeman et al. 1998). Importantly, a transgenic mouse expressing a PKC inhibitor peptide, PKC-[19–31] in the Purkinje cells displays no LTD (De Zeeuw et al. 1998). Among the four classical PKC isoforms (α, βI, βII and γ) whose activation requires Ca2+ elevation, PKCα, PKCβI and PKCγ are strongly expressed in the Purkinje cells of the entire cerebellum (Hirono et al. 2001a). Although PKCγ knockout mice display ataxia and a defect in developmental synapse elimination (see below; Kano et al. 1995), LTD was intact in their Purkinje cells (Chen et al. 1995). Therefore, PKCα and/or PKCβI may be the probable candidate that plays a role in LTD induction.
IP3-induced Ca2+ release is also shown to be required for LTD induction in cerebellar slices (Hemart et al. 1995; Khodakhah & Armstrong 1997; Inoue et al. 1998; Daniel et al. 1999; Miyata et al. 2000), although it is not required for LTD of glutamate responsiveness in certain cultured Purkinje cell preparations (Narasimhan et al. 1998). Several studies indicate that pharmacological blockade of IP3 receptors or depletion of Ca2+ stores blocks LTD or LTD-like synaptic depression in cerebellar slices (Hemart et al. 1995; Khodakhah & Armstrong 1997; Inoue et al. 1998). Mikoshiba and co-workers created a type-1 IP3 receptor knockout mouse that exhibits seizure and ataxia and mostly dies during the first three post-natal weeks. LTD is clearly deficient in the Purkinje cells in cerebellar slices from the type-1 IP3 receptor knockout mice (Inoue et al. 1998). Furthermore, Miyata et al. (2000) demonstrated that IP3-mediated Ca2+ signalling in the Purkinje cell dendritic spines and concomitantly LTD were absent in those mice and rats with mutations in myosin Va, in which the endoplasmic reticulum did not enter the dendritic spines. LTD was restored in these animals by photolysis of caged Ca2+ compound in the Purkinje cells (Miyata et al. 2000). Daniel et al. (1999) showed that photolytic release of IP3 restored LTD in the mGluR1 knockout mice and this LTD was sensitive to a PKC inhibitor. These results unequivocally indicate that IP3-induced local Ca2+ release within Purkinje cell dendritic spines is crucial for LTD induction.
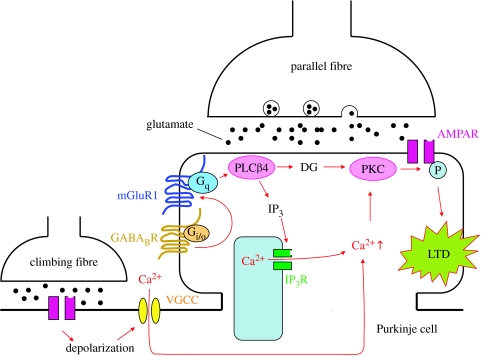
Figure 1 summarizes and schematically illustrates how mGluR1 and its downstream signal transduction cascade are involved in LTD induction. Other receptors and signalling molecules such as glutamate receptor δ2 subunit (GluRδ2) and nitric oxide known to be involved in LTD are not illustrated here for simplicity. The details of signal transduction underlying LTD are described in recent reviews (Ito 2001, 2002). However, the putative final common path to LTD is included in figure 1. It has been demonstrated that LTD results from the internalization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors from the postsynaptic membrane of the parallel fibre to Purkinje cell synapses (Matsuda et al. 2000; Wang & Linden 2000; Xia et al. 2000). This internalization is caused by the PKC-dependent phosphorylation of Ser880 of AMPA receptor GluR2 subunit. This leads to the unbinding of GluR2 from glutamate receptor-interacting protein (GRIP), which usually anchors AMPA receptors to cytoskeleton lining the postsynaptic membrane (Matsuda et al. 2000). As described above, Japanese neuroscientists have made major contributions to the elucidation of the LTD signal transduction cascade as illustrated in figure 1.
Figure 1.
Contribution of mGluR1 to LTD induction. LTD is induced following conjunctive activation of parallel and climbing fibre synapses. During LTD induction, glutamate released from parallel fibre terminals binds to mGluR1 at the perisynaptic region of Purkinje cell dendritic spines. The molecular cascade involving Gq and PLCβ4 is activated in the spines, and DG and IP3 are produced. IP3 triggers Ca2+ release from internal stores in the spines. Climbing fibre-induced depolarization of Purkinje cell dendrites opens voltage-gated Ca2+ channels (VGCC) and induces Ca2+ influx. As a result of these two events, local Ca2+ levels in the spines are elevated such that they can activate PKC together with DG. PKC phosphorylates Ser880 of the GluR2 subunit of AMPA receptor, and then the phosphorylated AMPA receptors are internalized. GABABR activated presumably by extracellular Ca2+ and/or ambient GABA spilt over from neighbouring inhibitory synapses enhances mGluR1 signalling through its direct interaction with mGluR1 and/or through the action of Gi/o protein. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor.
3. Endocannabinoid signalling
(a) Retrograde synaptic suppression triggered by postsynaptic mGluR1 activation
It has been shown that bath application of mGluR1 agonists to a cerebellar slice induces presynaptic inhibition of excitatory inputs to Purkinje cells (Conquet et al. 1994; Maejima et al. 2001). However, since mGluR1 is not detected at excitatory presynaptic terminals in immunoelectron microscopic studies (Baude et al. 1993; Nusser et al. 1994; Petralia et al. 1998; Lopez-Bendito et al. 2001), how mGluR1 agonists affect glutamate release from presynaptic terminals was a puzzling problem. Maejima et al. (2001) at our laboratory injected a non-hydrolyzable analogue of GTP or GDP (GTPγ-S or GDPβ-S) into the recorded Purkinje cell through a patch-clamp recording pipette and inactivated the G-protein signalling. This manipulation completely eliminated the presynaptic inhibition of climbing fibre-mediated EPSCs induced by the mGluR1 agonist 3,5-dihydroxyphenylglycine (DHPG; Maejima et al. 2001). This result unequivocally indicates that mGluR1 signalling in the postsynaptic Purkinje cell is responsible for the presynaptic inhibition. Therefore, some retrograded messenger must exist that transmits signals from Purkinje cells to climbing fibre terminals. After a 3-year struggle, we have finally revealed that antagonists of cannabinoid CB1 receptor abolish the DHPG-induced presynaptic suppression (Maejima et al. 2001), indicating that endogenous cannabinoids (endocannabinoids) mediate the retrograde signal.
(b) An endocannabinoid, 2-arachidonoylglycerol, as a retrograde messenger
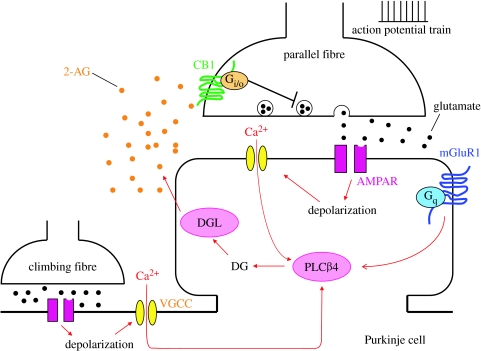
Five months prior to Maejima's report, the three groups including the authors' laboratory published independently and simultaneously that strong depolarization and the resultant elevation of intracellular Ca2+ concentration in hippocampal neurons (Ohno-Shosaku et al. 2001; Wilson & Nicoll 2001) and cerebellar Purkinje cells (Kreitzer & Regehr 2001) produce endocannabinoids, which in turn retrogradely suppress transmitter release from inhibitory and excitatory presynaptic terminals. These phenomena, termed depolarization-induced suppression of inhibition (DSI) and excitation (DSE), can be triggered by Ca2+ elevation alone without the activation of mGluRs. By contrast, mGluR1-mediated endocannabinoid release occurs even in the Purkinje cells dialysed with the Ca2+ chelator BAPTA (Maejima et al. 2001), indicating that mGluR1 activation produces endocannabinoids without the elevation of Ca2+. These results indicate that there are two distinct modes of endocannabinoid release from neurons. Later, it was shown that Ca2+ elevation and activation of Gq/11-coupled receptors cooperatively produce endocannabinoids in hippocampal neurons (Ohno-Shosaku et al. 2002, 2003; Varma et al. 2002). Hashimotodani et al. (2005) at our laboratory have revealed that this cooperativity is attributable to PLCβ activity that depends on both intracellular Ca2+ and Gq/11 protein. In cerebellar Purkinje cells, a brief burst of parallel fibre stimulation causes mGluR1 activation and local Ca2+ elevation due to AMPA receptor-mediated depolarization, and induces endocannabinoid-mediated retrograde suppression of glutamate release from parallel fibres (Maejima et al. 2001; Brown et al. 2003; Marcaggi & Attwell 2005, 2007). We have demonstrated that this suppression results from the Ca2+ dependency of PLCβ4 (Maejima et al. 2005). We also show that mGluR1 activation combined with depolarization leads to the production of an endocannabinoid, 2-arachidonoyl glycerol (2-AG) (Maejima et al. 2005), which is known to be produced from DG by diacylglycerol lipase (Sugiura et al. 2006). Brenowits and Regehr observed that coactivation of parallel and climbing fibres markedly facilitated endocannabinoid-mediated suppression of the parallel fibre synapses, presumably owing to the cooperativity of parallel fibre-induced mGluR1 activation and climbing fibre-induced Ca2+ elevation (Brenowitz & Regehr 2005). Thus, endocannabinoid-mediated retrograde suppression, dependent on both mGluR1 and Ca2+, may constitute a local negative feedback loop to prevent the hyper-excitation of Purkinje cells. Figure 2 schematically illustrates how this putative negative feedback loop works at parallel fibre to Purkinje cell synapses.
Figure 2.
Contribution of mGluR1 to endocannabinoid signalling. A brief burst of parallel fibre stimulation causes the release of a sufficient amount of glutamate to activate mGluR1 and induce Ca2+ influx through voltage-gated Ca2+ channels opened by AMPA receptor-mediated local depolarization. Then, Gq and Ca2+ synergistically activate PLCβ4. When climbing fibre stimulation is paired with parallel fibre stimulation, a shorter burst (e.g. two pulses) is sufficient to activate PLCβ4 because the Ca2+ flowing into the dendrites by climbing fibre-induced depolarization further sensitizes PLCβ4. Activated PLCβ4 produces DG, which in turn is converted to the endocannabinoid 2-AG by diacylglycerol lipase (DGL). 2-AG is released from the postsynaptic membrane and retrogradely binds to CB1 receptors at the parallel fibre terminals. Activated CB1 suppresses the release of glutamate presumably inhibiting voltage-gated Ca2+ channels and release machinery in the presynaptic terminals. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor.
(c) Endocannabinoid signalling involved in cerebellar LTD and motor learning
Endocannabinoid signalling is also involved in the LTD of parallel fibre to Purkinje cell synapses. Safo and Regehr observed that LTD was blocked by a CB1 receptor antagonist and inhibiting diacylglycerol lipase, and LTD was deficient in a CB1 knockout mouse (Safo & Regehr 2005). Although the CB1 knockout mouse exhibits no apparent deficit in motor coordination, Kishimoto & Kano (2006) found that delay eyeblink conditioning was severely impaired in these mice and in wild-type mice injected with a CB1 antagonist. These results suggest that mGluR1-dependent endocannabinoid signalling is required for LTD induction and cerebellum-dependent discrete motor learning.
4. Synapse elimination
(a) Maturation of climbing fibre synapses during post-natal development
A widely accepted scheme of functional neural circuit formation during post-natal development is that immature neurons initially make synaptic connections not only to their final targets, but also to other neurons. Then, functionally important synapses are strengthened, and less important synapses are weakened relative to the important ones. The weakened synapses are finally eliminated morphologically (Purves & Lichtman 1980; Lichtman & Colman 2000). The climbing fibre to Purkinje cell synapse is a typical example in the CNS, which undergoes such changes during post-natal development. In early post-natal days, all Purkinje cells are innervated by multiple climbing fibres (multiple innervation) (Crepel et al. 1976; Crepel 1982; Lohof et al. 1996). These surplus climbing fibres are eliminated eventually with the progress of post-natal development, and most Purkinje cells become innervated by single climbing fibres (mono-innervation) by the end of the third post-natal week in mice (Kano et al. 1995, 1997, 1998; Offermanns et al. 1997).
In the mature cerebellum, a single climbing fibre forms synapses on the proximal dendrites of a Purkinje cell. Stimulation of such a mono-innervating climbing fibre elicits very large EPSPs that are enough to activate voltage-gated Ca2+ channels over whole dendrites (Konnerth et al. 1992; Miyakawa et al. 1992; Hashimoto et al. 2001a). By contrast, multiple climbing fibres initially form synapses around the somata of Purkinje cells in newborn mice (Chedotal & Sotelo 1993; Morando et al. 2001). EPSCs elicited by stimulating such multiply innervating climbing fibres are much smaller than those of mature climbing fibres (Kano et al. 1995, 1997, 1998; Hashimoto & Kano 2003). Therefore climbing fibre inputs become stronger during post-natal development, while redundant climbing fibres are eliminated during the same period. Mariani and Changeux showed that some Purkinje cells had two climbing fibre-mediated EPSPs, the amplitudes of which were quite different around P10–P13 (Mariani & Changeux 1981), suggesting that only one climbing fibre is strengthened relative to others prior to the completion of synapse elimination.
We analysed changes in the relative synaptic strengths of multiple climbing fibres innervating the same Purkinje cell in mice (Hashimoto & Kano 2003). In the Purkinje cells from newborn mice, the amplitudes of climbing fibre-mediated EPSCs (CF-EPSCs) are similar, indicating that multiple climbing fibres have similar strengths. In the Purkinje cells from mice of approximately two weeks of age, one CF-EPSC is much larger than other few CF-EPSCs. Our detailed quantitative analysis of the developmental course indicates that the disparity among multiply innervating CF-EPSC amplitudes progressively becomes lager from P3 to P6 and reaches a plateau after P7 (Hashimoto & Kano 2003). A morphological study by Sugihara has shown that the innervation pattern of climbing fibres over Purkinje cells drastically changes during this post-natal period in rats. At P4, climbing fibres have many creeping terminals in the Purkinje cell layer and their swellings do not aggregate at particular Purkinje cell somata (creeper type). Then, from P4 to P7, climbing fibres surround several specific Purkinje cell somata and form aggregated terminals on them (nest type; Sugihara 2005). This result indicates that one of the multiple climbing fibres innervating the same Purkinje cell is strengthened during the first post-natal week (Hashimoto & Kano 2003).
By contrast, Scelfo and Strata reported a conflicting result that the disparity among multiply innervating CF-EPSC amplitudes becomes smaller from P4 to P7, and then progressively increases from P7 to P10 (Scelfo & Strata 2005). The reason for the apparent discrepancy between the two reports is not clear. One possibility is a difference between preparations and/or techniques used in these studies. Scelfo and Strata reported that the average number of climbing fibres innervating individual Purkinje cells at P4–P7 was approximately 3.3 in CD1 mice. This value is much smaller than that of our measurements in C57BL/6 mice (approx. 5.9; Hashimoto & Kano 2005). This may be attributed to the difference in mouse strain (Scelfo & Strata 2005). Alternately, Scefo and Strata might have missed more climbing fibres than we might have when searching for multiple climbing fibres innervating individual Purkinje cells.
We examined the physiological parameters of CF-EPSCs among the strongest and weaker climbing fibre inputs in individual multiply innervated Purkinje cells as well as those of the single climbing fibre inputs in mono-innervated Purkinje cells. The size of glutamate transients at the synaptic clefts from weaker climbing fibres were consistently smaller than those for the strongest climbing fibres (Hashimoto & Kano 2003). This difference is attributable to the fact that the number of simultaneously released vesicles is smaller (i.e. the probability of multivesicular release is lower in weaker climbing fibres) when compared with the strongest climbing fibre innervating the same Purkinje cell or climbing fibres of mono-innervated Purkinje cells. The occurrence of multivesicular release is affected by the release probability and the number of release sites. We show that the paired-pulse ratio, which reflects release probability, is not different among the three types of climbing fibres in a condition in which postsynaptic AMPA receptors should not be saturated (i.e. with a low external Ca2+ concentration; Hashimoto & Kano 2003). Therefore, it is concluded that the number of functional release sites facing a narrow postsynaptic region of Purkinje cell is smaller in the weaker climbing fibres than in the strongest climbing fibre.
In addition to functional differences, we show that the innervation territories over Purkinje cell dendrites are different between the strongest and weaker climbing fibres. We also show that Ca2+ signals elicited by stimulating the strongest climbing fibres spread over the whole dendritic trees, indicating that these climbing fibres form synaptic contacts in a similar manner to mono-innervating mature climbing fibres in the adult (Konnerth et al. 1992; Miyakawa et al. 1992; Hashimoto et al. 2001a). By contrast, Ca2+ signals elicited by stimulating weaker climbing fibres were confined to the most proximal parts of the dendritic trees (Hashimoto & Kano 2003). This result indicates that synapses formed by weaker climbing fibres are confined to the proximal part of dendrites near the soma.
(b) Elimination of redundant climbing fibre synapses
Earlier studies in the 1970s and 1980s clarified that multiple innervation persists into adulthood in several spontaneously occurring mutant mice that are devoid of granule cells or have a defect in parallel fibre synaptogenesis. These mice include reeler (Mariani et al. 1977), weaver (Crepel & Mariani 1976; Puro & Woodward 1977) and staggerer (Crepel et al. 1980; Mariani & Changeux 1980). The multiple innervation also persists in the animals whose migrating granule cells are destroyed by X-ray irradiation (Woodward et al. 1974; Crepel et al. 1981) or by infection of a mink enteritis virus (Benoit et al. 1987). These results suggest that the regression of surplus climbing fibres is critically dependent on the normal formation of granule cell to Purkinje cell connections (Crepel 1982; Lohof et al. 1996). Moreover, an analysis of the developmental course of multiple innervation in X-ray irradiated rats revealed that there are at least two distinct phases in climbing fibre elimination process (Crepel et al. 1981). In these rats, initial climbing fibre innervation and subsequent elimination until P8 are normal, but further regression of climbing fibres does not progress in the following period (Crepel et al. 1981). This indicates that the early phase of synapse elimination is independent of the formation of granule cell to Purkinje cell connection, but the late phase is critically dependent on it. This notion is confirmed by the analysis of a GluRδ2 knockout mouse. GluRδ2 belongs to a family of ionotropic glutamate receptors, which is expressed selectively in Purkinje cells (Araki et al. 1993; Lomeli et al. 1993; Takayama et al. 1996; Landsend et al. 1997). In the GluRδ2 null mutant mice, the density of parallel fibre synapses is less than half of that of the wild-type mice, whereas the abnormalities in cerebellar structures, Purkinje cell dendrites and granule cell density are mild (Kashiwabuchi et al. 1995; Kurihara et al. 1997). Kashiwabuchi et al. (1995) showed that the climbing fibre synapse elimination was impaired, supporting that parallel fibre synapse formation is essential for the climbing fibre synapse elimination. In addition, Hashimoto and co-workers revealed that the spatial pattern of climbing fibre innervation over Purkinje cell dendrites was abnormal in the GluRδ2 knockout mice. In the adult cerebellum, parallel fibres and a climbing fibre normally make synapses on distal and proximal dendrites, respectively. In the GluRδ2 knockout mice, climbing fibres invade into the distal dendrites and form ectopic synapses there (Hashimoto et al. 2001a; Ichikawa et al. 2002). These ectopic climbing fibre synapses appear around P10 when parallel fibre synapse formation and Purkinje cell dendritic arborization occur most vigorously. A similar type of multiple climbing fibre innervation was also found in a mutant mouse deficient in cerebellin in which parallel fibre to Purkinje cell synapse formation is severely impaired (Hirai et al. 2005). These results suggest that parallel fibres compete with climbing fibres for the innervation territory during development and play a role in restricting climbing fibre innervation to proximal dendrites.
(c) Parallel fibres activate mGluR1 signalling cascades for driving the regression of surplus climbing factor synapses
Another role of parallel fibre synapse is to activate mGluR1 and its downstream signalling cascades in the Purkinje cell to drive the process of climbing fibre synapse elimination. Kano, Hashimoto and co-workers show that the mutant mice deficient in mGluR1, Gαq, PLCβ4 or PKCγ are all impaired in climbing fibre synapse elimination (Kano et al. 1995, 1997, 1998; Offermanns et al. 1997; Hashimoto et al. 2000, 2001b; Ichise et al. 2000). Levenes et al. (1997) also reported incomplete synapse elimination in the mGluR1 knockout mice. In these mice, the regression of climbing fibre synapse normally occurs until around P10, indicating that the early phase of climbing fibre synapse elimination (Crepel et al. 1981) is independent of these signalling molecules. However, these mice display an abnormality in synapse elimination thereafter. These results suggest that the signalling cascade from mGluR1 to PKCγ is essential for the late phase of climbing fibre synapse elimination. Importantly, the formation and function of parallel fibre to Purkinje cell synapses are normal in these mutant mice, indicating that the multiple climbing fibre innervation is not caused secondarily by the defect in parallel fibre synaptogenesis.
The defect of the climbing fibre synapse elimination in the mGluR1 knockout mice is restored in mGluR1-rescue mice in which mGluR1a has been introduced specifically into Purkinje cells (Ichise et al. 2000). Regression of climbing fibre synapses is impaired in mice by Purkinje cell-specific expression of a PKC inhibitor peptide (De Zeeuw et al. 1998). Furthermore, the distribution of multiply innervated Purkinje cells in the PLCβ4 knockout mouse cerebellum exactly matches that of the Purkinje cells predominantly expressing PLCβ4 in the wild-type mouse cerebellum (Kano et al. 1998; Hashimoto et al. 2000).These lines of evidence clearly indicate that the signalling from mGluR1 to PKCγ in Purkinje cells but not other cell types plays a central role in mediating climbing fibre synapse elimination.
Although mGluR1 is concentrated at perisynaptic annuli of the postsynaptic Purkinje cell spines facing parallel and climbing fibre terminals (Baude et al. 1993; Nusser et al. 1994; Petralia et al. 1998; Lopez-Bendito et al. 2001), mGluR1 activation at parallel fibre synapses appears to be important for the late phase of climbing fibre synapse elimination. This could be correlated to the fact that mGluR1 can readily be activated by parallel fibre inputs (Finch & Augustine 1998; Takechi et al. 1998) but hardly by climbing fibre inputs without blockade of glutamate transporters (Dzubay & Otis 2002). Furthermore, we demonstrate that chronic blockade of NMDA receptors within the cerebellum specifically impairs the late phase of climbing fibre synapse elimination (Kakizawa et al. 2000). Because NMDA receptors are not present at excitatory synapses of Purkinje cells but are abundantly expressed at mossy fibre to granule cell synapses, the chronic blockade of NMDA receptors within the cerebellum should affect mossy fibre to granule cell transmission. These results suggest that neural activity along mossy fibre (granule cell) and parallel fibre (Purkinje cell) pathway, and subsequent activation of mGluR1 is a prerequisite for the late phase of climbing fibre synapse elimination (Kakizawa et al. 2000).
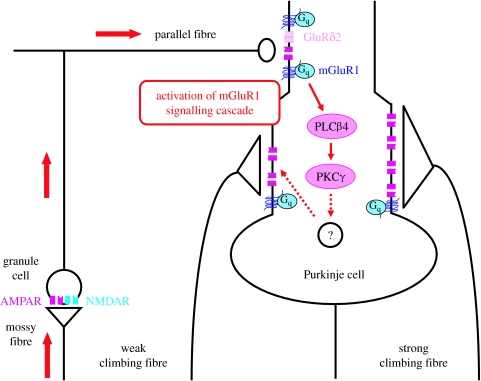
Figure 3 illustrates the current model for mechanisms underlying the late phase of climbing fibre synapse elimination. First, parallel fibre synapses confine the climbing fibre innervation sites to the proximal dendrites of Purkinje cells. Second, parallel fibre activity involving NMDA receptor at mossy fibre to granule cell synapses drives mGluR1 to PKCγ signalling cascades in the Purkinje cells to eliminate weaker climbing fibres. The signalling molecules downstream of PKCγ are currently unknown. Furthermore, we know little about the molecular mechanisms underlying the morphological elimination of the weaker climbing fibres. Some mechanisms must convey trans-synaptic retrograde signalling from Purkinje cells to weaker climbing fibres. One possibility would be that some cytokines such as insulin-like growth factor 1 (IGF-1) supports climbing fibre synapses (Kakizawa et al. 2003); the climbing fibres that do not receive a large enough amount of such cytokines may undergo regression. Another possibility would be that climbing fibre synapses may be maintained by protein–protein interaction between surface-expressed proteins such as cell adhesion molecules; disruption of such interaction may lead to the regression of climbing fibres.
Figure 3.
Contribution of mGluR1 to synapse elimination during cerebellar development. Elimination of redundant climbing fibre synapses on Purkinje cells consists of two phases, the early phase (until P10) and the late phase (P10–P20) in mice. The mGluR1 signal is required for the late phase. Neural activity along mossy fibre (granule cell) and parallel fibre (Purkinje cell) pathway, which is gated by NMDA receptor at the mossy fibre to granule cell synapses, drives the mGluR1 signalling cascades in Purkinje cells. Subsequently, the downstream signalling cascade involving Gq, PLCβ4 and PKCγ is activated and, through unknown mechanisms, it facilitates the elimination of weaker climbing fibre synapses while leaving stronger climbing fibre synapses intact. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; NMDAR, N-methyl-D-aspartate receptor.
5. Conclusion
In this article, we have made an overview of the three functional roles of mGluR1 in cerebellar Purkinje cells: LTD at parallel fibre to Purkinje cell synapses; endocannabinoid-mediated retrograde suppression of transmitter release; and climbing fibre synapse elimination during post-natal development. Japanese neuroscientists have made seminal contributions to the discovery and the elucidation of the mechanisms of these phenomena. Based on the theory that LTD underlies cerebellar motor learning, Ito demonstrated LTD experimentally and paved a way to the subsequent researches on LTD. The presence of metabotropic glutamate receptor was first demonstrated by Sugiyama and co-workers, and the molecular identity and functions of mGluR family receptors were clarified by Nakanishi and his pupils. In collaboration with Aiba who created the mGluR1 knockout mouse and the mGluR1-rescue mouse, we elucidated that mGluR1 signal transduction in the Purkinje cell is crucial for LTD induction. We also discovered the mGluR1-mediated endocannabinoid release and resultant retrograde suppression of transmitter release. Finally, we show that mGluR1 signal transduction in the Purkinje cell is indispensable for the elimination of redundant climbing fibre synapses that occurs during second and third post-natal weeks in mice. The massive outcome in this field is due to the efforts of Japanese scientists from various disciplines including neurophysiology, neuroanatomy, neuropharmacology, molecular biology, computational neuroscience and genetic engineering.
Acknowledgments
We thank D. Kawakami for preparing the figures. This work was supported by grants-in-aid for scientific research 17023021 and 17100004 (M.K.), 17700305 and 18019022 (T.T.) and 16680014 (K.H.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
One contribution of 17 to a Theme Issue ‘Japan: its tradition and hot topics in biological sciences’.
References
- Aiba A, Kano M, Chen C, Stanton M.E, Fox G.D, Herrup K, Zwingman T.A, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. doi:10.1016/0092-8674(94)90204-6 [PubMed] [Google Scholar]
- Albus J.S. A theory of cerebellar function. Math. Biosci. 1971;10:25–61. doi:10.1016/0025-5564(71)90051-4 [Google Scholar]
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel delta 2 subunit in cerebellar Purkinje cells. Biochem. Biophys. Res. Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. doi:10.1006/bbrc.1993.2614 [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. doi:10.1016/0896-6273(92)90096-V [DOI] [PubMed] [Google Scholar]
- Batchelor A.M, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. doi: 10.1038/385074a0. doi:10.1038/385074a0 [DOI] [PubMed] [Google Scholar]
- Batchelor A.M, Madge D.J, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63:911–915. doi: 10.1016/0306-4522(94)90558-4. doi:10.1016/0306-4522(94)90558-4 [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts J.D, Mulvihill E, McIlhinney R.A, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. doi:10.1016/0896-6273(93)90086-7 [DOI] [PubMed] [Google Scholar]
- Benoit P, Mariani J, Delhaye-Bouchaud N, Chappuis G. Evidence for a multiple innervation of cerebellar Purkinje cells by climbing fibers in adult ferrets infected at birth by a mink enteritis virus. Brain Res. 1987;431:51–57. doi: 10.1016/0165-3806(87)90194-5. [DOI] [PubMed] [Google Scholar]
- Brenowitz S.D, Regehr W.G. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. doi:10.1016/j.neuron.2004.12.045 [DOI] [PubMed] [Google Scholar]
- Brown S.P, Brenowitz S.D, Regehr W.G. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat. Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. doi:10.1038/nn1126 [DOI] [PubMed] [Google Scholar]
- Chedotal A, Sotelo C. The ’creeper stage’ in cerebellar climbing fiber synaptogenesis precedes the ’pericellular nest’-ultrastructural evidence with parvalbumin immunocytochemistry. Brain Res. Dev. Brain Res. 1993;76:207–220. doi: 10.1016/0165-3806(93)90209-s. doi:10.1016/0165-3806(93)90209-S [DOI] [PubMed] [Google Scholar]
- Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim J.J, Hashimoto K, Thompson R.F, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC gamma mutant mice. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. doi:10.1016/0092-8674(95)90148-5 [DOI] [PubMed] [Google Scholar]
- Conn P.J, Pin J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. doi:10.1146/annurev.pharmtox.37.1.205 [DOI] [PubMed] [Google Scholar]
- Conquet F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. doi:10.1038/372237a0 [DOI] [PubMed] [Google Scholar]
- Crepel F. Regression of functional synapses in the immature mammalian cerebellunm. Trends Neurosci. 1982;5:266–269. doi:10.1016/0166-2236(82)90168-0 [Google Scholar]
- Crepel F, Jaillard D. Protein kinases, nitric oxide and long-term depression of synapses in the cerebellum. Neuroreport. 1990;1:133–136. doi: 10.1097/00001756-199010000-00013. doi:10.1097/00001756-199010000-00013 [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J. Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the Weaver Mutant Mouse. J Neurobiol. 1976;7:579–582. doi: 10.1002/neu.480070610. doi:10.1002/neu.480070610 [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J. Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. doi:10.1002/neu.480070609 [DOI] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, Guastavino J.M, Sampaio I. Multiple innervation of cerebellar Purkinje cells by climbing fibres in staggerer mutant mouse. Nature. 1980;283:483–484. doi: 10.1038/283483a0. doi:10.1038/283483a0 [DOI] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, Dupont J.L. Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, X-irradiated and hypothyroid rats. Brain Res. 1981;227:59–71. doi: 10.1016/0165-3806(81)90094-8. [DOI] [PubMed] [Google Scholar]
- Crepel F, Daniel H, Hemart N, Jaillard D. Effects of ACPD and AP3 on parallel-fibre-mediated EPSPs of Purkinje cells in cerebellar slices in vitro. Exp. Brain Res. 1991;86:402–406. doi: 10.1007/BF00228964. doi:10.1007/BF00228964 [DOI] [PubMed] [Google Scholar]
- Daniel H, Levenes C, Fagni L, Conquet F, Bockaert J, Crepel F. Inositol-1,4,5-trisphosphate-mediated rescue of cerebellar long-term depression in subtype 1 metabotropic glutamate receptor mutant mouse. Neuroscience. 1999;92:1–6. doi: 10.1016/s0306-4522(99)00136-0. doi:10.1016/S0306-4522(99)00136-0 [DOI] [PubMed] [Google Scholar]
- De Zeeuw C.I, Hansel C, Bian F, Koekkoek S.K, van Alphen A.M, Linden D.J, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. doi:10.1016/S0896-6273(00)80990-3 [DOI] [PubMed] [Google Scholar]
- Dufosse M, Ito M, Jastreboff P.J, Miyashita Y. A neuronal correlate in rabbit's cerebellum to adaptive modification of the vestibulo-ocular reflex. Brain Res. 1978;150:611–616. doi: 10.1016/0006-8993(78)90825-9. doi:10.1016/0006-8993(78)90825-9 [DOI] [PubMed] [Google Scholar]
- Dzubay J.A, Otis T.S. Climbing fiber activation of metabotropic glutamate receptors on cerebellar purkinje neurons. Neuron. 2002;36:1159–1167. doi: 10.1016/s0896-6273(02)01052-8. doi:10.1016/S0896-6273(02)01052-8 [DOI] [PubMed] [Google Scholar]
- Eccles J.C, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J. Physiol. 1966a;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J.C, Llinas R, Sasaki K. The inhibitory interneurones within the cerebellar cortex. Exp. Brain Res. 1966b;1:1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Eccles J.C, Llinas R, Sasaki K. Intracellularly recorded responses of the cerebellar Purkinje cells. Exp. Brain Res. 1966c;1:161–183. doi: 10.1007/BF00236869. [DOI] [PubMed] [Google Scholar]
- Eccles J.C, Llinas R, Sasaki K. The mossy fibre–granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp. Brain Res. 1966d;1:82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Eccles J.C, Llinas R, Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp. Brain Res. 1966e;1:17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J.C, Sasaki K, Strata P. The profiles of physiological events produced by a parallel fibre volley in the cerebellar cortex. Exp. Brain Res. 1966f;2:18–34. doi: 10.1007/BF00234358. doi:10.1007/BF00234358 [DOI] [PubMed] [Google Scholar]
- Eccles J.C, Ito M, Szentagothai J. Springer; Berlin, Germany; New York, NY: 1967a. The cerebellum as a neuronal machine. [Google Scholar]
- Eccles J.C, Sasaki K, Strata P. A comparison of the inhibitory actions of Golgi cells and of basket cells. Exp. Brain Res. 1967b;3:81–94. doi: 10.1007/BF00234471. [DOI] [PubMed] [Google Scholar]
- Eccles J.C, Sasaki K, Strata P. Interpretation of the potential fields generated in the cerebellar cortex by a mossy fibre volley. Exp. Brain Res. 1967c;3:58–80. doi: 10.1007/BF00234470. [DOI] [PubMed] [Google Scholar]
- Ekerot C.F, Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985;342:357–360. doi: 10.1016/0006-8993(85)91136-9. doi:10.1016/0006-8993(85)91136-9 [DOI] [PubMed] [Google Scholar]
- Ekerot C.F, Kano M. Stimulation parameters influencing climbing fibre induced long-term depression of parallel fibre synapses. Neurosci. Res. 1989;6:264–268. doi: 10.1016/0168-0102(89)90065-5. doi:10.1016/0168-0102(89)90065-5 [DOI] [PubMed] [Google Scholar]
- Finch E.A, Augustine G.J. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. doi:10.1038/25541 [DOI] [PubMed] [Google Scholar]
- Foster A.C, Fagg G.E. Acidic amino acid receptor nomenclature: time for change. Trends Neurosci. 1988;11:17–18. doi: 10.1016/0166-2236(88)90043-4. doi:10.1016/0166-2236(88)90043-4 [DOI] [PubMed] [Google Scholar]
- Freeman J.H, Jr, Shi T, Schreurs B.G. Pairing-specific long-term depression prevented by blockade of PKC or intracellular Ca2+ Neuroreport. 1998;9:2237–2241. doi: 10.1097/00001756-199807130-00016. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Highstein S.M, Ito M. Cerebellar inhibitory control of the vestibulo-ocular reflex investigated in rabbit 3rd nucleus. Exp. Brain Res. 1972;14:511–526. doi: 10.1007/BF00236593. doi:10.1007/BF00236593 [DOI] [PubMed] [Google Scholar]
- Ghelarducci B, Ito M, Yagi N. Impulse discharges from flocculus Purkinje cells of alert rabbits during visual stimulation combined with horizontal head rotation. Brain Res. 1975;87:66–72. doi: 10.1016/0006-8993(75)90780-5. doi:10.1016/0006-8993(75)90780-5 [DOI] [PubMed] [Google Scholar]
- Gilbert P.F, Thach W.T. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. doi:10.1016/0006-8993(77)90997-0 [DOI] [PubMed] [Google Scholar]
- Hartell N.A. cGMP acts within cerebellar Purkinje cells to produce long term depression via mechanisms involving PKC and PKG. Neuroreport. 1994;5:833–836. doi: 10.1097/00001756-199403000-00024. [DOI] [PubMed] [Google Scholar]
- Hartmann J, et al. Distinct roles of Galpha(q) and Galpha11 for Purkinje cell signaling and motor behavior. J. Neurosci. 2004;24:5119–5130. doi: 10.1523/JNEUROSCI.4193-03.2004. doi:10.1523/JNEUROSCI.4193-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796. doi: 10.1016/s0896-6273(03)00298-8. doi:10.1016/S0896-6273(03)00298-8 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci. Res. 2005;53:221–228. doi: 10.1016/j.neures.2005.07.007. doi:10.1016/j.neures.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, et al. Climbing fiber synapse elimination during postnatal cerebellar development requires signal transduction involving G alpha q and phospholipase C beta 4. Prog. Brain Res. 2000;124:31–48. doi: 10.1016/S0079-6123(00)24006-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, et al. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J. Neurosci. 2001a;21:9701–9712. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Miyata M, Watanabe M, Kano M. Roles of phospholipase Cbeta4 in synapse elimination and plasticity in developing and mature cerebellum. Mol. Neurobiol. 2001b;23:69–82. doi: 10.1385/MN:23:1:69. doi:10.1385/MN:23:1:69 [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin H.S, Kano M. Phospholipase Cb serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. doi:10.1016/j.neuron.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Hemart N, Daniel H, Jaillard D, Crepel F. Receptors and second messengers involved in long-term depression in rat cerebellar slices in vitro: a reappraisal. Eur. J. Neurosci. 1995;7:45–53. doi: 10.1111/j.1460-9568.1995.tb01019.x. doi:10.1111/j.1460-9568.1995.tb01019.x [DOI] [PubMed] [Google Scholar]
- Hesslow G, Yeo C. Cerebellum and learning: a complex problem. Science. 1998;280:1817–1819. doi: 10.1126/science.280.5371.1815e. doi:10.1126/science.280.5371.1815e [DOI] [PubMed] [Google Scholar]
- Hirai H, et al. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat. Neurosci. 2005;8:1534–1541. doi: 10.1038/nn1576. doi:10.1038/nn1576 [DOI] [PubMed] [Google Scholar]
- Hirano T. Depression and potentiation of the synaptic transmission between a granule cell and a Purkinje cell in rat cerebellar culture. Neurosci. Lett. 1990;119:141–144. doi: 10.1016/0304-3940(90)90818-t. doi:10.1016/0304-3940(90)90818-T [DOI] [PubMed] [Google Scholar]
- Hirono M, et al. Phospholipase Cbeta4 and protein kinase Calpha and/or protein kinase CbetaI are involved in the induction of long term depression in cerebellar Purkinje cells. J. Biol. Chem. 2001a;276:45 236–45 242. doi: 10.1074/jbc.M105413200. doi:10.1074/jbc.M105413200 [DOI] [PubMed] [Google Scholar]
- Hirono M, Yoshioka T, Konishi S. GABA(B) receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat. Neurosci. 2001b;4:1207–1216. doi: 10.1038/nn764. doi:10.1038/nn764 [DOI] [PubMed] [Google Scholar]
- Ichikawa R, Miyazaki T, Kano M, Hashikawa T, Tatsumi H, Sakimura K, Mishina M, Inoue Y, Watanabe M. Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor delta 2. J. Neurosci. 2002;22:8487–8503. doi: 10.1523/JNEUROSCI.22-19-08487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. doi:10.1126/science.288.5472.1832 [DOI] [PubMed] [Google Scholar]
- Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J. Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Neurophysiological aspects of the cerebellar motor control system. Int. J. Neurol. 1970;7:162–176. [PubMed] [Google Scholar]
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972;40:81–84. doi: 10.1016/0006-8993(72)90110-2. doi:10.1016/0006-8993(72)90110-2 [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar control of the vestibulo-ocular reflex-around the flocculus hypothesis. Annu. Rev. Neurosci. 1982;5:275–296. doi: 10.1146/annurev.ne.05.030182.001423. doi:10.1146/annurev.ne.05.030182.001423 [DOI] [PubMed] [Google Scholar]
- Ito M. Raven Press; New York, NY: 1984. The cerebellum and neural control. [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol. Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Ito M. The molecular organization of cerebellar long-term depression. Nat. Rev. Neurosci. 2002;3:896–902. doi: 10.1038/nrn962. doi:10.1038/nrn962 [DOI] [PubMed] [Google Scholar]
- Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci. Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. doi:10.1016/0304-3940(82)90380-9 [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M. The cerebellar-evoked monosynaptic inhibition of Deiters' neurones. Experientia. 1964;20:515–516. doi: 10.1007/BF02154085. doi:10.1007/BF02154085 [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M. The origin of cerebral-induced inhibition of Deiters neurones. I. Monosynaptic initiation of the inhibitory postsynaptic potentials. Exp. Brain Res. 1966;2:330–349. doi: 10.1007/BF00234779. [DOI] [PubMed] [Google Scholar]
- Ito M, Obata K, Ochi R. The origin of cerebellar-induced inhibition of Deiters neurones. II. Temporal correlation between the trans-synaptic activation of Purkinje cells and the inhibition of Dieters neurones. Exp. Brain Res. 1966;2:350–364. doi: 10.1007/BF00234780. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K. Monosynaptic inhibition of the intracerebellar nuclei induced rom the cerebellar cortex. Experientia. 1964;20:575–576. doi: 10.1007/BF02150304. doi:10.1007/BF02150304 [DOI] [PubMed] [Google Scholar]
- Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kim S.J, Kim J, Worley P.F, Linden D.J. Long-term depression of mGluR1 signaling. Neuron. 2007;55:277–287. doi: 10.1016/j.neuron.2007.06.035. doi:10.1016/j.neuron.2007.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J. Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Yamada K, Iino M, Watanabe M, Kano M. Effects of insulin-like growth factor I on climbing fibre synapse elimination during cerebellar development. Eur. J. Neurosci. 2003;17:545–554. doi: 10.1046/j.1460-9568.2003.02486.x. doi:10.1046/j.1460-9568.2003.02486.x [DOI] [PubMed] [Google Scholar]
- Kamikubo Y, Tabata T, Kakizawa S, Kawakami D, Watanabe M, Ogura A, Iino M, Kano M. Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells. J. Physiol. 2007;585:549–563. doi: 10.1113/jphysiol.2007.141010. doi:10.1113/jphysiol.2007.141010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987;325:276–279. doi: 10.1038/325276a0. doi:10.1038/325276a0 [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S. Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. doi:10.1016/0092-8674(95)90147-7 [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. doi:10.1016/S0896-6273(01)80047-7 [DOI] [PubMed] [Google Scholar]
- Kano M, et al. Phospholipase cbeta4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc. Natl Acad. Sci. USA. 1998;95:15 724–15 729. doi: 10.1073/pnas.95.26.15724. doi:10.1073/pnas.95.26.15724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabuchi N, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. doi:10.1016/0092-8674(95)90334-8 [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Armstrong C.M. Induction of long-term depression and rebound potentiation by inositol trisphosphate in cerebellar Purkinje neurons. Proc. Natl Acad. Sci. USA. 1997;94:14 009–14 014. doi: 10.1073/pnas.94.25.14009. doi:10.1073/pnas.94.25.14009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J. Neurosci. 2006;26:8829–8837. doi: 10.1523/JNEUROSCI.1236-06.2006. doi:10.1523/JNEUROSCI.1236-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Fujimichi R, Araishi K, Kawahara S, Kano M, Aiba A, Kirino Y. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. Eur. J. Neurosci. 2002;16:2416–2424. doi: 10.1046/j.1460-9568.2002.02407.x. doi:10.1046/j.1460-9568.2002.02407.x [DOI] [PubMed] [Google Scholar]
- Konnerth A, Dreessen J, Augustine G.J. Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc. Natl Acad. Sci. USA. 1992;89:7051–7055. doi: 10.1073/pnas.89.15.7051. doi:10.1073/pnas.89.15.7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer A.C, Regehr W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. doi:10.1016/S0896-6273(01)00246-X [DOI] [PubMed] [Google Scholar]
- Kubo Y, Miyashita T, Murata Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. doi:10.1126/science.279.5357.1722 [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R. Distinct localization of GABA(B) receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur. J. Neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. doi:10.1046/j.0953-816x.2001.01855.x [DOI] [PubMed] [Google Scholar]
- Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M. Impaired parallel fiber->Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J. Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsend A.S, Amiry-Moghaddam M, Matsubara A, Bergersen L, Usami S, Wenthold R.J, Ottersen O.P. Differential localization of delta glutamate receptors in the rat cerebellum: coexpression with AMPA receptors in parallel fiber-spine synapses and absence from climbing fiber-spine synapses. J. Neurosci. 1997;17:834–842. doi: 10.1523/JNEUROSCI.17-02-00834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenes C, Daniel H, Jaillard D, Conquet F, Crepel F. Incomplete regression of multiple climbing fibre innervation of cerebellar Purkinje cells in mGLuR1 mutant mice. Neuroreport. 1997;8:571–574. doi: 10.1097/00001756-199701200-00038. doi:10.1097/00001756-199701200-00038 [DOI] [PubMed] [Google Scholar]
- Lichtman J.W, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. doi:10.1016/S0896-6273(00)80893-4 [DOI] [PubMed] [Google Scholar]
- Linden D.J, Connor J.A. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 1991;254:1656–1659. doi: 10.1126/science.1721243. doi:10.1126/science.1721243 [DOI] [PubMed] [Google Scholar]
- Linden D.J, Dickinson M.H, Smeyne M, Connor J.A. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–89. doi: 10.1016/0896-6273(91)90076-c. doi:10.1016/0896-6273(91)90076-C [DOI] [PubMed] [Google Scholar]
- Lohof A.M, Delhaye-Bouchaud N, Mariani J. Synapse elimination in the central nervous system: functional significance and cellular mechanisms. Rev. Neurosci. 1996;7:85–101. doi: 10.1515/revneuro.1996.7.2.85. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie D.J, Kohr G, Herb A, Seeburg P.H, Wisden W. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. doi:10.1016/0014-5793(93)81186-4 [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Shigemoto R, Lujan R, Juiz J.M. Developmental changes in the localisation of the mGluR1alpha subtype of metabotropic glutamate receptors in Purkinje cells. Neuroscience. 2001;105:413–429. doi: 10.1016/s0306-4522(01)00188-9. doi:10.1016/S0306-4522(01)00188-9 [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. doi:10.1016/S0896-6273(01)00375-0 [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase C b4 signaling cascade in the cerebellum. J. Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. doi:10.1523/JNEUROSCI.0945-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa K, Simpson J.L. Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J. Neurophysiol. 1973;36:649–666. doi: 10.1152/jn.1973.36.4.649. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat. Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. doi:10.1038/nn1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Short- and long-term depression of rat cerebellar parallel fibre synaptic transmission mediated by synaptic crosstalk. J. Physiol. 2007;578:545–550. doi: 10.1113/jphysiol.2006.115014. doi:10.1113/jphysiol.2006.115014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Changeux J.P. Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the adult staggerer mutant mouse. J. Neurobiol. 1980;11:41–50. doi: 10.1002/neu.480110106. doi:10.1002/neu.480110106 [DOI] [PubMed] [Google Scholar]
- Mariani J, Changeux J.P. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J. Neurosci. 1981;1:696–702. doi: 10.1523/JNEUROSCI.01-07-00696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Crepel F, Mikoshiba K, Changeux J.P, Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Phil. Trans. R. Soc. B. 1977;281:1–28. doi: 10.1098/rstb.1977.0121. doi:10.1098/rstb.1977.0121 [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J. Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. doi:10.1038/349760a0 [DOI] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. doi:10.1093/emboj/19.12.2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa H, Lev-Ram V, Lasser-Ross N, Ross W.N. Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. J. Neurophysiol. 1992;68:1178–1189. doi: 10.1152/jn.1992.68.4.1178. [DOI] [PubMed] [Google Scholar]
- Miyata M, et al. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. doi:10.1016/S0896-6273(00)00099-4 [DOI] [PubMed] [Google Scholar]
- Miyata M, et al. Deficient long-term synaptic depression in the rostral cerebellum correlated with impaired motor learning in phospholipase C beta4 mutant mice. Eur. J. Neurosci. 2001;13:1945–1954. doi: 10.1046/j.0953-816x.2001.01570.x. doi:10.1046/j.0953-816x.2001.01570.x [DOI] [PubMed] [Google Scholar]
- Morando L, Cesa R, Rasetti R, Harvey R, Strata P. Role of glutamate delta -2 receptors in activity-dependent competition between heterologous afferent fibers. Proc. Natl Acad. Sci. USA. 2001;98:9954–9959. doi: 10.1073/pnas.171098398. doi:10.1073/pnas.171098398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur. J. Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. doi:10.1111/j.1460-9568.2004.03768.x [DOI] [PubMed] [Google Scholar]
- Narasimhan K, Pessah I.N, Linden D.J. Inositol-1,4,5-trisphosphate receptor-mediated Ca mobilization is not required for cerebellar long-term depression in reduced preparations. J. Neurophysiol. 1998;80:2963–2974. doi: 10.1152/jn.1998.80.6.2963. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Meek J.L, Iadarola M.J, Chuang D.M, Roth B.L, Costa E. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J. Neurochem. 1986;46:40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. doi:10.1111/j.1471-4159.1986.tb12922.x [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. doi:10.1016/0306-4522(94)90421-9 [DOI] [PubMed] [Google Scholar]
- Obata K, Ito M, Ochi R, Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters neurones. Exp. Brain Res. 1967;4:43–57. doi: 10.1007/BF00235216. doi:10.1007/BF00235216 [DOI] [PubMed] [Google Scholar]
- Offermanns S, Hashimoto K, Watanabe M, Sun W, Kurihara H, Thompson R.F, Inoue Y, Kano M, Simon M.I. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq. Proc. Natl Acad. Sci. USA. 1997;94:14 089–14 094. doi: 10.1073/pnas.94.25.14089. doi:10.1073/pnas.94.25.14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. doi:10.1016/S0896-6273(01)00247-1 [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo M.M, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur. J. Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. doi:10.1046/j.1460-9568.2003.02732.x [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur. J. Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. doi:10.1046/j.1460-9568.2002.01929.x [DOI] [PubMed] [Google Scholar]
- Palay S.L, Chan-Palay V. Springer; New York, NY: 1974. Cerebellar Cortex. [Google Scholar]
- Petralia R.S, Zhao H.M, Wang Y.X, Wenthold R.J. Variations in the tangential distribution of postsynaptic glutamate receptors in Purkinje cell parallel and climbing fiber synapses during development. Neuropharmacology. 1998;37:1321–1334. doi: 10.1016/s0028-3908(98)00118-x. doi:10.1016/S0028-3908(98)00118-X [DOI] [PubMed] [Google Scholar]
- Puro D.G, Woodward D.J. The climbing fiber system in the Weaver mutant. Brain Res. 1977;129:141–146. doi: 10.1016/0006-8993(77)90976-3. doi:10.1016/0006-8993(77)90976-3 [DOI] [PubMed] [Google Scholar]
- Purves D, Lichtman J.W. Elimination of synapses in the developing nervous system. Science. 1980;210:153–157. doi: 10.1126/science.7414326. doi:10.1126/science.7414326 [DOI] [PubMed] [Google Scholar]
- Safo P.K, Regehr W.G. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. doi:10.1016/j.neuron.2005.09.020 [DOI] [PubMed] [Google Scholar]
- Sakurai M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J. Physiol. 1987;394:463–480. doi: 10.1113/jphysiol.1987.sp016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M. Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc. Natl Acad. Sci. USA. 1990;87:3383–3385. doi: 10.1073/pnas.87.9.3383. doi:10.1073/pnas.87.9.3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Strata P. Responses evoked in the cerebellar cortex by stimulating mossy fibre pathways to the cerebellum. Exp. Brain Res. 1967;3:95–110. doi: 10.1007/BF00233255. doi:10.1007/BF00233255 [DOI] [PubMed] [Google Scholar]
- Scelfo B, Strata P. Correlation between multiple climbing fibre regression and parallel fibre response development in the postnatal mouse cerebellum. Eur. J. Neurosci. 2005;21:971–978. doi: 10.1111/j.1460-9568.2005.03933.x. doi:10.1111/j.1460-9568.2005.03933.x [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron. 1994;12:1245–1255. doi: 10.1016/0896-6273(94)90441-3. doi:10.1016/0896-6273(94)90441-3 [DOI] [PubMed] [Google Scholar]
- Sladeczek F, Pin J.P, Recasens M, Bockaert J, Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985;317:717–719. doi: 10.1038/317717a0. doi:10.1038/317717a0 [DOI] [PubMed] [Google Scholar]
- Sugihara I. Microzonal projection and climbing fiber remodeling in single olivocerebellar axons of newborn rats at postnatal days 4–7. J. Comp. Neurol. 2005;487:93–106. doi: 10.1002/cne.20531. doi:10.1002/cne.20531 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. doi:10.1016/j.plipres.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Ito I, Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987;325:531–533. doi: 10.1038/325531a0. doi:10.1038/325531a0 [DOI] [PubMed] [Google Scholar]
- Tabata T, Aiba A, Kano M. Extracellular calcium controls the dynamic range of neuronal metabotropic glutamate receptor responses. Mol. Cell Neurosci. 2002;20:56–68. doi: 10.1006/mcne.2002.1118. doi:10.1006/mcne.2002.1118 [DOI] [PubMed] [Google Scholar]
- Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, Kano M. Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc. Natl Acad. Sci. USA. 2004;101:16 952–16 957. doi: 10.1073/pnas.0405387101. doi:10.1073/pnas.0405387101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, et al. G protein-independent neuromodulatory action of adenosine on metabotropic glutamate signaling in mouse cerebellar Purkinje cells. J. Physiol. 2007;581:693–708. doi: 10.1113/jphysiol.2007.129866. doi:10.1113/jphysiol.2007.129866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama C, Nakagawa S, Watanabe M, Mishina M, Inoue Y. Developmental changes in expression and distribution of the glutamate receptor channel delta 2 subunit according to the Purkinje cell maturation. Brain Res. Dev. Brain Res. 1996;92:147–155. doi: 10.1016/0165-3806(95)00212-x. doi:10.1016/0165-3806(95)00212-X [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. doi:10.1038/25547 [DOI] [PubMed] [Google Scholar]
- Tanaka J, et al. Gq protein alpha subunits Galphaq and Galpha11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur. J. Neurosci. 2000;12:781–792. doi: 10.1046/j.1460-9568.2000.00959.x. doi:10.1046/j.1460-9568.2000.00959.x [DOI] [PubMed] [Google Scholar]
- Varma N, Brager D, Morishita W, Lenz R.A, London B, Alger B. Presynaptic factors in the regulation of DSI expression in hippocampus. Neuropharmacology. 2002;43:550–562. doi: 10.1016/s0028-3908(02)00168-5. doi:10.1016/S0028-3908(02)00168-5 [DOI] [PubMed] [Google Scholar]
- Wang Y.T, Linden D.J. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. doi:10.1016/S0896-6273(00)81066-1 [DOI] [PubMed] [Google Scholar]
- Wilson R.I, Nicoll R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. doi:10.1038/35069076 [DOI] [PubMed] [Google Scholar]
- Woodward D.J, Hoffer B.J, Altman J. Physiological and pharmacological properties of Purkinje cells in rat cerebellum degranulated by postnatal x-irradiation. J. Neurobiol. 1974;5:283–304. doi: 10.1002/neu.480050402. doi:10.1002/neu.480050402 [DOI] [PubMed] [Google Scholar]
- Xia J, Chung H.J, Wihler C, Huganir R.L, Linden D.J. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. doi:10.1016/S0896-6273(00)00128-8 [DOI] [PubMed] [Google Scholar]