Abstract
Dried colonies of the Antarctic rock-inhabiting meristematic fungi Cryomyces antarcticus CCFEE 515, CCFEE 534 and C. minteri CCFEE 5187, as well as fragments of rocks colonized by the Antarctic cryptoendolithic community, were exposed to a set of ground-based experiment verification tests (EVTs) at the German Aerospace Center (DLR, Köln, Germany). These were carried out to test the tolerance of these organisms in view of their possible exposure to space conditions outside of the International Space Station (ISS). Tests included single or combined simulated space and Martian conditions. Responses were analysed both by cultural and microscopic methods. Thereby, colony formation capacities were measured and the cellular viability was assessed using live/dead dyes FUN 1 and SYTOX Green. The results clearly suggest a general good resistance of all the samples investigated. C. minteri CCFEE 5187, C. antarcticus CCFEE 515 and colonized rocks were selected as suitable candidates to withstand space flight and long-term permanence in space on the ISS in the framework of the LIchens and Fungi Experiments (LIFE programme, European Space Agency).
Keywords: Astrobiology, cryptoendolithic community, fungi, ground-based experiments, lithopanspermia, panspermia, space conditions, stress resistance, viability
INTRODUCTION
Astrobiology raises the fascinating question about the possible existence of life forms on other planets, survival in space conditions and possible interplanetary transfers. This emerging field of research encounters evident challenges in carrying out in situ experiments. Most of the information on the behaviour of organism under extraterrestrial conditions has been obtained by space simulation experiments performed on Earth (Nicholson et al. 2000, Rettberg et al. 2004, de Vera et al. 2003, 2004). Earth is our only reference for studying the possibility of life on other planetary bodies: extreme terrestrial environments host specifically adapted organisms, among which anhydrobionts are generally considered the best models for exobiological studies (Finster et al. 2007). Extreme environments include the cold and dry polar regions, permafrost soils, deep sea, alkaline and hypersaline habitats, arid regions, or acidic sites (González-Toril et al. 2003, McKay et al. 2003, Gunde-Cimerman et al. 2005, Gilichinsky et al. 2007, Onofri et al. 2007a). Organisms able to thrive in extreme environments are generally defined as `extremophiles'. The existence of extremophiles has led to speculations about the survival of organisms during interplanetary transfer and that life could be present even on other planets of the Solar system, where water might be present e.g. under the icy surfaces of some of Jupiter's moons or in the underground caverns of Mars (Miller 2005, Mustard et al. 2008).
Antarctica is a continent where a combination of dry, cold, and oligotrophic extremes exists, and huge fluxes of dangerous radiations such as UV radiation can be present as well. These conditions become harsher in the ice-free McMurdo Dry Valleys area in continental Antarctica where cryptoendolithic microbial communities are almost the only life-form possible. Black meristematic microfungi of these communities were already suggested as eukaryotic models for the biological exploration of Mars (Onofri et al. 2004). The McMurdo Dry Valleys area, also known as Ross Desert, is the largest ice-free area in Antarctica. It is located within the Transantarctic Mountains in the Southern part of Victoria Land. Winter air temperature fluctuates between –20 and –50 °C (occasionally lower), rising to mean daily values of about –15 °C in the summer, up to 15 °C or higher values at ground surfaces. Wide and repeated thermal fluctuations are a stress factor, more than the minimum values reached. Dryness is also extreme: water is mainly supplied by snow (less than 100 mm water equivalent / yr), that mostly sublimes without visibly wetting the ground or it is blown away (Nienow & Friedmann 1993). High evaporation leads to high salt concentration on rock surfaces and a poor nutrient soil is occasionally present because of the scarcity of organic matter. Finally, UV radiation is high, mainly in the springtime, as a consequence of stratospheric ozone depletion during this period. As for it's oxygenic atmosphere no place on Earth is truly comparable to what is present on Mars, the McMurdo Dry Valleys could be called the closest terrestrial analogue. Therefore, they are one of the best investigated areas as a model environment for astrobiological studies since the 1970ies (Horowitz et al. 1972, Wynn-Williams & Edwards 2000, Onofri et al. 2004).
Friedmann and co-workers (Friedmann & Ocampo-Friedmann 1976, Friedmann 1982, Nienow & Friedmann 1993) discovered various microbial communities in the Ross Desert. The communities live sheltered under the rock surface, where they find a more favorable nanoclimate in the rock pore-spaces. Among these, the “lichen dominated cryptoendolithic community” is the most studied and known (Friedmann 1982). Under a reddish superficial crust, eukaryotic and prokaryotic autotrophic and heterotrophic microorganisms form a clearly stratified community. Antarctic black non-lichenised rock fungi, showing meristematic growth, are recurrent components of this community and common inhabitants of the black zone immediately below the lichen crust. Their biodiversity has been studied only recently. Sampling is still limited, yet some new genera were discovered in these habitats, viz. Friedmanniomyces, Cryomyces, Recurvomyces and Elasticomyces (Onofri et al. 1999, 2004, Selbmann et al. 2005, 2008).
The results from freeze-thaw experiments, after UV exposures and osmotic stress tolerance of two isolates of C. antarcticus (CCFEE 515 and CCFEE 534 = CBS 116301), and one isolate of C. minteri (CCFEE 5187 = CBS 116902), revealed an unusual ability to survive under these pressures (Onofri et al. 2007b).
The opportunity to expose these isolates to space conditions by the “EXPOSE-E” facility of the European Space Agency (ESA) on the EuTEF platform (part of the European Columbus Laboratory) outside of the International Space Station (ISS) inspired ground-based experiments that will be described in this contribution. The experiments have been carried out at the German Aerospace Center (DLR, Köln, Germany), where we tested single and combined simulated space and Martian conditions. Furthermore, we extended the experiments to the entire Antarctic crytptoendolithic community as well, also to test the potential protective role of the rock substratum.
We considered this as a preliminary and essential step before launching Antarctic black fungi and cryptoendolithic communities to outer space and for exposure on the ISS in the framework of the Lichens and Fungi Experiments (LIFE, European Space Agency).
MATERIALS AND METHODS
Biological material
The biological material used for the Experiment Verification Tests (EVTs) consisted of two isolates of C. antarcticus (CCFEE 515 and CCFEE 534), one isolate of C. minteri (CCFEE 5187), and colonised rock fragments.
Cryomyces antarcticus CCFEE 515 was isolated by R. Ocampo-Friedmann from sandstone collected at Linnaeus Terrace (Southern Victoria Land) by H. Vishniac, in the Antarctic expedition 1980-81; C. antarcticus CCFEE 534 was isolated by R. Ocampo-Friedmann from weathered rock collected at Linnaeus Terrace by E.I. Friedmann, during the Antarctic expedition 1981–82; C. minteri CCFEE 5187 was isolated by S. Pagano from weathered rocks collected by S. Onofri at Battleship Promontory (Southern Victoria Land) on Dec 28, 1996. For the EVTs, dehydrated fungal colonies were prepared as follows: cell suspensions were spread on MEA (malt extract agar: malt extract, powdered 30 g/L; peptone 5 g/L; agar 15 g/L; Applichem, GmbH) medium (5 mm thick) in Petri dishes and, once grown, maintained at 15 °C for 1 yr. Agar disks (12 mm diameter) containing 1–3 colonies each were then drilled and used for tests.
The colonised sandstone sample, with a well developed and stratified colonisation, was collected by L. Zucconi at Battleship Promontory (76°54'37.6”S 160°55'27.5”E), Southern Victoria Land, on Jan. 24, 2004. Colonised rock fragments (11 mm wide, maximum 6 mm thick) were obtained by hitting the rock lengthwise, and then dehydrated at room temperature.
Tests facilities and exposure conditions
Two sets of ground-based Experiment Verification Tests for EXPOSE-E (EVT-E1 and EVT-E2) were performed, using the Planetary and Space Simulation facilities (PSI) at the Institute of Aerospace Medicine (German Aerospace Center, DLR, Köln, Germany). Twenty-seven samples of each isolate and colonised rock for the EVT-E1 (9 tests) and 12 for the EVT-E2 (4 tests) were prepared, plus 3 controls each. The aim of EVT-E1 was to test the response of rock fungi and cryptoendolithic communities to exposure to the following space conditions: vacuum, temperature fluctuations (–20 / +20 °C), laboratory standard monochromatic UV-C radiation and high polychromatic UV radiation (Table 1).
Table 1.
Test parameters (EVT-E1 and EVT-E2).
| EVT | Parameters | Duration/exposure | No. samples | |
|---|---|---|---|---|
| E1 | ||||
| Vacuum 10-5 Pa | 1 h | 1.3 × 10-5 Pa | 3 | |
| 1 wk | 2.3 × 10-6 Pa | 3 | ||
| Temperature oscillation 50 cycles | ||||
| -20 °C to +20 °C, 1 atm air | 2 wk | 3 | ||
| UV-C irradiation monochromatic | 14 s | 10 Jm-2 | 3 | |
| 254 nm, 1 atm air, 71.4 μW/cm2 | 2 min 20 s | 100 Jm-2 | 3 | |
| 23 min 20 s | 1000 Jm-2 | 3 | ||
| UV irradiation polychromatic | 3 s (SOL2000) | 1.44 kJm-2 | 3 | |
| 200-400 nm, 1 atm air | 52 min (SOL2000) | 1.5 × 103 kJm-2 | 3 | |
| 87 h (SOL2000) | 1.5 × 105 kJm-2 | 3 | ||
| total number of samples EVT-E1 | 27 | |||
| E2 | ||||
| Vacuum 10-5 Pa (dark) | 22 d | 3 | ||
| Vacuum 10-5 Pa | 22 d | |||
| + | 1.5 × 105 kJm-2 | |||
| UV irradiation polychromatic | 244.5 h (SOL1000) | |||
| (200-400 nm) | 3 | |||
| Mars atmosphere 600 Pa (dark) | 21 d | 3 | ||
| Simulated CO2 Mars atmosphere | 21 d | |||
| 600 Pa | ||||
| + | 18 min (SOL2000) + | |||
| UV irradiation polychromatic | 10 d 3 h 40 min 48 s (SOL1000) | |||
| (200-400 nm) | 1.5 × 105 kJm-2 | 3 | ||
| total number of samples EVT-E2 | 12 | |||
| Control | ||||
| Room temperature, dark, 1 atm air | 2 mo | 3 | ||
The aim of EVT-E2 was to test the responses of rock fungi and cryptoendolithic community to simulated space vacuum, simulated CO2 Martian atmosphere and pressure, simulated space vacuum combined with polychromatic UV radiation, and simulated CO2 Martian atmosphere combined with polychromatic UV radiation (Table 1). All tests were performed in triplicate.
Vacuum (E1 and E2)
The pressure was set at the value of 10–5 Pa as expected to prevail during the space flight. Samples were accommodated in a vacuum facility, called PSI 6, and exposed to vacuum for 1 h and 1 wk for E1, and 22 d for E2, after reaching 10–5 Pa (monitored by a Pirani cold cathode inserted into the vacuum chamber).
Temperature fluctuation (E1)
Temperature fluctuations (–20 / +20 °C) are expected during the space flight. 50 cycles were therefore performed inside a facility, called PSI 2, within 2 wk, programming 2 h heating, 2.5 h cooling, and maintenance at –20 °C and +20 °C respectively for 1 h. The temperature was monitored with a sensor attached to the inner side of a sample carrier.
UV Radiation conditions and fluences (E1 and E2)
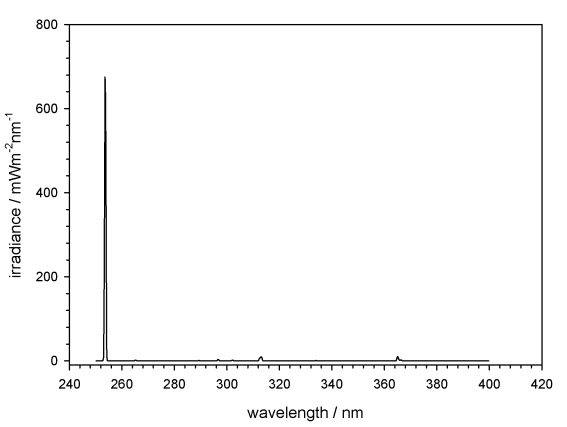
E1. The monochromatic UV-C at 254 nm was obtained using a Hg-low-pressure lamp, the spectral irradiance was measured using a Bentham spectroradiometer (Fig. 1) giving a total irradiance of 0.7 W/m2; in addition the irradiance was controlled before and after each exposure with a calibrated UVX-meter at the sample site. Samples were arranged within the homogeneously irradiated area. Due to the low infrared (IR) output of this lamp, no cooling was required. The exposure was performed additively, by covering the samples that received their assigned UV-C fluence of 10, 100 and 1000 Jm–2, with UV opaque filters.
Fig. 1.
Spectral irradiance of the monochromatic UV source, a Hg-low-pressure lamp, mainly emitting at 254 nm (data provided by DLR).
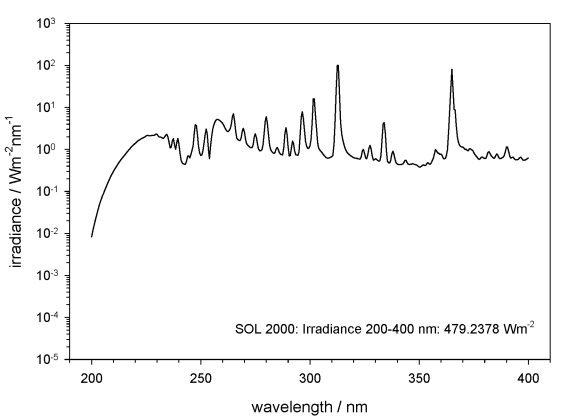
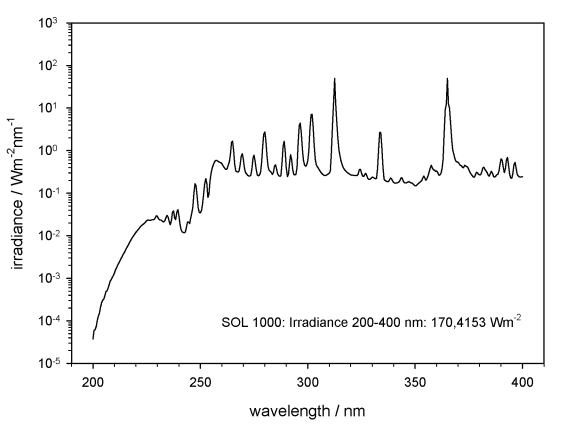
The polychromatic UV irradiance was obtained using the solar simulators SOL2000 and SOL1000 (the latter only used in EVT-E2). Figs 2, 3 show only the polychromatic UV irradiation spectra in the range 200–400 nm. The samples were accommodated on a cold plate inside the homogeneously irradiated area inside the facility PSI 2 (Fig. 4), and kept below 30 °C during the whole irradiation, by cooling the cold plate to 10 °C. Temperature was monitored during the irradiation by a sensor attached to the sample carrier (Fig. 5). The fluences for irradiation with polychromatic UV in EVT-E1 were selected to simulate the final dose of irradiation (1.5 × 105 kJm–2) and the attenuated fluences beneath 1 % and 0.01 % neutral density filters, expected on the ISS during the mission in this wavelength range. Similar fluences were reached with the solar simulator SOL2000 after 87 h irradiation for 1.5 × 105 kJm–2, 52 min for 1.5 × 103 kJm–2 and 3 s for 1.5 kJm–2 (Table 1).
Fig. 2.
Spectrum of the solar simulator SOL2000 in the range of the polychromatic UV, 200–400 nm (data provided by DLR).
Fig. 3.
Spectrum of the solar simulator SOL1000 in the range of the polychromatic UV, 200–400 nm (data provided by DLR).
Fig. 4.
Planetary and Space Simulation facility PSI 2: irradiation with solar simulator SOL2000.
Fig. 5.
Sample carrier, composed of three trays, 16 wells each, in PSI 2.
E2. A combination of polychromatic UV irradiation and vacuum or simulated Martian atmosphere was applied (Table 1). For the polychromatic UV irradiance in vacuum (10–5 Pa) as well as in a simulated CO2 Martian atmosphere (composed of argon 1.56 %, oxygen 0.16 %, nitrogen 2.72 %, carbon dioxide 95.56 %; pressure of 600 Pa), the samples were accommodated on the cold plate kept at 10 °C inside the homogeneous irradiated area inside the facility PSI 2. Temperature was monitored during the irradiation by a sensor attached to the sample carrier. The IR radiation of the solar simulators leads to heating the irradiated samples in the low pressure and hence low-convection environment. Because the SOL2000 caused a high heating of the samples, for more prolonged irradiation times the weaker SOL1000 solar simulator was used (Table 1).
The measured and integrated irradiance of the SOL2000 for the polychromatic UV range 200–400 nm was 479.2 W/m2, and 170.4 W/m2 for the SOL1000, at the sample site. The selected final dose of 1.5 × 105 kJm–2 (full polychromatic irradiation) was reached after a total irradiation time of 18 min with SOL2000 plus 243 h with SOL1000 in simulated CO2 Martian atmosphere, and after a total irradiation time of 244.5 h with SOL1000 irradiation in simulated space vacuum.
Analyses of responses to tested parameters
Responses to test parameters were analysed both by cultural and staining methods. Viability of isolates of C. antarcticus (CCFEE 515 and CCFEE 534) and of C. minteri (CCFEE 5187) after EVTs by cultural methods was evaluated as number of formed colony. Tested colonies were collected, preliminarily treated in a rotator (9 g/min) with 1 mL of a sterile solution of Tween 20 (0.35 %) for 15 min to remove eventual external contaminants, and then washed with 1 mL of sterile physiological solution (4 times), for 15 min, to remove Tween 20. Fungal suspensions were obtained by crumbling colonies with a sterile needle, followed by serial dilutions up to a final concentration of about 104 cells per mL of inoculum in physiological solution (0.9 % NaCl). Cultural tests were performed by spreading 0.15 mL of standardised inocula on MEA (Malt Extract Agar, Applichem Gmbh), with the addition of chloramphenicol (100 ppm) to prevent bacterial growth. Cultures were then incubated at 15 °C. The number of growing microcolonies was recorded after 1 and 2 mo of incubation. Data are reported as percentage of colonies compared to the untreated control. All tests were performed in triplicate.
Viability of samples from rocks was demonstrated by directly spreading small fragments of rocks on 5 different cultural media: MEA (Malt Extract Agar; Applichem Gmbh) and DRBC (Oxoid) media (Dichloran Rose-Bengal Chloramphenicol agar: peptone 5 g/L; dextrose 10 g/L; biacid potassium phosphate 1 g/L; magnesium sulphate 0.5 g/L; dichloran 0.002 g/L; rose-bengal 0.025 g/L; agar 15 g/L) for fungal growth (filamentous and black fungi respectively); TM medium (Trebouxia Medium: Bold's Basal Medium 970 mL; proteose peptone 2.5 g/L; glucose 5 g/L; agar 15 g/L) for algal growth; TY and BG11 media (Trypton Yeast Medium: trypton 5 g/L; yeast extract 3 g/L; CaCl2 anhydrous 0.4 g/L; agar 17 g/L. Blue Green Algae Medium: NaNO3 1.5 g/L; K2HPO4 0.04 g/L; MgSO4 × 7H2O 0.075 g/L; CaCl2 × 2H2O 0.036 g/L; citric acid 0.006 g/L; ammonium ferric citrate 0.006 g/L; EDTA 0.001 g/L; metal traces 1 mL/L: H3BO3 2.86 g/L, MnCl2 × 4H2O 1.81 g/L, ZnSO4 × 7H2O 0.222 g/L, NaMoO4 × 2H2O 0.39 g/L, CuSO4 × 5H2O 0.079 g/L, Co(NO3)2 × 6H2O 49.4 mg/L; agar 10 g/L) for bacterial and cyanobacterial growth, respectively. The occurrence / absence of growing colonies was simply recorded after 3 mo of incubation in the dark for fungi and bacteria, and in the light for algae and cyanobacteria.
Viability was also evaluated by staining methods using two dyes, the LIVE / DEAD dye FUN 1 (30 μM) and SYTOX Green (10 μM) in PBS (Dulbecco's Phosphate Buffered Saline: NaCl 8 g/L; KCl 0.2 g/L; Na2HPO4 anhydrous 1.15 g/L; KH2PO4 0.259 g/L). This enabled us to detect viable and metabolically active cells as well as non-viable or damaged cells. Staining methods were applied only to Cryomyces minteri isolate.
Each tested colony was rehydrated with a PBS solution for 1 h, included in a polyethylene glycol mixture (Killik, Bio-Optica) inside a cryostat chamber (Leica, CM1510 S) at –20 °C for 10 min, cut into sections about 25 μm thick by a microtome, and coloured on microscope slides. The best dye penetration for FUN 1 and SYTOX Green was obtained with an incubation of 1–3 h and 1.5 h respectively, under dark conditions at room temperature.
A fluorescence microscope Axioskop 2 plus (Zeiss), provided with long-pass filters (488 nm with emission ≥ 530 nm for fluorescein isothiocyanate, 546 nm with emission ≥ 580 nm for the rhodamine), was used to analyse the viability of the C. minteri samples after exposure to test parameters. Red (viable and metabolically active) and green (viable but not metabolically active) emitting images of stained samples were separately acquired by a mounted Axiocam using AxioVision v. 4 software. The combined image obtained by overlapping both images allows to distinguish clearly the viability state. The SYTOX Green dye (S-7020, Molecular Probes) stains the DNA of non-viable cells green when excited at 450–500 nm, and using an appropriate filter (504 nm with emission ≥ 524 nm). The imagines were imported in Adobe Photoshop whereas the quantitative analyses were performed using KS300 software and expressed as percentage of live / dead cells.
Responses to high temperatures
Thermal model calculations by ESA estimated that during the space flight the temperature may reach high values (up to 90 °C without any coverage). Therefore we tested the resistance of C. antarcticus CCFEE 515 and CCFEE 534 and C. minteri CCFEE 5187 at 60 °C (selected as limit value for closing automatically the EXPOSE sample carrier lids) and of C. antarcticus CCFEE 515 and C. minteri CCFEE 5187, previously selected for flight, also at 80 and 90 °C. Six-mo-old dried colonies of all the isolates were incubated at 60 °C for 15 and 60 min (3 replicates for each test) and six-mo old dried colonies of C. antarcticus CCFEE 515 and C. minteri CCFEE 5187 were incubated for 60 min at 80 and 90 °C (5 replicates). All colonies were subsequently maintained overnight in a desiccator, to allow a slow cooling to room temperature. Survival was tested by cultural methods, as described above, as number of colonies growing after two mo of incubation on MEA at 15 °C, for the tests at 60 °C, and as presence / absence of growing colonies for tests at 80 and 90 °C. Viability after exposure at 80 and 90 °C was also evaluated in C. minteri by staining methods.
RESULTS
Fungal isolates
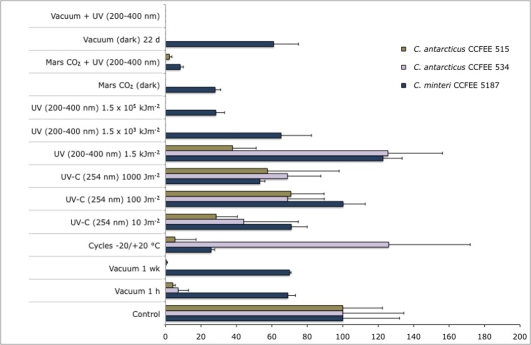
Figure 6 shows cell viability of fungal isolates after Experiment Verification Tests E1 and E2, analysed by cultural methods.
Fig. 6.
Percentage variations, compared to untreated control, in the number of colonies of C. antarcticus CCFEE 534 and CCFEE 515 and C. minteri CCFEE 5187 after exposure to single or combined simulated space and Mars conditions (EVT-E1 and E2), evaluated by cultural methods.
Vacuum
The viability of dried isolates after 1 h, 1 wk (EVT-E1) and 22 d (EVT-E2) of exposure to simulated space vacuum is reported as percentage variations, compared to the untreated controls, in the number of colonies grown after 2 mo of incubation on MEA at 15 °C. Colony formation of all isolates was negatively affected by the vacuum treatment, more markedly in C. antarcticus than in C. minteri. Cryomyces antarcticus CCFEE 534 is more markedly affected, with a complete loss of growth ability already after 1 wk of exposure, whereas isolate CCFEE 515 maintains the ability to grow also after 22 d of exposure.
Freeze and thawing cycles
Results of the viability of dried isolates after 50 repeated freeze and thawing cycles, recorded as above and compared to the untreated controls, showed different responses: C. antarcticus CCFEE 515 was the most negatively affected with a strong reduction of growth ability, C. minteri CCFEE 5187 showed a 75 % reduction, while C. antarcticus CCFEE 534 seemed to be almost unaffected.
UV radiation at different spectral ranges
With respect to the capability of dried fungal isolates to form colonies after increasing monochromatic or polychromatic radiation doses, respectively, C. antarcticus isolates were both negatively affected by the higher doses of polychromatic UV, whereas the species showed diversified response to the monochromatic irradiation (Fig. 6). CCFEE 515 remained practically unaffected, and CCFEE 534 was totally inhibited after the highest exposure only. Cryomyces minteri CCFEE 5187 showed a good survival after both radiation types and doses, with a certain decrease only at the highest doses.
UV radiation plus vacuum or Martian atmosphere and pressure
Figure 6 shows the results of exposure of dried samples to polychromatic UV spectrum in space-simulating vacuum as well as in simulated Martian atmosphere compared to vacuum and Martian atmosphere exposure, respectively. Growth obtained with both isolates of C. antarcticus was scarce, with a complete inhibition of isolate CCFEE 534 and a substantial reduction in viability in CCFEE 515. This response was consistent with negative results obtained after vacuum and 1.5 × 105 kJm–2 polychromatic irradiation. Propagules of C. minteri CCFFEE 5187 showed a higher survival than both C. antarcticus strains after exposure either to vacuum or Martian CO2, and a reduction, but not complete inhibition, at combined UV radiation and Martian atmosphere. A total absence in viability apparently appears in the combined test of vacuum and maximum UV (200–400 nm) dose of 1.5 × 105 kJm–2, but this result was disproved by the staining techniques.
The LIVE / DEAD dye FUN1 (F-7030, Molecular Probes) passively penetrates the cytoplasm and stains cells with a yellowish green to green fluorescence: the intravacuolar enzymatic activity of viable and metabolically active cells is indicated by the appearance of compact cylindrical intravacuolar structures (CIVS) with an orange to red fluorescence (when a λex between 470 and 590 nm is used) and a concomitant reduction of the green to yellow cytoplasmatic fluorescence.
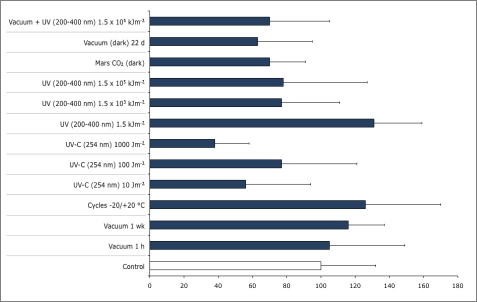
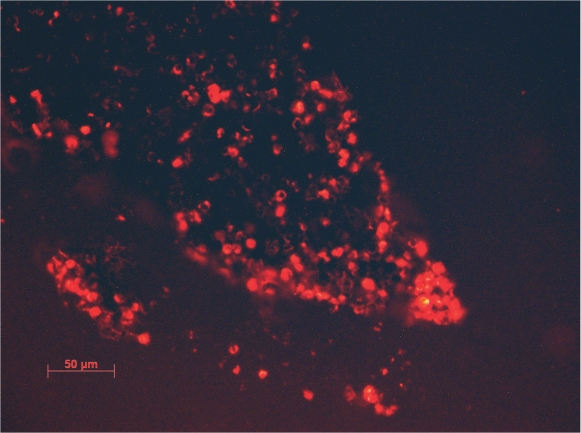
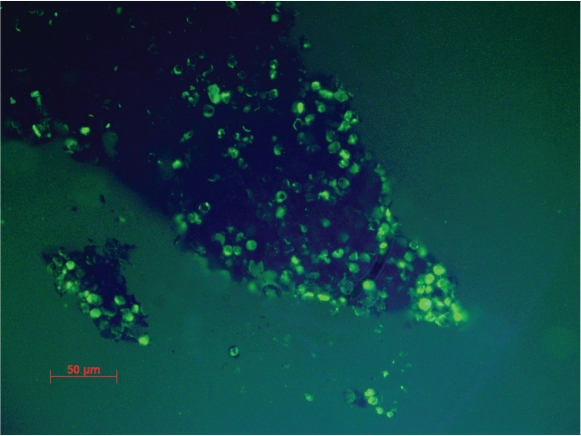
The staining procedure failed with the isolates of C. antarcticus because the thickness of cell wall prevented the penetration of the dyes, while the same procedure was successful with C. minteri. Good viability percentages obtained in all EVTs with the LIVE / DEAD dye FUN 1 (Fig. 7) confirmed the positive responses obtained by the cultural methods and the high resistance of C. minteri to simulated space and Martian conditions in both EVTs, while combined vacuum and maximum UV (200–400 nm) exposure gave ambiguous results when the responses were tested with cultural or staining approach. In fact, even though C. minteri was unable to grow after this treatment, cell viability in a 25 μm section of a colony was suggested by the red and green fluorescence shown in Fig. 8. Data (not shown) obtained by the SYTOX Green dye, confirmed results reported in Fig. 7.
Fig. 7.
Percentage variations, compared to untreated control, of C. minteri CCFEE 5187 cell viability after experiment verification tests E1 and E2, evaluated in vivo by LIVE / DEAD dye FUN 1.
Fig. 8.
Red and green fluorescence of viable and metabolic active cells in 25 μm sections of a C. minteri colony treated with LIVE / DEAD dye FUN 1, after combined exposure to vacuum and maximum polychromatic UV 200–400 nm dose (bar = 50 μm).
High temperatures
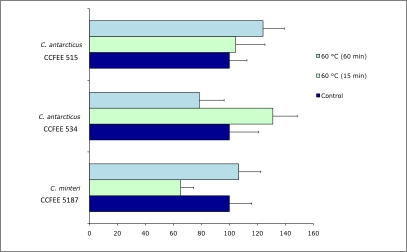
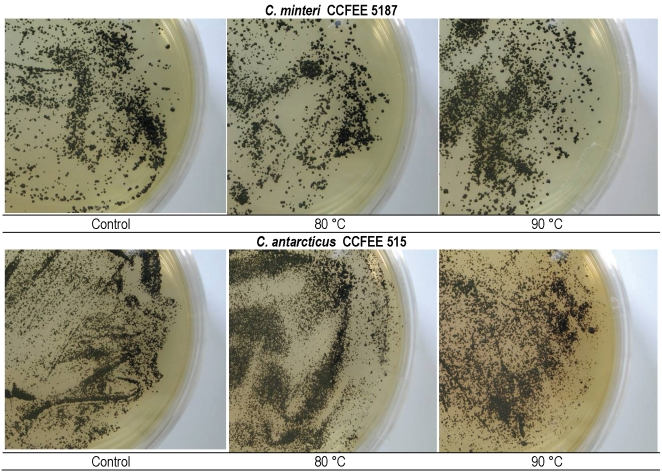
Effects of incubation at 60 °C for 15 and 60 min, and of 80 and 90 °C for 1 h respectively, on the viability of the tested isolates, was evaluated by counting the number of colonies (Fig. 9) and the presence / absence of grown colonies (Fig. 10). A good resistance of all strains was found at 60 °C for both 15 min and 60 min, with low variations compared to the controls. Both tested isolates of C. minteri CCFEE 5187 and C. antarcticus CCFEE 515 showed an unexpected high viability of growing colonies after exposure to 80 and 90 °C, already after one mo incubation (Fig. 10). Viability of C. minteri was confirmed by LIVE / DEAD dye FUN1 staining (Fig. 11).
Fig. 9.
Percentage variations, compared to controls, in the number of colonies of C. antarcticus CCFEE 534 and CCFEE 515 and C. minteri CCFEE 5187 after incubation at 60 °C for 15 and 60 min.
Fig. 10.
Colonies on MEA of C. minteri CCFEE 5187 and C. antarcticus CCFEE 515 after incubation at 80 °C (center) and 90 °C (right) for 60 min, compared to the controls (left).
Fig. 11.
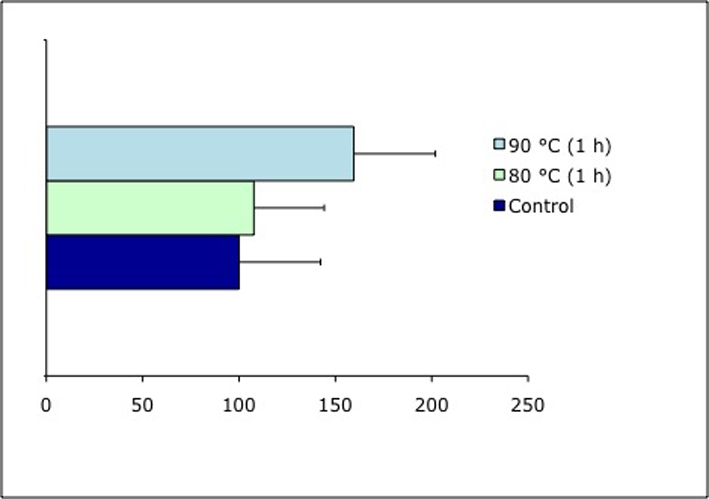
Percentage of cell viability of C. minteri CCFEE 5187 after incubation at 80 and 90 °C for 60 min, compared to the control, evaluated by using FUN 1 in vivo.
Cryptoendolithic community
Table 2 reports the presence or absence of colonies grown from rock fragment samples after EVTs. Eleven verification tests E1 and E2 were carried out, and colony appearance was recorded in 8 of them. Negative results are those concerning polychromatic irradiation at higher doses and vacuum plus polychromatic UV-irradiation at the lower dose. Some samples treated with simulated Martian atmosphere with or without combined polychromatic UV radiation have been lost and EVT-E2 data for these experiments are missing.
Table 2.
Presence (+) / absence (-) of colonies of fungi (filamentous fungi and black yeast fungi), algae, and bacteria (cyanobacteria included) from rock fragments after EVTs.
|
|
TEST
|
Colony formation
|
|||||
|---|---|---|---|---|---|---|---|
| EVT-E1 | Algae | Fungi | Bacteria | ||||
| Vacuum | 10-5 Pa | 1 h | - | - | + | ||
| 1 wk | + | - | + | ||||
| Freeze and thawing cycles | 2 wk | + | + | + | |||
| UV (254 nm), 1 atm | |||||||
| Fluence: | 10 Jm-2 | + | + | - | |||
| 100 Jm-2 | + | + | + | ||||
| 1000 Jm-2 | + | + | + | ||||
| UV (200-400 nm), 1 atm | |||||||
| Fluence: | 1.5 kJ m-2 | - | + | - | |||
| 1.5 × 103 kJ m-2 | - | - | - | ||||
| 1.5 × 105 kJ m-2 | - | - | - | ||||
| EVT-E2 | |||||||
| Vacuum | 10-5 Pa | 22 d | + | + | - | ||
| Vacuum + UV (200-400 nm) | |||||||
| Fluence: | 1.5 × 105 kJ m-2 | - | - | - | |||
| Control | |||||||
| Room temperature, dark, 1 atm, air | 2 mo | + | + | + | |||
DISCUSSION
This is the first report on resistance of fungal isolates and cryptoendolithic communities from terrestrial extreme environments, to simulated space and Martian conditions, which were applied individually or in different combinations. Selected fungal isolates were previously demonstrated to survive some extreme terrestrial factors, such as repeated freezing and thawing cycles, high salt concentrations and UV-B irradiation (Onofri et al. 2007b). This study demonstrates high resistance of all isolates to simulated space or Martian conditions, despite wide standard deviations. Thirteen verification tests were carried out in both EVT-E1 and E2. Reporting results as survival (+) or non-survival (-) (Table 3), 13 positive responses were recorded for C. minteri CCFEE 5187, 10 for C. antarcticus CCFEE 515 and 6 for C. antarcticus CCFEE 534. Moreover, results obtained by staining methods in C. minteri (Fig. 7) showed no significant reductions of living cells both in control and treated cultures, with the exception of the UV-C treatment (254 nm, 1000 Jm–2). The higher resistance of C. minteri CCFEE 5187 to both EVTs and the good survival shown to heat shocks suggested that this isolate might be selected as a good candidate to withstand space flight and long-term permanence in space. Under the most selective combined condition of vacuum and maximum dose of polychromatic UV used, this strain was unable to grow. Its positive response in staining techniques but absence of growth might be due to the transition to a state of “viable but non-culturable cells” (VBNC), as described for bacteria (Weichart 1999). Apparently cells can maintain their integrity and viability but nevertheless may lose reproductive ability. Growth of C. minteri may escape detection because of the transition to a specific survival state characterised by deceleration of vital activity (DVA) (Feofilova 2003). Because C. antarcticus cells were not stainable, we did not have the opportunity to verify the occurrence of viable but not culturable cells after exposure to stressing conditions. Particularly surprising were the results concerning single and combined irradiation of C. minteri at the maximum UV polychromatic dose, since it corresponds to the irradiation expected during the whole planned space exposure of 1.5 yr without attenuation, i.e. without neutral density filter.
Table 3.
Summarisation of responses of the tested isolates to the EVTs, reporded as survival (+) or non-survival (-).
| C. antarcticus CCFEE 515 | C. antarcticus CCFEE 534 | C. minteri CCFEE 5187 | ||
|---|---|---|---|---|
| EVT-E1 | Vacuum 10-5 Pa/1h | + | + | + |
| Vacuum 10-5 Pa/1 wk | + | - | + | |
| 50 cycles -20/+20 °C/2 wk | + | + | + | |
| UV-C (254 nm) 10 Jm-2 | + | + | + | |
| UV-C (254 nm) 100 Jm-2 | + | + | + | |
| UV-C (254 nm) 1000 Jm-2 | + | + | + | |
| UV (200-400 nm) 1.5 kJm-2 | + | + | + | |
| UV (200-400 nm) 1.5 × 103 kJm-2 | - | - | + | |
| UV (200-400 nm) 1.5 × 105 kJm-2 | - | - | + | |
| EVT-E2 | Vacuum/22 d (dark) | + | - | + |
| Vacuum + UV (200-400 nm) 1.5 × 105 kJm-2 | - | - | +(*) | |
| Mars CO2/21 d (dark) | + | - | + | |
| Mars CO2 + UV (200-400 nm) 1.5 × 105 kJm-2 | + | - | + | |
| Control | Room temperature, dark, 1 atm air | + | + | + |
Result obtained only by staining techniques.
Cryomyces antarcticus CCFEE 515, which gave better results compared with CCFEE 514 of the same species, was also selected to investigate short and long term resistance to space conditions on the ISS.
The high resistance to space conditions shown by the three isolates tested could be ascribed to the peculiar morpho- and physiological features of black meristematic fungi. These microorganisms produce slowly expanding, cauliflower-like colonies, barely differentiated structures, and thick and heavily pigmented cell walls (Selbmann et al. 2005, Onofri et al. 2007a). These characteristics convey to high tolerance to extreme terrestrial environments and, by coincidence, spacial conditions. Melanin, for instance, is a biological macromolecule, ubiquitous in nature, mainly known for its protective role against UV and ionising radiation, extreme temperatures, and desiccation (Sterflinger 2005). The high tolerance to the UV-B exposure of single cells of the tested fungi has been reported recently (Onofri et al. 2007b). The thickness of the colony itself may represent an additional protection for the cells in the inner layers.
The high resistance of tested isolates to temperatures up to 90 °C is in agreement with literature on survival of dehydrated colonies of other meristematic fungi subjected to high temperatures (Sterflinger & Krumbein 1995, Sterflinger et al. 1999, Sterflinger 2005). The ability to enter a cryptobiotic state under poikilohydric conditions could be aided by the presence of abundant extracellular polymeric substances (EPS) in many species. EPS production may be abundant (Selbmann et al. 2005) and may appear as a gelatinous matrix in lichen thalli (de Vera et al. 2004) which may serve as a water reservoir to survive long dry periods (de los Ríos et al. 2004, 2005). Not surprisingly, many meristematic black fungi are commonly recorded from Mediterranean areas and hot deserts, where substrate surface temperatures can reach very high values. The ability to survive long-term desiccation makes these isolates pre-adapted to the extreme conditions of space, since high-vacuum conditions produce an extreme dehydrating effect.
Recent studies also show that lichens, as well as their isolated photobionts and mycobionts, cope with the extreme conditions of outer space in ground-based experiments (de Vera et al. 2003, 2004). Xanthoria elegans was able to photosynthesise under simulated Martian conditions with light in visible wave-lengths and in the presence of water (de Vera et al. 2007). Finally, samples of the lichens Rhizocarpon geographicum and X. elegans survived 16 d of exposure to space in the BIOPAN-5 facility of the European Space Agency located on the outer shell of the Earth-orbiting FOTON-M2 Russian satellite (Sancho et al. 2007).
By means of the NASA Space Shuttle Atlantis flight launched on Feb 7, 2008, these fungi are now exposed to actual space in the EXPOSE facility (created by Kaiser-Threde - DE) on the outside platform EuTEF of the International Space Station, orbiting round the Earth at a height of ∼300 km, where space conditions include pressures of 10–5 pa, temperatures ranges between –20 and +20 °C, and full solar (including UV-A, UV-B, and UV-C) and cosmic radiation. These conditions are normally prohibitive for life.
Lithopanspermia postulates the feasibility of interplanetary transfer of living material, protected against extraterrestrial solar UV and possibly heat within asteroids, comets and meteorites (Nicholson et al. 2000). Our knowledge on the limits of life has largely expanded in the last decades. The discovery of extremophiles, the high survival of Bacillus subtilis spores over six yr in space (Horneck et al. 1994), the survival of lichens after ground-based experiments (de Vera et al. 2003, 2004), as well as in space for 2 wk during the Biopan experiments (Sancho et al. 2007), and our results with the Experiment Verification Tests on meristematic fungi and cryptoendolithic communities give additional support to the idea of lithopanspermia. Considering that 1 yr is the minimum flight time estimated for Martian meteorites landing on Earth (Mileikowsky et al. 2000), the eventual survival after 1.5 yr permanence in space planned in the LIFE experiment represents a further contribution in the scenario of interplanetary transfer of life.
Acknowledgments
The authors would like to thank PNRA (Italian National Program for Antarctic Research) for supporting samples collection, Italian National Antarctic Museum “Felice Ippolito” for supporting CCFEE (Culture Collection of Fungi From Extreme Environments), the BMWi for financial support of the Astrobiological working group at the Heinrich-Heine-University, Düsseldorf (project 50WB0614), ESA (European Space Agency), and DLR (German Aerospace Center) for organising and supporting Experiment Verification Tests.
References
- Feofilova EP (2003). Deceleration of vital activity as a universal biochemical mechanism ensuring adaptation of microorganisms to stress factors: a review. Applied Biochemistry and Microbiology 39: 1–18. [PubMed] [Google Scholar]
- Finster K, Hansen AA, Liengaard L, Mikkelsen K, Kristoffersen T, Merrison J, Nörnberg P, Lomstein BAa (2007). Mars simulation experiments with complex microbial soil communities. In: ROME: Response of Organisms to the Martian Environment (Cockell C, Horneck G, ed). ESA Communications. ESTEC, Noordwijk, The Netherlands: 59–71.
- Friedmann EI (1982). Endolithic microorganisms in the Antarctic cold desert. Science 215: 1045–1053. [DOI] [PubMed] [Google Scholar]
- Friedmann EI, Ocampo-Friedman R (1976). Endolithic blue-green algae in the dry valleys: primary producers in the Antarctic desert ecosystem. Science 103: 1247–1249. [DOI] [PubMed] [Google Scholar]
- Gilichinsky DA, Wilson GS, Friedmann EI, McKay CP, Sletten RS, Rivkina EM, Vishnivetskaya TA, Erokhina LG, Ivanushkina NE, Kochkina GA, Shcherbakova VA, Soina VS, Spirina EV, Vorobyova EA, Fyodorov-Davydov DG, Hallet B, Ozerskaya SM, Sorokovikov VA, Laurinavichyus KS, Shatilovich AV, Chanton JP, Ostroumov VE, Tiedje JM (2007). Microbial populations in Antarctic permafrost: biodiversity, state, age, and implication for astrobiology. Astrobiology 7: 275–311. [DOI] [PubMed] [Google Scholar]
- González-Toril E, Llobet-Brossa E, Casamayor EO, Amann R, Amils R (2003). Microbial ecology of an extreme acidic environment, the Tinto river. Applied and Environmental Microbiology 69: 4853–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunde-Cimerman N, Oren A, Plemenitaš A (2005). Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya. Springer, The Netherlands.
- Hornek G, Bücker H, Reitz G (1994). Long-term survival of bacterial spores in space. Advances in Space Research 14: 41–45. [DOI] [PubMed] [Google Scholar]
- Horowitz NH, Cameron RE, Hubbard JS (1972). Microbiology of the Dry Valleys of Antarctica. Science 193: 242–245. [DOI] [PubMed] [Google Scholar]
- McKay CP, Friedmann EI, Gomez-Silva B, Caceres-Villanueva L, Andersen DT, Landheim R (2003). Temperature and moisture conditions for life in the extreme arid region of the Atacama Desert: four yr of observations including the El Niño of 1997–1998. Astrobiology 3: 393–406. [DOI] [PubMed] [Google Scholar]
- Mileikowsky C, Cucinotta F, Wilson JW, Gladman B, Horneck G, Lindegren L, Melosh J, Rickman H, Valtonen M, Zheng JQ (2000). Natural transfer of viable microbes in space, Part 1: from Mars to Earth and Earth to Mars. Icarus 145: 391–427. [DOI] [PubMed] [Google Scholar]
- Miller RW (2005) Viewpoint: Millennial Fever, Extremophiles, NASA, Astroenvironmentalism, and Planetary Protection. Electronic Green Journal 1 (22): 1-9 [Google Scholar]
- Mustard JF, Murchie SL, Pelkey SM, Ehlmann BL, Milliken RE, Grant JA, Bibring J-P, Poulet F, Bishop J, Noe Dobrea E, Roach L, Seelos F, Arvidson RE, Wiseman S, Green R, Hash C, Humm D, Malaret E, McGovern JA, Seelos K, Clancy T, Clark R, Marais DD, Izenberg N, Knudson A, Langevin Y, Martin T, McGuire R, Morris M, Robinson T, Roush M, Smith G, Swayze P, Taylor H, Titus T, Wolff M (2008). Hydrated silicate minerals on Mars observed by the Mars reconnaissance orbiter CRISM instrument. Nature 454: 305–309. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000). Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiology and Molecular Biology Reviews 64: 548–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienow JA, Friedmann EI (1993). Terrestrial lithophytic (rock) communities. In: Antarctic Microbiology (Friedmann EI, ed). Wiley-Liss, New York: 343–412.
- Onofri S, Pagano S, Zucconi L, Tosi S (1999). Friedmanniomyces endolithicus (Fungi, Hyphomycetes), anam.-gen. and sp.nov., from continental Antarctica. Nova Hedwigia 68: 175–181. [Google Scholar]
- Onofri S, Selbmann L, Hoog GS de, Grube M, Barreca D, Ruisi S, Zucconi L (2007b). Evolution and adaptation of fungi at boundaries of life. Advances in Space Research 40: 1657–1664. [Google Scholar]
- Onofri S, Selbmann L, Zucconi L, Pagano S (2004). Antarctic microfungi as models for exobiology. Planetary and Space Science 52: 229–237. [Google Scholar]
- Onofri S, Zucconi L, Selbmann L, Hoog GS de, de los Ríos A, Ruisi S, Grube M (2007a). Fungal Association at the cold edge of life. In: Algae and Cyanobacteria in Extreme Environments. Series: Cellular Origin, Life in Extreme Habitats and Astrobiology (Seckbach J, ed.). Springer, Berlin.
- Rettberg P, Rabbow E, Panitz C, Horneck G (2004). Biological space experiments for the simulation of Martian conditions: UV radiation and Martian soil analogues. Advances in Space Research 3: 1294–1301. [DOI] [PubMed] [Google Scholar]
- Ríos de los A, Wiezchos J, Sancho LG, Ascaso C (2004). Exploring the physiological state of continental Antarctic endolithic microorganisms by microscopy. FEMS Microbiology Ecology 50: 143–152. [DOI] [PubMed] [Google Scholar]
- Ríos de los A, Wiezchos J, Sancho LG, Green TGA, Ascaso C (2005). Ecology of endolithic lichens colonizing granite in continental Antarctic. Lichenologist 37: 383–395. [Google Scholar]
- Sancho LG, de la Torre R, Horneck G, Ascaso C, de los Rios A, Pintado A, Wierzchos J, Schuster M (2007). Lichens survive in space: results from the 2005 LICHENS experiment. Astrobiology 7(3): 443–454. [DOI] [PubMed] [Google Scholar]
- Selbmann L, Hoog GS de, Mazzaglia A, Friedmann EI, Onofri S (2005). Fungi at the edge of life: cryptoendolithic black fungi from Antarctic deserts. Studies in Mycology 51: 1–32. [Google Scholar]
- Selbmann L, Hoog GS de, Zucconi L, Isola D, Ruisi S, Gerrits van den Ende AHG, Ruibal C, De Leo F, Urzì C, Onofri S (2008). Drought meets acid: three new genera in a dothidealean clade of extremotolerant fungi. Studies in Mycology 61: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterflinger K (2005). Black yeasts and meristematic fungi: ecology, diversity and identification. In: Yeast Handbook: Biodiversity and Ecophysiology of Yeasts (Rosa C, Gabor P, eds). Springer, New York: 505–518.
- Sterflinger K, Krumbein WE (1995). Dematiaceous fungi as a major agent for biopitting on Mediterranean marbles and limestones. Geomicrobiology Journal 14: 219–230. [Google Scholar]
- Sterflinger K, Hoog GS de, Haase G (1999). Phylogeny and ecology of meristematic ascomycetes. Studies in Mycology 43: 5–22. [Google Scholar]
- Vera de J-P, Horneck G, Rettberg P, Ott S (2003). The potential of the lichen symbiosis to cope with extreme conditions of outer space – I. Influence of UV radiation and space vacuum on the vitality of lichen symbiosis germination capacity. International Journal of Astrobiology 1: 285–293. [Google Scholar]
- Vera de J-P, Horneck G, Rettberg P, Ott S (2004). The potential of the lichen symbiosis to cope with the extreme conditions of outer space – II: germination capacity of lichen ascospores in response to simulated space conditions. Advances in Space Research 33: 1236–1243. [DOI] [PubMed] [Google Scholar]
- Vera de J-P, Tilmes F, Heydenreich T, Meyer C, Horneck G, Ott S (2007). Potential of prokaryotic and eukaryotic organisms in a Mars like environment and as system for the search of life on other planets. Proceeding of DGLR Int. Symp. To the Moon and beyond, March 2007 (available as CD).
- Weichart DH (1999). Stability and survival of VBNC cells – conceptual and practical implications. In: Microbial Biosystems: New Frontiers. Proceedings of the 8th International Symposium on Microbial Ecology (Bell CR, Brylinsky M, Johnson-Green P, eds). Atlantic Canada Society for Microbial Ecology, Halifax, Canada.
- Wynn-Williams DD, Edwards HGM (2000). Antarctic ecosystems as models from extraterrestrial surface habitats. Planetary and Space Science 48: 1065–1075. [Google Scholar]