Abstract
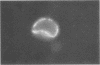
The protozoan parasite Giardia muris colonizes the mouse small intestinal lumen. This parasite is cleared immunologically from the intestine of normal mice. In contrast, T-lymphocyte-deficient (nude) mice have an impaired immunological response to G. muris and become chronically infected. In the present study, trophozoites were harvested from the intestinal lumen of immunocompetent BALB/c mice and nude mice and examined for surface-bound mouse immunoglobulins by immunofluorescence microscopy. Immunoglobulin A (IgA) and IgG, but not IgM, were detected on trophozoites obtained from BALB/c mice, from day 10 of the infection onwards. Trophozoites from nude mice showed very little evidence of surface-bound mouse immunoglobulin at any time during the 5-week period immediately following infection of these animals with G. muris cysts. Intestinal G. muris infection was cleared by the BALB/c mice but not by the nude animals. The data suggest that parasite-specific IgA and IgG bind to G. muris trophozoites in the intestinal lumen of immunocompetent BALB/c mice. Intestinal antibodies that bind to trophozoite surfaces are likely to play an important part in the clearance of G. muris infection by immunocompetent mice. The inability of nude mice to clear this infection at a normal rate is likely to be due to impairment of Giardia-specific intestinal antibody production.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Roberts-Thomson I. C., Mitchell G. F. Giardiasis in mice: analysis of humoral and cellular immune responses to Giardia muris. Parasite Immunol. 1982 Jan;4(1):47–57. doi: 10.1111/j.1365-3024.1982.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cox F. E. Immunological aspects of Giardia muris and Spironucleus muris infections in inbred and outbred strains of laboratory mice: a comparative study. Parasitology. 1982 Aug;85(Pt 1):85–99. doi: 10.1017/s0031182000054172. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Savage D. C., Dubois R. S., Alp M. H., Mallory A., Kern F., Jr Intestinal microflora of immunoglobulin-deficient and normal human subjects. Gastroenterology. 1972 Jun;62(6):1143–1152. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Galfrè G., Milstein C., Wright B. Rat x rat hybrid myelomas and a monoclonal anti-Fd portion of mouse IgG. Nature. 1979 Jan 11;277(5692):131–133. doi: 10.1038/277131a0. [DOI] [PubMed] [Google Scholar]
- Hermans P. E., Diaz-Buxo J. A., Stobo J. D. Idiopathic late-onset immunoglobulin deficiency. Clinical observations in 50 patients. Am J Med. 1976 Aug;61(2):221–237. doi: 10.1016/0002-9343(76)90173-x. [DOI] [PubMed] [Google Scholar]
- Heyworth M. F., Owen R. L., Jones A. L. Comparison of leukocytes obtained from the intestinal lumen of Giardia-infected immunocompetent mice and nude mice. Gastroenterology. 1985 Dec;89(6):1360–1365. doi: 10.1016/0016-5085(85)90656-0. [DOI] [PubMed] [Google Scholar]
- Heyworth M. F., Owen R. L., Seaman W. E., Schaefer F. W., 3rd, Jones A. L. Harvesting of leukocytes from intestinal lumen in murine giardiasis and preliminary characterization of these cells. Dig Dis Sci. 1985 Feb;30(2):149–153. doi: 10.1007/BF01308202. [DOI] [PubMed] [Google Scholar]
- LoGalbo P. R., Sampson H. A., Buckley R. H. Symptomatic giardiasis in three patients with X-linked agammaglobulinemia. J Pediatr. 1982 Jul;101(1):78–80. doi: 10.1016/s0022-3476(82)80188-1. [DOI] [PubMed] [Google Scholar]
- Loftness T. J., Erlandsen S. L., Wilson I. D., Meyer E. A. Occurrence of specific secretory immunoglobulin A in bile after inoculation of Giardia lamblia trophozoites into rat duodenum. Gastroenterology. 1984 Nov;87(5):1022–1029. [PubMed] [Google Scholar]
- Milstein C., Pink J. R. Structure and evolution of immunoglobulins. Prog Biophys Mol Biol. 1970;21:209–263. doi: 10.1016/0079-6107(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Gillin F. D., Smith P. D. Excretory-secretory products of Giardia lamblia. J Immunol. 1983 Oct;131(4):2004–2010. [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Mitchell G. F. Giardiasis in mice. I. Prolonged infections in certain mouse strains and hypothymic (nude) mice. Gastroenterology. 1978 Jul;75(1):42–46. [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976 Jul;71(1):57–61. [PubMed] [Google Scholar]
- Smith P. D., Gillin F. D., Brown W. R., Nash T. E. IgG antibody to Giardia lamblia detected by enzyme-linked immunosorbent assay. Gastroenterology. 1981 Jun;80(6):1476–1480. [PubMed] [Google Scholar]
- Snider D. P., Gordon J., McDermott M. R., Underdown B. J. Chronic Giardia muris infection in anti-IgM-treated mice. I. Analysis of immunoglobulin and parasite-specific antibody in normal and immunoglobulin-deficient animals. J Immunol. 1985 Jun;134(6):4153–4162. [PubMed] [Google Scholar]
- Stohrer R., Kearney J. F. Fine idiotype analysis of B cell precursors in the T-dependent and T-independent responses to alpha 1-3 dextran in BALB/c mice. J Exp Med. 1983 Dec 1;158(6):2081–2094. doi: 10.1084/jem.158.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdown B. J., Roberts-Thomson I. C., Anders R. F., Mitchell G. F. Giardiasis in mice: studies on the characteristics of chronic infection in C3h/He mice. J Immunol. 1981 Feb;126(2):669–672. [PubMed] [Google Scholar]