Abstract
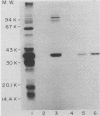
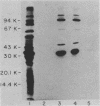
The chinchilla experimental model of otitis media was used to examine the importance of serum antibodies in protection against disease caused by nontypable Haemophilus influenzae. An immune serum pool was prepared by immunizing chinchillas with killed bacterial cells of nontypable H. influenzae 3245. Pooled preimmune or immune serum from these immunized animals was administered intravenously to a group of nonimmune chinchillas 1 day before intrabullar challenge with strain 3245. Of 5 animals receiving preimmune serum, 5 developed otitis media compared with 0 of 10 animals receiving immune serum (P = 0.008). The immune serum pool contained antibodies directed against both surface-exposed outer membrane proteins and lipopolysaccharide (LPS). The 39-kilodalton major outer membrane protein was the immunodominant surface protein. Anti-LPS antibodies were removed from the immune serum pool by affinity chromatography, and affinity-purified anti-LPS antibodies were recovered. Immune serum, immune serum absorbed of LPS antibodies, or affinity-purified LPS antibodies were then administered to another group of experimental animals 1 day before bacterial challenge. Of four animals that received the affinity-purified LPS antibodies, four developed otitis compared with zero of four animals that received the immune serum or zero of four animals that received the LPS-absorbed immune serum (P = 0.028). These studies indicate that passive immunization with immune serum is protective in experimental nontypable H. influenzae otitis media and that bacterial outer membrane proteins may be the principal targets of protective antibody.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982 May;36(2):535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Payne E. E., Mills E. L., Juhn S. K., Quie P. G. Experimental otitis media due to Streptococcus pneumoniae: immunopathogenic response in the chinchilla. J Infect Dis. 1976 Dec;134(6):595–604. doi: 10.1093/infdis/134.6.595. [DOI] [PubMed] [Google Scholar]
- Giebink G. S. The pathogenesis of pneumococcal otitis media in chinchillas and the efficacy of vaccination in prophylaxis. Rev Infect Dis. 1981 Mar-Apr;3(2):342–353. doi: 10.1093/clinids/3.2.342. [DOI] [PubMed] [Google Scholar]
- Gnehm H. E., Pelton S. I., Gulati S., Rice P. A. Characterization of antigens from nontypable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Invest. 1985 May;75(5):1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie V. M., Ploussard J. H., Lester R. L., Jr Otitis media: a clinical and bacteriological correlation. Pediatrics. 1970 Jan;45(1):29–35. [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Karasic R. B., Trumpp C. E., Gnehm H. E., Rice P. A., Pelton S. I. Modification of otitis media in chinchillas rechallenged with nontypable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis. 1985 Feb;151(2):273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Antigenic heterogeneity of outer membrane proteins of nontypable Haemophilus influenzae is a basis for a serotyping system. Infect Immun. 1985 Oct;50(1):15–21. doi: 10.1128/iai.50.1.15-21.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Hague-Park M., Baughn R. E., Wallace R. J., Jr, Cowley B. Opsonizing and bactericidal effects of normal human serum on nontypable Haemophilus influenzae. Infect Immun. 1983 Jan;39(1):297–304. doi: 10.1128/iai.39.1.297-304.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982 Jul;70(1):157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riding K. H., Bluestone C. D., Michaels R. H., Cantekin E. I., Doyle W. J., Poziviak C. S. Microbiology of recurrent and chronic otitis media with effusion. J Pediatr. 1978 Nov;93(5):739–743. doi: 10.1016/s0022-3476(78)81069-5. [DOI] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Granoff D. M. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J Infect Dis. 1982 Feb;145(2):181–190. doi: 10.1093/infdis/145.2.181. [DOI] [PubMed] [Google Scholar]
- Shurin P. A., Pelton S. I., Tager I. B., Kasper D. L. Bactericidal antibody and susceptibility to otitis media caused by nontypable strains of Haemophilus influenzae. J Pediatr. 1980 Sep;97(3):364–369. doi: 10.1016/s0022-3476(80)80182-x. [DOI] [PubMed] [Google Scholar]
- Sloyer J. L., Jr, Cate C. C., Howie V. M., Ploussard J. H., Johnston R. B., Jr The immune response to acute otitis media in children. II. Serum and middle ear fluid antibody in otitis media due to Haemophilus influenza. J Infect Dis. 1975 Dec;132(6):685–688. doi: 10.1093/infdis/132.6.685. [DOI] [PubMed] [Google Scholar]
- Teele D. W., Pelton S. I., Klein J. O. Bacteriology of acute otitis media unresponsive to initial antimicrobial therapy. J Pediatr. 1981 Apr;98(4):537–539. doi: 10.1016/s0022-3476(81)80755-x. [DOI] [PubMed] [Google Scholar]
- Ulsø C., Hentzer E. Surgical treatment of recurrent perilymph fistula with anacusis. Ann Otol Rhinol Laryngol. 1980 Mar-Apr;89(2 Pt 1):121–123. doi: 10.1177/000348948008900204. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M., Septimus E. J., McGowan J. E., Jr, Quinones F. J., Wiss K., Vance P. H., Trier P. A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and beta-lactamase production. J Infect Dis. 1981 Aug;144(2):101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Briggs B. R., Lim D. J. Experimental otitis media in chinchillas. I. Baseline immunological investigation. Ann Otol Rhinol Laryngol Suppl. 1982 May-Jun;93:1–8. [PubMed] [Google Scholar]