Abstract
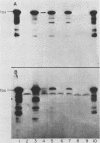
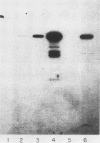
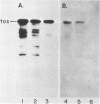
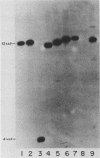
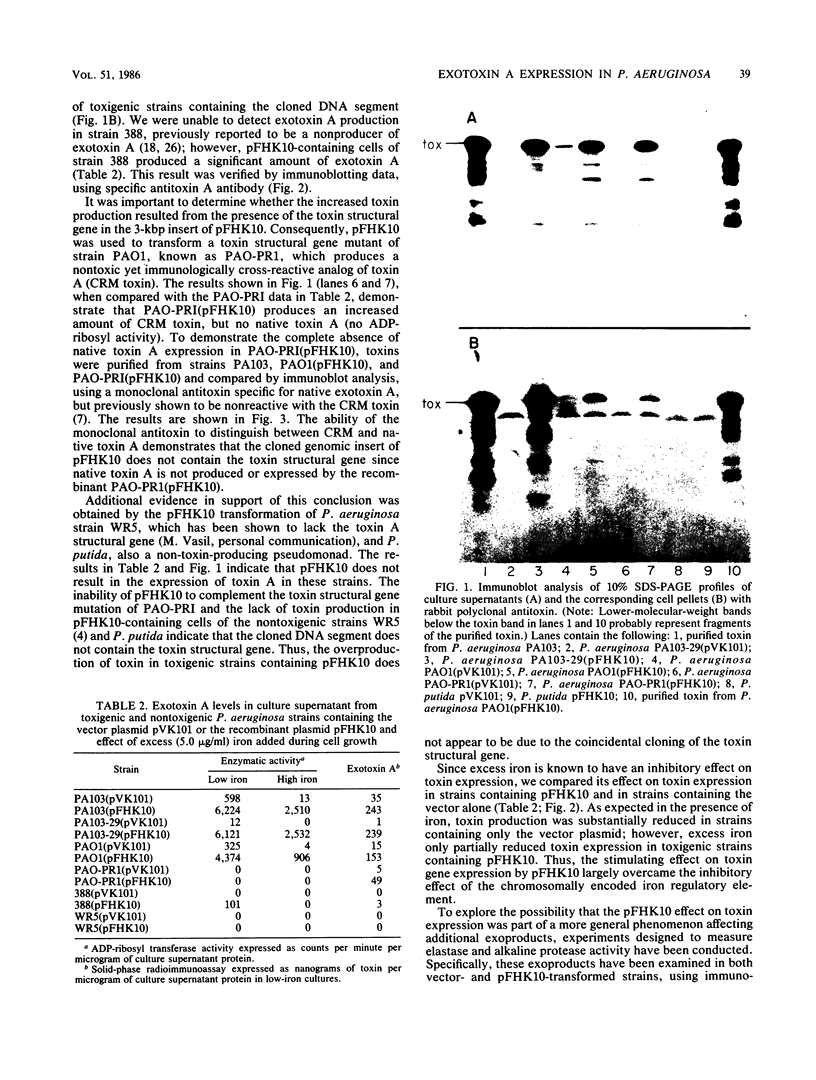
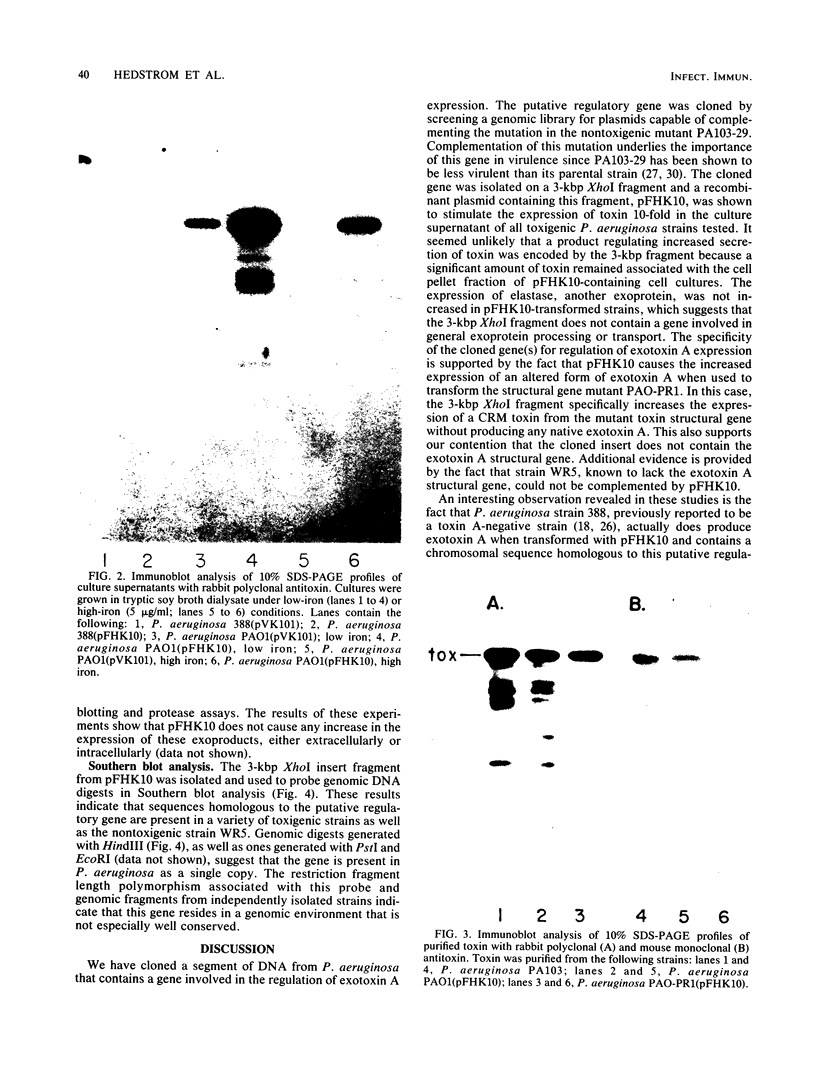
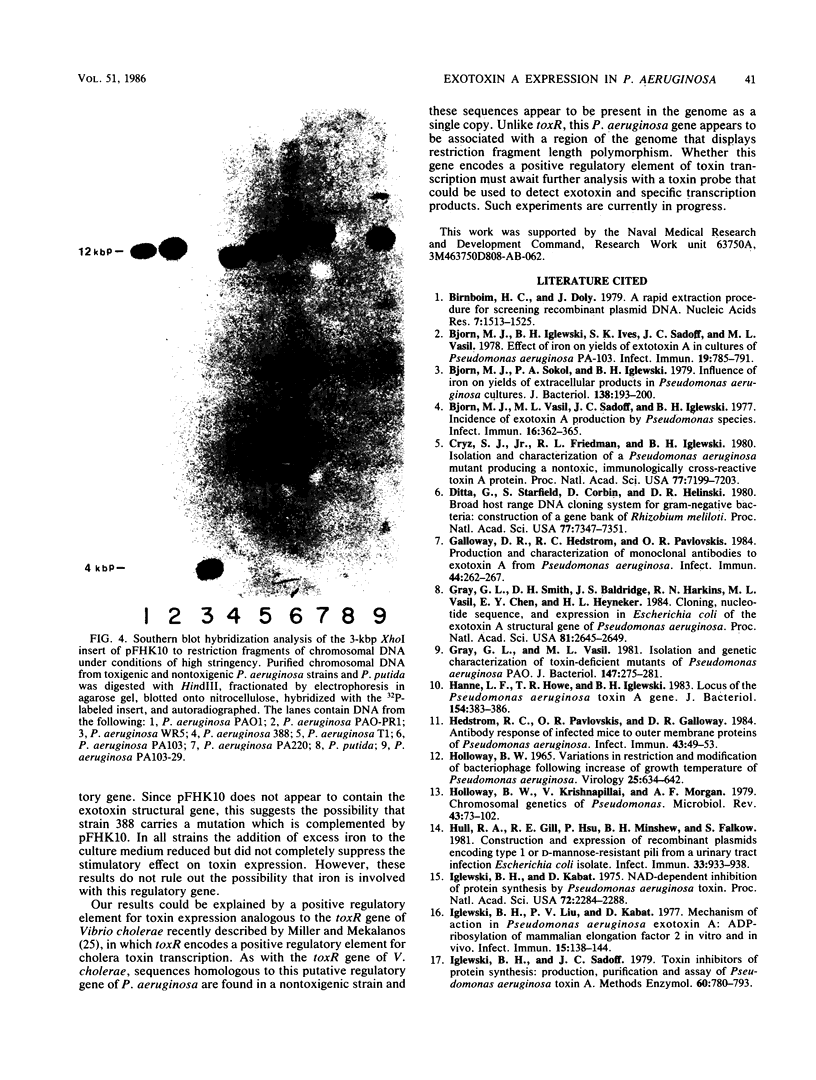
We have cloned a gene from Pseudomonas aeruginosa that stimulates the expression of exotoxin A. A recombinant library of genomic DNA from strain PA103 constructed with a broad-host-range plasmid vector containing chromosomal insert fragments generated by Sau3A was used to transform the hypotoxigenic mutant strain PA103-29. A recombinant plasmid, pFHK6, was isolated from a PA103-29 transformant which displayed increased toxin production. From pFHK6, which contained a 20-kilobase-pair chromosomal insert, a 3-kilobase-pair XhoI fragment was isolated and subcloned into the plasmid cloning vector pVK101 to give pFHK10. In toxigenic P. aeruginosa strains containing pFHK10, toxin expression was increased 10-fold and high levels of iron in the culture medium only partially inhibited the overproduction. Expression studies suggested that pFHK10 did not contain the toxin structural gene. In addition, Southern analysis with the 3-kilobase-pair XhoI fragment suggested that the putative toxin regulatory gene is common among different strains of P. aeruginosa including previously reported nontoxigenic strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Vasil M. L., Sadoff J. C., Iglewski B. H. Incidence of exotoxin production by Pseudomonas species. Infect Immun. 1977 Apr;16(1):362–366. doi: 10.1128/iai.16.1.362-366.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Friedman R. L., Iglewski B. H. Isolation and characterization of a Pseudomonas aeruginosa mutant producing a nontoxic, immunologically crossreactive toxin A protein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7199–7203. doi: 10.1073/pnas.77.12.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. R., Hedstrom R. C., Pavlovskis O. R. Production and characterization of monoclonal antibodies to exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):262–267. doi: 10.1128/iai.44.2.262-267.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Vasil M. L. Isolation and genetic characterization of toxin-deficient mutants of Pseudomonas aeruginosa PAO. J Bacteriol. 1981 Aug;147(2):275–281. doi: 10.1128/jb.147.2.275-281.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W. VARIATIONS IN RESTRICTION AND MODIFICATION OF BACTERIOPHAGE FOLLOWING INCREASE OF GROWTH TEMPERATURE OF PSEUDOMONAS AERUGINOSA. Virology. 1965 Apr;25:634–642. doi: 10.1016/0042-6822(65)90091-7. [DOI] [PubMed] [Google Scholar]
- Hanne L. F., Howe T. R., Iglewski B. H. Locus of the Pseudomonas aeruginosa toxin A gene. J Bacteriol. 1983 Apr;154(1):383–386. doi: 10.1128/jb.154.1.383-386.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R. C., Pavlovskis O. R., Galloway D. R. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect Immun. 1984 Jan;43(1):49–53. doi: 10.1128/iai.43.1.49-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J., Bjorn M. J., Maxwell E. S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect Immun. 1984 Aug;45(2):470–474. doi: 10.1128/iai.45.2.470-474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Burns R. P., Iglewski B. H. Corneal infections in mice with toxin A and elastase mutants of Pseudomonas aeruginosa. J Infect Dis. 1980 Oct;142(4):547–555. doi: 10.1093/infdis/142.4.547. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980 Jun;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Cox C. D., Iglewski B. H. Pseudomonas aeruginosa mutants altered in their sensitivity to the effect of iron on toxin A or elastase yields. J Bacteriol. 1982 Aug;151(2):783–787. doi: 10.1128/jb.151.2.783-787.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]