Abstract
In outbred rats, increases in brain neuropeptide Y (NPY) activity suppress ethanol consumption in a variety of access conditions, but only following a history of ethanol dependence. NPY reliably suppresses ethanol drinking in alcohol-preferring (P) rats and this effect is augmented following a period of ethanol abstinence. The purpose of this experiment was to examine the effects of NPY on 2-bottle choice ethanol drinking and feeding in Wistar rats that had undergone chronic ethanol vapor exposure, cycles of ethanol abstinence, or both. Ethanol-drinking Wistars were given six weeks of access to 15% (v/v) ethanol and water followed by either: two cycles of one week ethanol vapor exposure and two weeks with no ethanol; two cycles of one week ethanol bottle availability and two weeks with no ethanol; or two weeks of ethanol vapor exposure. Rats were infused ICV with one of four NPY doses (0.0, 2.5, 5.0, or 10.0 µg) following the ethanol exposure patterns described above, and tested for ethanol drinking and feeding in a 2-bottle choice situation. NPY dose-dependently increased food intake regardless of ethanol exposure history, but suppressed ethanol drinking only in rats that underwent cycles of ethanol access and ethanol abstinence. These results support the notion that dysregulation of brain NPY systems during chronic intermittent ethanol exposure is important in the motivational drive for subsequent relapse to ethanol drinking.
Keywords: Neuropeptide Y, Dependence, Ethanol Vapor, Ethanol Abstinence
INTRODUCTION
Neuropeptide Y (NPY) has potent anxiolytic (Heilig et al., 1989), sedative (Heilig and Murison, 1987), and orexigenic effects (Clark et al., 1984) in rodents. More recently, a role has been elucidated for NPY in ethanol-drinking behavior. This role of NPY appears to be affected by the ethanol exposure history and genetic background of the organism.
Intracerebroventricular (ICV) NPY does not alter ethanol drinking in outbred Wistar rats in limited-access situations (Badia-Elder et al., 2001; Slawecki et al., 2000). However, following chronic intermittent vapor exposure, increases in brain NPY activity suppress ethanol drinking in outbred rats exposed to ethanol vapor but not ambient air (Rimondini et al., 2005; Thorsell et al., 2005a, 2005b). In agreement with these latter findings, ICV NPY suppresses free-choice ethanol drinking in selectively bred alcohol-preferring (P) rats, and the magnitude and duration of that effect is augmented following periods of imposed ethanol abstinence (Gilpin et al., 2003, 2005).
The dual purpose of the present investigation was to examine (1) the separate and combined effects of continuous (24 hrs/day) ethanol vapor exposure and abstinence periods on relapse ethanol drinking by Wistar rats in a 2-bottle choice situation, and (2) the dose-dependent effects of ICV administered NPY on this relapse behavior. A dependence protocol was used in which animals are trained to self-administer ethanol, and then exposed to high ethanol doses for extended periods of time on multiple schedules. Ethanol vapor inhalation procedures allow for precise control of the dose, duration, and pattern of ethanol exposure (i.e. minimal oscillation of blood-ethanol levels), and reliably produce functional tolerance and dependence on ethanol (Rogers et al., 1979). For example, animals exposed to chronic continuous ethanol vapor exhibit elevated ethanol intake and anxiety-like behavior during acute withdrawal and also 4 weeks following termination of ethanol vapor exposure (Roberts et al., 1996, 2000; Valdez et al., 2002, 2003).
In the present investigation, one group of Wistar rats underwent cycles of ethanol vapor exposure and ethanol abstinence, and two other groups served as controls for either the amount of ethanol exposure or the pattern of ethanol exposure. Consistent with prior literature, it was hypothesized that Wistar rats in all ethanol-experienced groups would exhibit increases in ethanol intake in a relapse situation. It was further hypothesized that NPY would effectively suppress ethanol drinking only in Wistar rats that had been exposed to chronic ethanol vapor, and that this effect would be amplified following periods of imposed ethanol abstinence.
MATERIALS AND METHODS
Subjects
Subjects were 206 experimentally naïve female Wistar rats (Harlan, Indianapolis, IN) that were between 8 and 10 weeks of age and weighed between 172–327 g (mean ± SEM = 225.63 ± 1.92) at the start of the experiment. All rats were individually housed in plastic tub-style cages in a vivarium maintained on a 12:12 hr light/dark cycle (lights off at 1400 hrs). Food (Lab Diet 5001, PMI Nutrition International Inc., Brentwood, MO) and water were available ad libitum at all times except where otherwise stated. The protocol for this study was approved by the IUPUI School of Science IACUC and was conducted in accordance with NIH guidelines (1996).
Stereotaxic Surgeries
Surgical implantation of intracerebroventricular cannulae was conducted using aseptic procedures as previously described (Badia-Elder et al., 2001), with the exception that rats were anesthetized via inhalation of isoflourane (IsoFlo, Abbott Laboratories, North Chicago, IL) before and during surgery. The stereotaxic coordinates were adjusted to accommodate the smaller female rats used in the present study (AP-1.0, ML±1.5, DV-3.8). At the completion of all experimental manipulations, anatomic localization was confirmed after euthanization. Following euthanization, a 1% solution of bromphenol blue dye in aCSF (5.0 µl) was injected into the ventricles. The brain was immediately removed, sliced, and placement was verified in a procedure that was blind to the treatment histories of the animals.
Microinfusions
A Harvard 33 microinfusion pump was used for all drug infusions at a rate of 2.5 µl/minute for a period of two minutes, and the injection cannula was left in the guide cannula for one additional minute to allow for adequate diffusion of the solution. Infusions were delivered to the cannula via polyethylene tubing (PE 50) that was connected to a Hamilton 25 µl syringe. Immediately following infusion, rats were placed in a clean plastic cage, where ethanol as well as water and food were available ad libitum.
Procedure
Baseline Ethanol Drinking Period
To train the rats to drink ethanol solution, a modified sucrose-fading procedure was used as previously described (Badia-Elder et al., 2003). Rats were then allowed 6 weeks of 24-hour continuous access to 15% (v/v) ethanol and water. On days 19 and 20 of the 6-week baseline drinking period, rats were deprived of water (forced ethanol self-administration in order to optimize ethanol drinking) except during the 30-minute period when ethanol bottles were weighed. Fluid intakes during the final 6 days of the initial 6-week drinking period were used as baseline measurements. Ethanol and water bottle positions were alternated daily throughout the experiment to control for side preferences.
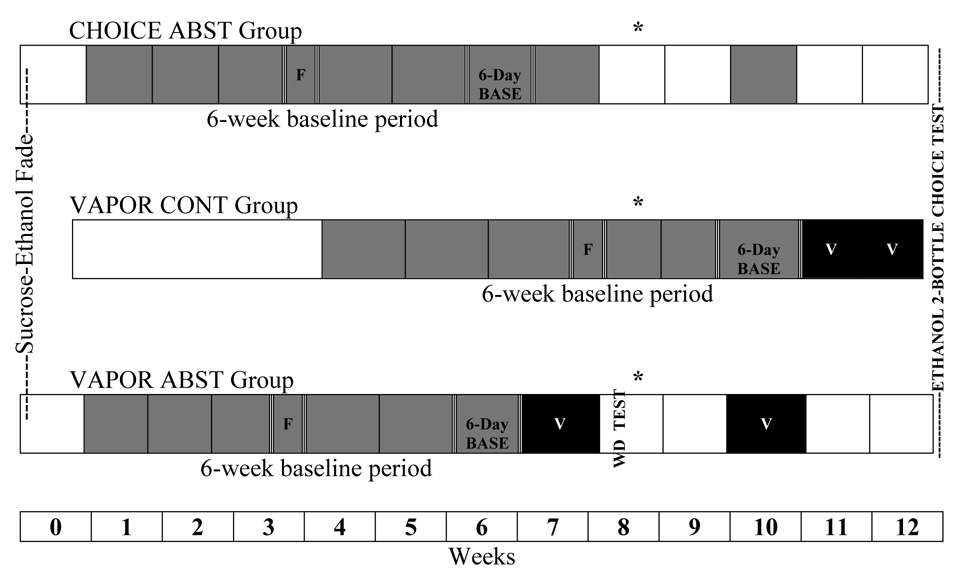
Initially, rats were randomly assigned to one of two groups. The first of these two groups contained rats that would undergo periods of ethanol abstinence later in the experiment, and eventually be further subdivided into two subgroups. The second of these two groups contained rats that would not undergo periods of ethanol abstinence (continuous group). Figure 1 illustrates the schedule of access to ethanol solution and exposure to ethanol vapor in the various groups of Wistar rats, as well as surgery and behavioral test days. Rats in the first group described above, the abstinence group, underwent the sucrose-fading procedure and were given 6 weeks of continuous (24hr/day) access to 15% (v/v) ethanol and water. Ethanol intakes (g ethanol/kg body weight) from the final 6 days of this baseline period were used to subdivide these rats into two groups matched for ethanol intake g/kg/day): the ethanol vapor-exposed abstinence (VAPOR ABST) group and the 2-bottle choice abstinence (CHOICE ABST) group. Rats in the second group described above, the ethanol vapor-exposed continuous (VAPOR CONT) group, received exactly the same amount of ethanol exposure as rats in the VAPOR ABST group, but the pattern of exposure was altered such that the sucrose-ethanol fade was initiated 4 weeks later (in order to maintain age-matched groups) and exposure to ethanol solution and ethanol vapor was not interrupted by imposed periods of abstinence.
Figure 1.
Timeline of ethanol access schedules (by weeks) for Wistar rats that were given 2-bottle choice (CHOICE ABST) between ethanol and water, and ethanol vapor-exposed rats that did (VAPOR ABST) and did not (VAPOR CONT) undergo periods of imposed ethanol abstinence. Segments of the lines that are filled in gray represent periods during which ethanol solution was available to rats in a 2-bottle choice situation. Segments of the lines that are filled in black represent periods during which rats were exposed to ethanol vapor (denoted by “V”). Segments of the lines that are not filled represent periods without ethanol. Six-week 2-bottle choice training and six-day baseline periods are denoted appropriately. All rats were forced to consume ethanol on days 19 and 20 of the training period, denoted by “F.” The time point at which rats underwent stereotaxic surgeries is marked by an asterisk (*).
VAPOR ABST Group
Following the initial 6 weeks of ethanol access, rats were exposed to ethanol vapor for a period of 7 days as described below. Rats then underwent a 14-day period with no ethanol (abstinence period 1), were exposed to ethanol vapor for 7 more days, and underwent another 14-day period with no ethanol (abstinence period 2). Stereotaxic surgeries were conducted during days 7–9 of abstinence period 1. During days 12 and 13 of abstinence period 2, sham infusions (rats treated as if receiving infusion but nothing infused) were implemented daily at 1400 hrs to acclimate the rats to the infusion procedure. On the fourteenth day of abstinence period 2 and immediately preceding the return of ethanol solution, rats previously assigned to NPY dose groups matched for ethanol intake (g/kg/day over final 6 days of baseline) were ICV-infused with either aCSF (5.0 µl) or one of three NPY doses (2.5, 5.0, or 10.0 µg/5.0 µl aCSF).
CHOICE ABST Group
Rats in the CHOICE ABST group were treated in a similar manner as the VAPOR ABST group of Wistar rats except that during the periods when VAPOR ABST rats were exposed to ethanol vapor, CHOICE ABST rats were given access to ethanol solution in a 2-bottle choice situation (i.e. the two groups differed only in amount of ethanol exposure). Rats underwent ethanol access schedules, stereotaxic surgeries, NPY dose group assignments, sham infusions, NPY microinfusions, and behavioral testing in parallel with rats in the VAPOR ABST group.
VAPOR CONT Group
Rats in the VAPOR CONT group were treated in a similar manner as the VAPOR ABST group of Wistar rats except that they did not undergo any periods of imposed ethanol abstinence (i.e. the two groups differed only in pattern of ethanol exposure). Therefore, at the end of the initial 6-week baseline period, rats in this group were immediately exposed to ethanol vapor for a period of two weeks. Rats underwent stereotaxic surgeries at the end of week 5 of the baseline drinking period. In an attempt to match the treatment timeline with the VAPOR ABST group, rats were briefly removed from the vapor chambers and underwent sham infusions during the two days directly preceding NPY infusion. Rats were assigned to NPY dose groups in parallel with rats in the VAPOR ABST group. Rats were infused with either aCSF (5.0 µl) or one of three doses (2.5, 5.0, 10.0 µg/5.0 µl aCSF) of NPY and tested for ethanol drinking immediately following removal from ethanol vapor chambers.
Ethanol Vapor Exposure
Ethanol vapor inhalation (24 hrs/day) was employed to gradually increase blood-ethanol levels to 200 mg% over the first 12 hours of exposure, and then maintain blood-ethanol levels between 150 and 200 mg% over periods of 7 or 14 days. Rats were exposed to ethanol/air vapor in a rodent alcohol inhalation system (La Jolla Alcohol Research, Inc., San Diego, CA) that has been described in detail elsewhere (Lee et al., 2000). During vapor exposure, rats were dually housed in standard plastic tub style cages with opaque walls. At all times in ethanol vapor cages, rats sharing the same cage were separated by a dividing wall, and each rat within a cage had free access to food and water. Rats were monitored daily and weighed twice per week, food and water were replenished as necessary, room temperature and humidity settings were monitored daily, and vapor settings were adjusted according to ethanol concentrations within the cages as well as observed blood-ethanol levels and behavior of rats.
Blood-Ethanol Level (BEL) Determination
In order to determine BELs at different time points during intoxication and withdrawal, blood was collected from animals by cutting the tip of the tail. Tail bloods were collected from VAPOR ABST rats at 4 hr, 8 hr, 12 hr, 24 hr, 84 hr and 96 hr following the start of the first week of ethanol vapor exposure to record the ascent and stabilization of BELs within the target range, and to make appropriate adjustments to settings on the ethanol vapor inhalation units. Tail bloods were also collected from VAPOR ABST rats 6 hr, 8 hr and 10 hr following removal from ethanol vapor inhalation chambers in conjunction with the first three time points at which physical dependence was assessed (see below). BELs were determined with an alko-analyzer (AM1 series, Analox Instruments Ltd., MA) that measures oxygen levels (proportional to ethanol concentrations) following oxidation of ethanol to acetaldehyde and hydrogen peroxide by alcohol oxidase.
Assessment of Physical Dependence
Following the first 7-day period of exposure to ethanol vapor, and during acute withdrawal from ethanol vapor exposure, rats in the VAPOR ABST group were tested for behavioral signs of CNS hyperexcitability indicative of physical dependence on ethanol. In a blind procedure, rats in only the first cohort (n=8; see results section) of the CHOICE ABST group were tested in parallel with rats in the VAPOR ABST group. Testing occurred at 6 hr, 8 hr, 10 hr, 12 hr, 14 hr, 16 hr and 24 hr following removal from ethanol vapor inhalation chambers. Tail blood samples were collected at the 6 hr, 8 hr and 10 hr test time points. Behavior was scored by two treatment-blind observers, one at each time point, in an alternating pattern. Behavior was observed for two minutes in the home cage at each test time point.
The withdrawal testing protocol and behavioral scoring rubric evaluated the presence, specificity, frequency, and severity of an array of ethanol withdrawal signs. According to Majchrowicz (1975), these signs include, in order of ascending withdrawal severity: general hyperactivity, tail spasticity/tremors, general spasticity/tremors, head tremors, wet shakes and teeth chattering, and spontaneous tonic-clonic convulsions. Five categories of behavior were scored by treatment-blind observers: ventromedial dorsal limb flexion (defined as retraction of limbs toward body when rat is lifted into the air), abnormal body posture (defined as broad-based gait, immobility, and slow or unsteady forward locomotion), tail stiffness (defined as presence of rigid tail that extends parallel to the back of the rat or up toward the ceiling), hyperlocomotion (defined as excessive running, vigorous escape attempts, spontaneous convulsions), and tremors/stereotypic movements (defined as the presence of body/head/tail tremors, wet-dog shakes, chattering teeth, stereotyped repetitive behavior, aimless circling, restless head turning). At each test time point, behaviors in each of these five categories were assigned one of three scores: 0 (normal), 1 (moderately abnormal), or 2 (severely abnormal). As such, total withdrawal scores ranged from zero to 10, where 10 indicated the most severe form of withdrawal and zero indicated the absence of any withdrawal signs.
Data Analysis
The amount of fluid consumed was determined by weighing the drinking bottles at 1400 hrs. every day, just before the start of the dark cycle, for the twenty-four hour measurement, and at 1800 hrs. for the four-hour measurement. Ethanol intake (ml and g/kg), water intake (ml), and ethanol preference (E:T) ratio by CHOICE ABST rats during baseline (average values across 6 days) and on the first days of reinstatement period 1 were analyzed using a one-way (day) repeated-measures (RM) ANOVA.
Ethanol intake (ml and g/kg), water intake (ml), ethanol preference (E:T) ratio, total fluid intake (ml), and food intake (g/kg) were analyzed at 4 and 24 hours following NPY infusion. Data for VAPOR ABST rats were analyzed relative to each of the control groups using two-way ANOVAs: in the 2 vapor-exposed groups (ethanol access schedule [VAPOR ABST vs VAPOR CONT] × NPY dose), and separately in the 2 abstinent groups (ethanol administration mode [VAPOR ABST vs CHOICE ABST] × NPY dose). In cases where ANOVAs for 24-hr measurements yielded significant effects, data from post-infusion day 1 (24 hr time point to 48 hr time point) were analyzed using identical two-way ANOVAs. All post-hoc analyses were conducted using Bonferroni multiple comparison tests. In all cases, significance was determined at p<0.05.
The number of rats in each group in post-NPY ethanol and food intake analyses was as follows: VAPOR ABST 10.0 µg (n=18), VAPOR ABST 5.0 µg (n=17), VAPOR ABST 2.5 µg (n=17), VAPOR ABST aCSF (n=17); CHOICE ABST 10.0 µg (n=16), CHOICE ABST 5.0 µg (n=15), CHOICE ABST 2.5 µg (n=16), CHOICE ABST aCSF (n=16), VAPOR CONT 10.0 µg (n=16), VAPOR CONT 5.0 µg (n=20), VAPOR CONT 2.5 µg (n=19), VAPOR CONT aCSF (n=19).
RESULTS
Blood-Ethanol Levels
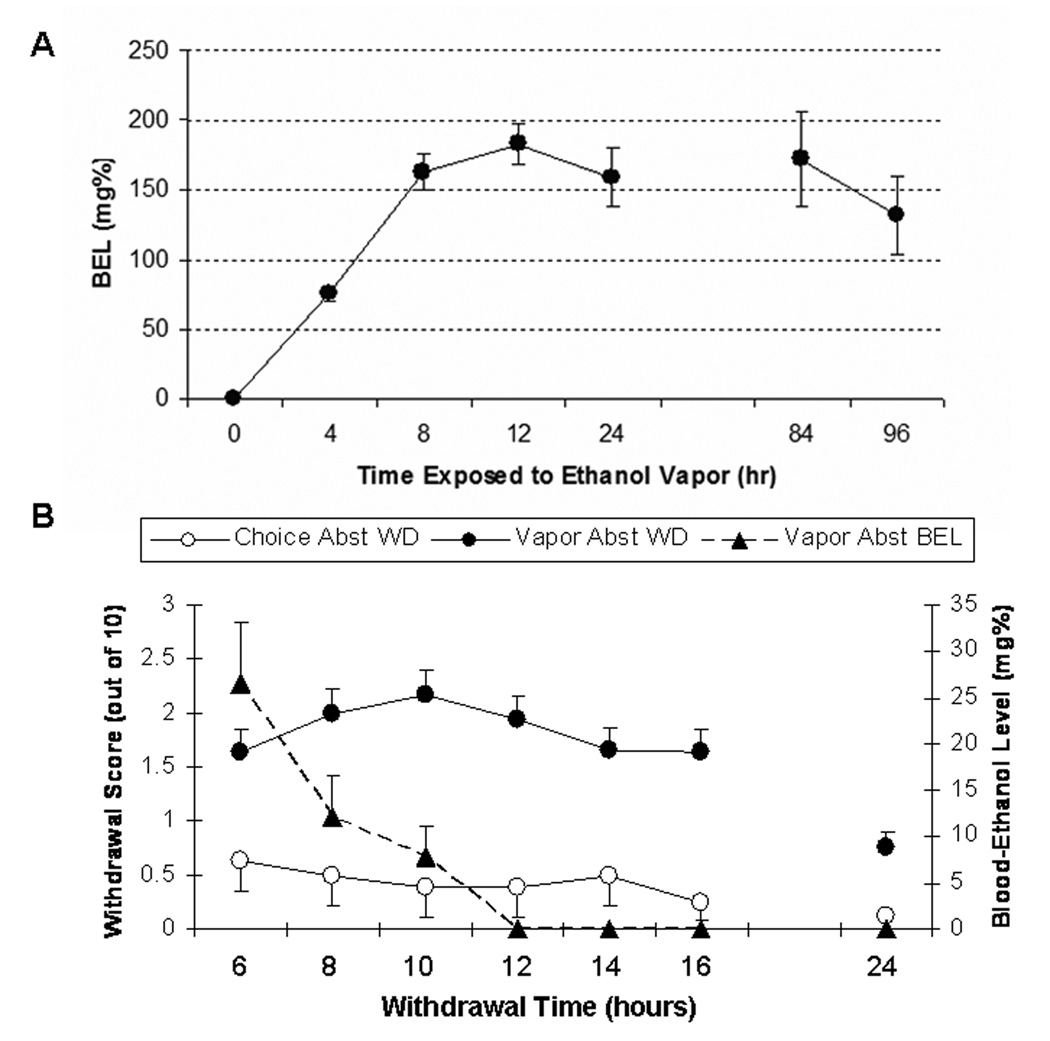
Figure 2a illustrates BELs during the first week of ethanol vapor exposure in VAPOR ABST rats. Tail bloods were taken from the VAPOR ABST group at 4 hr, 8 hr, 12 hr, 24 hr, 84 hr and 96 hr during the first week of exposure. Ethanol evaporation rates were adjusted such that BELs were maintained between 150 mg% and 200 mg%. The vapor inhalation system allowed for reasonable resolution and precision of control over the BELs in these rats.
Figure 2.
(A) Timeline of mean (±SEM) blood-ethanol levels in VAPOR ABST Wistar rats exposed to ethanol vapor over a period of 7 days. Dashed lines are included to denote the boundaries of the target BEL range (150–200 mg%). Tail bloods were taken from the VAPOR ABST group at 4 hr, 8 hr, 12 hr, 24 hr, 84 hr and 96 hr during the first week of exposure. The 84 hr and 96 hr time points were chosen as representative midweek time points time points at which “maximal” and “minimal” BELs, respectively, were targeted. (B) Mean (±SEM) withdrawal scores from test of physical dependence in VAPOR ABST (closed circles) and CHOICE ABST (closed triangles) rats, as well as mean (±SEM) blood-ethanol levels (open circles) for VAPOR ABST rats following removal from first week of ethanol vapor exposure. Withdrawal scores were out of 10 total possible points. Withdrawal scores were inversely related to BELs at the 6-, 8- and 10-hr time points.
Withdrawal Testing
Figure 2b illustrates withdrawal test scores and blood-ethanol levels during the 24 hours following removal of VAPOR ABST rats from the first week of ethanol vapor exposure. A one-way (time) RM ANOVA revealed that withdrawal scores for VAPOR ABST rats changed as a function of time post-ethanol vapor, F(6,492)=19.97, p<0.001. Withdrawal scores were inversely related to BELs at the 6, 8 and 10-hr time points. Maximum withdrawal scores occurred at 10 hrs post-ethanol vapor and then descended toward zero. BELs approached zero by the 10-hr time point. Identical withdrawal testing was also conducted in a single group of CHOICE ABST rats (n=8), but withdrawal scores and BELs were negligible and are not shown. Table 1 shows mean withdrawal scores for each of the 5 behavioral scoring categories at various time points post-ethanol vapor.
Table 1.
Mean (± SEM) scores (out of 2 possible points) for each of the 5 behavioral scoring categories at each withdrawal test time point for VAPOR ABST rats following the first week of ethanol vapor exposure.
| VMD | Abnormal body posture | Tail Stiffness | Hyperactivity | Tremors/Stereotype | |
|---|---|---|---|---|---|
| 6 hr | 0.51 (0.09) | 0.43 (0.08) | 0.18 (0.05) | 0.01 (0.01) | 0.52 (0.07) |
| 8 hr | 0.49 (0.09) | 0.64 (0.09) | 0.14 (0.04) | 0.00 (0.00) | 0.72 (0.08) |
| 10 hr | 0.47 (0.09) | 0.64 (0.09) | 0.23 (0.05) | 0.01 (0.01) | 0.82 (0.08) |
| 12 hr | 0.39 (0.08) | 0.61 (0.09) | 0.13 (0.04) | 0.00 (0.00) | 0.81 (0.07) |
| 14 hr | 0.34 (0.08) | 0.52 (0.08) | 0.08 (0.03) | 0.04 (0.03) | 0.69 (0.08) |
| 16 hr | 0.36 (0.08) | 0.43 (0.08) | 0.10 (0.03) | 0.02 (0.02) | 0.73 (0.08) |
| 24 hr | 0.08 (0.04) | 0.20 (0.06) | 0.08 (0.04) | 0.01 (0.01) | 0.37 (0.06) |
Ethanol Relapse Drinking
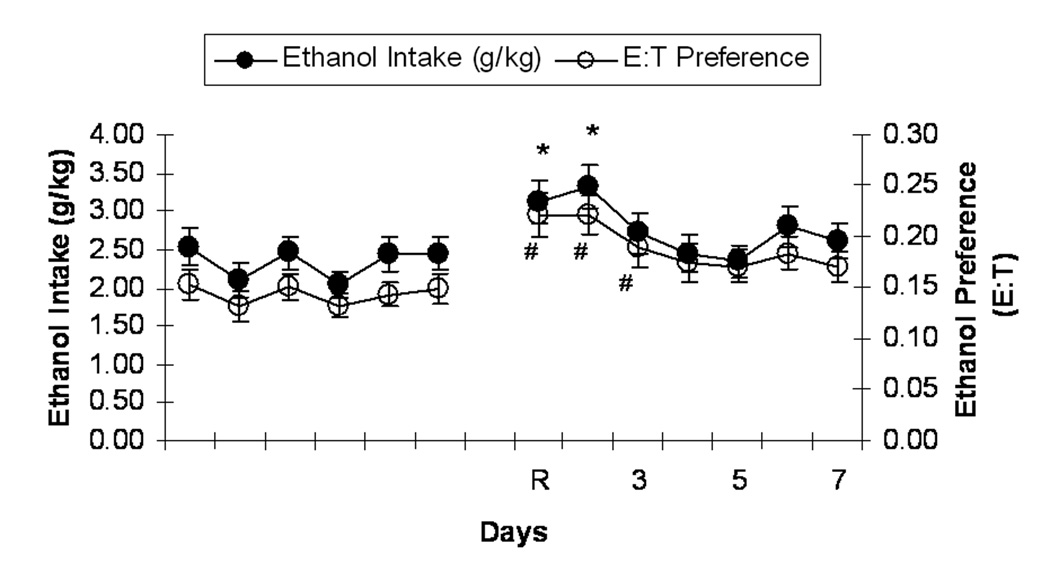
Rats in the CHOICE ABST group underwent a single cycle of ethanol abstinence and reinstatement to two-bottle choice drinking preceding NPY testing. Figure 3 shows that, upon reinstatement of ethanol availability, rats exhibited an alcohol deprivation effect (transient increase in ethanol intake following a period of deprivation relative to pre-deprivation intake levels; Sinclair and Senter, 1967) that lasted several days. A one-way (day) RM ANOVA of data from the baseline period and the first 4 days of reinstatement revealed a significant increase in ethanol consumption (ml) upon reinstatement, F(4,260)=14.55, p<0.001. Bonferroni post-hoc analyses revealed that rats consumed more ethanol (ml) on days one (mean ± SEM = 8.41±0.76), two (mean ± SEM = 9.01±0.78), and three (mean ± SEM = 7.24±0.69) of reinstatement (p<0.05 in all cases) relative to 6-day average baseline intake (mean ± SEM = 5.91±0.49). A separate one-way (day) RM ANOVA revealed a similar increase in ethanol intake (g/kg), F(4,260)=13.15, p<0.001, that lasted through day two of reinstatement (p<0.001), and a similar increase in ethanol preference, F(4,260)=14.05, p<0.001, that lasted through day three of reinstatement (p<0.01). A one-way RM ANOVA revealed a compensatory decrease in water intake (ml), F(4,260)=7.35, p<0.001. Bonferroni post-hoc analyses revealed that rats consumed less water (ml) on days one (mean ± SEM = 32.06±2.07), two (mean ± SEM = 32.23±1.52), and three (mean ± SEM = 32.61±1.43) of reinstatement (p<0.05 in all cases) relative to 6-day average baseline intake (mean ± SEM = 37.27±1.36). The results of a paired-samples t-test, t(62)=11.56, p<0.001, indicated that rats weighed more on reinstatement day (mean ± SEM = 321.52±4.79) relative to baseline (mean ± SEM = 303.78±4.41).
Figure 3.
Mean (± SEM) intake of 15% (v/v) ethanol (g ethanol/kg body weight; closed circles) and mean (± SEM) ethanol preference (ethanol consumed in ml/total fluid consumed in ml; open circles) by CHOICE ABST Wistar rats during the final 6 days of the initial 6-week baseline drinking period and during the 7-day reinstatement period. “R” denotes ethanol reinstatement day, and subsequent numbers indicate subsequent days of that drinking period. *p<0.001 significant difference from baseline ethanol intake (g/kg). # p<0.01 significant difference from baseline ethanol preference (E:T).
Post-NPY Ethanol Drinking and Feeding
Table 2 shows ethanol preference (E:T), water and total fluid intake (ml), food intake (g/kg), and body weights for rats in each of the three ethanol history groups during the 4 hours and 24 hours following infusion with one of four NPY doses (0.0, 2.5, 5.0, or 10.0 µg). The following analyses are separated by the ethanol history groups being compared. The VAPOR ABST group of Wistar rats was compared against two sets of controls: total ethanol exposure controls (VAPOR CONT group) and ethanol exposure pattern controls (CHOICE ABST group). Therefore, the following sections are separated according to the group included in analyses along with the VAPOR ABST group. Analyses are 2-way (Ethanol history × NPY dose) ANOVAs conducted on 4 hr and 24 hr fluid intake from NPY infusion day. Where effects lasted 24 hrs following infusion, analyses were replicated with the exception that they included data from the second 24 hrs (instead of the first 24 hours) following infusion in order to determine whether effects lasted 48 hrs following infusion. In all cases, there were 4 NPY doses (0.0, 2.5, 5.0, 10.0 µg) tested.
Table 2.
Mean (±SEM) ethanol preference (E:T), water intake (ml), total fluid intake (ml), food intake (g/kg body weight), and body weight for rats in each of the ethanol history groups during the 4 hours and 24 hours following infusion with one of four NPY doses (0.0, 2.5, 5.0, or 10.0 µg). Analyses of data from VAPOR ABST and CHOICE ABST rats revealed that 5.0 µg & 2.5 µg NPY suppressed 4-hr ethanol preference (p<0.05) and increased 4-hr water intake (p<0.001) relative to aCSF controls and also increased 4-hr total fluid intake in VAPOR ABST rats (p<0.001) relative to aCSF controls; all doses of NPY increased food intake (p<0.05) at 4 hrs and 24 hrs post-infusion; and VAPOR ABST rats weighed less than CHOICE ABST rats on infusion day (p<0.05). Analyses of data from VAPOR ABST and VAPOR CONT rats revealed that all NPY doses suppressed 4-hr ethanol preference (p<0.001), increased 4-hr water intake (p<0.05), increased 4-hr fluid intake (p<0.05), and increased food intake at 4 and 24 hours (p<0.05) post-infusion relative to aCSF controls; the 5.0 µg & 2.5 µg NPY doses increased 24-hr water intake (p<0.05) and 24-hr total fluid intake (p<0.05) relative to aCSF controls; and VAPOR ABST rats weighed more than VAPOR CONT rats on infusion day (p<0.001).
| Ethanol History | Time Post-Inf. | NPY Dose | Ethanol Preference | Water Intake (ml) | Food Intake (g/kg) | Total Fluid Intake (ml) | Body Weight (g) |
|---|---|---|---|---|---|---|---|
| VAPOR | 4hr | aCSF | 0.25 (0.03) | 10.51 (1.14) | 17.41 (1.33) | 13.63 (1.12) | 301.18 (7.67) |
| ABST | 2.5 µg | 0.17 (0.03) | 17.79 (1.14) | 29.52 (2.03) | 21.17 (0.99) | 329.53 (9.08) | |
| 5.0 µg | 0.12 (0.01) | 21.05 (1.04) | 40.19 (1.91) | 23.85 (0.96) | 324.41 (7.99) | ||
| 10.0 µg | 0.18 (0.03) | 13.66 (1.65) | 33.59 (2.88) | 16.29 (1.77) | 317.11 (5.69) | ||
| 24 hr | aCSF | 0.19 (0.04) | 28.52 (2.49) | 53.76 (3.93) | 34.71 (2.06) | ||
| 2.5 µg | 0.18 (0.03) | 36.10 (2.50) | 66.87 (2.52) | 43.78 (2.17) | |||
| 5.0 µg | 0.11 (0.01) | 38.09 (2.22) | 70.88 (3.06) | 42.64 (2.10) | |||
| 10.0 µg | 0.18 (0.03) | 28.81 (3.55) | 65.59 (5.84) | 33.78 (3.72) | |||
| CHOICE | 4 hr | aCSF | 0.22 (0.03) | 14.09 (1.59) | 18.23 (1.26) | 17.71 (1.62) | 340.13 (10.77) |
| ABST | 2.5 µg | 0.15 (0.01) | 18.44 (1.29) | 31.10 (1.88) | 21.71 (1.42) | 331.00 (11.60) | |
| 5.0 µg | 0.15 (0.02) | 17.74 (1.34) | 33.67 (3.20) | 20.55 (1.21) | 325.33 (6.05) | ||
| 10.0 µg | 0.17 (0.02) | 12.78 (1.33) | 30.11 (2.09) | 14.99 (1.36) | 326.38 (9.15) | ||
| 24 hr | aCSF | 0.26 (0.05) | 31.94 (2.66) | 51.05 (2.45) | 39.36 (2.59) | ||
| 2.5 µg | 0.26 (0.04) | 35.04 (1.91) | 65.44 (2.58) | 42.44 (2.51) | |||
| 5.0 µg | 0.20 (0.04) | 35.66 (3.48) | 64.45 (4.03) | 42.49 (2.94) | |||
| 10.0 µg | 0.18 (0.04) | 27.99 (2.92) | 61.06 (4.66) | 32.46 (3.10) | |||
| VAPOR | 4 hr | aCSF | 0.34 (0.06) | 10.69 (1.74) | 14.70 (1.61) | 14.48 (1.91) | 312.00 (8.38) |
| CONT | 2.5 µg | 0.17 (0.02) | 16.64 (1.42) | 22.17 (1.98) | 19.83 (1.38) | 307.84 (8.66) | |
| 5.0 µg | 0.19 (0.04) | 20.00 (1.86) | 32.35 (2.99) | 23.84 (1.62) | 286.05 (7.55) | ||
| 10.0 µg | 0.19 (0.03) | 16.94 (1.59) | 32.64 (2.58) | 20.34 (1.78) | 291.13 (7.34) | ||
| 24 hr | aCSF | 0.23 (0.03) | 29.91 (3.52) | 40.50 (4.76) | 38.49 (4.17) | ||
| 2.5 µg | 0.17 (0.02) | 40.43 (3.85) | 53.08 (4.50) | 47.85 (3.91) | |||
| 5.0 µg | 0.18 (0.04) | 43.73 (3.73) | 65.59 (4.78) | 52.38 (3.13) | |||
| 10.0 µg | 0.16 (0.02) | 39.52 (4.00) | 64.29 (6.01) | 47.33 (4.93) | |||
VAPOR ABST vs CHOICE ABST
The following sets of analyses include only data from rats in the VAPOR ABST and CHOICE ABST groups. The main purpose of the following analyses was to determine whether the amount of prior ethanol exposure affects fluid and food intake, and the effects of NPY on both of these when administered directly prior to the reinstatement of ethanol availability.
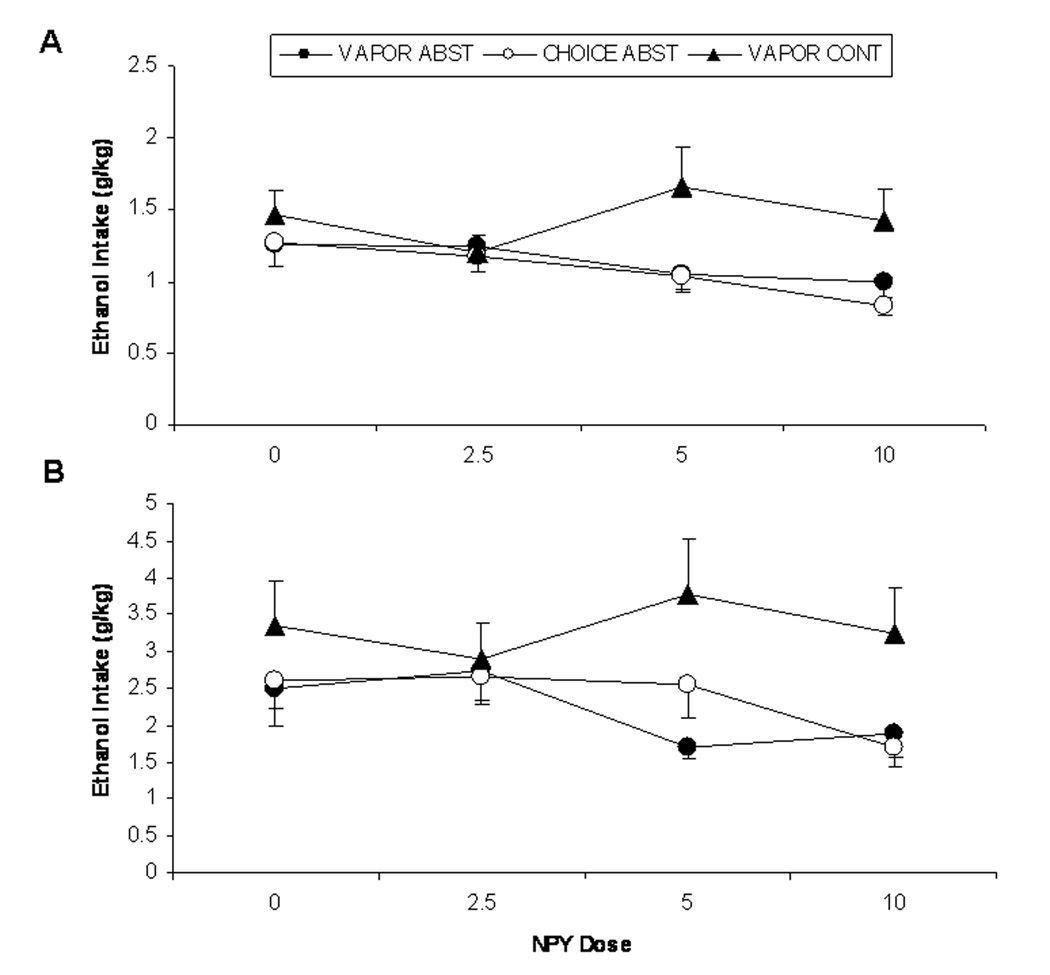
4-hr & 24-hr Post-Infusion Ethanol Intake (g/kg)
Figure 4 shows ethanol intake (g/kg) by Wistar rats in the VAPOR ABST and CHOICE ABST groups during the 4 hours following NPY infusion. A 2-way (ethanol history × NPY dose) ANOVA revealed a significant effect of NPY, F(3,124)=3.04, p=0.031, on ethanol intake (g/kg). Bonferroni post-hoc analyses revealed that 10.0 µg NPY produced a decrease in ethanol intake (g/kg) relative to aCSF controls (p<0.05). There was no effect of ethanol history, nor an interaction effect of NPY dose and ethanol history on ethanol intake (g/kg). Figure 4 also shows ethanol intake (g/kg) by Wistar rats in the VAPOR ABST and CHOICE ABST groups during the 24 hours following NPY infusion. A 2-way (ethanol history × NPY dose) ANOVA of data from the 24 hours following infusion revealed a significant effect of NPY, F(3,124)=2.70, p=0.05, on ethanol intake (g/kg). Again, there was no effect of ethanol history, nor an interaction effect of NPY dose and ethanol history on ethanol intake (g/kg). A separate 2-way (ethanol history × NPY dose) ANOVA of data from the second 24-hour period following infusion (24 to 48 hrs post-infusion) revealed a tendency toward an effect of NPY, F(3,124)=2.57, p=0.06, on ethanol intake (g/kg).
Figure 4.
Mean (± SEM) intake of 15% (v/v) ethanol (g ethanol/kg body weight) by Wistar rats in the VAPOR ABST (closed circles), CHOICE ABST (open circles), and VAPOR CONT (closed triangles) groups during the 4 hours (A) and 24 hours (B) following infusion of four NPY doses (0.0, 2.5, 5.0, 10.0 µg). Across VAPOR ABST and CHOICE ABST rats, there was a significant decrease in 4-hr ethanol intake (g/kg) following infusion of 10.0 µg NPY relative to aCSF controls. Across NPY doses, VAPOR CONT rats drank more ethanol (g/kg) than VAPOR ABST rats at 4 and 24 hrs.
4-hr & 24-hr Post-Infusion Ethanol Consumption (ml), Preference, and Total Fluid Intake
A 2-way (ethanol history × NPY dose) ANOVA revealed a significant effect of NPY, F(3,124)=3.30, p=0.023, on 4-hr ethanol consumption (ml). Bonferroni post-hoc analyses revealed that 10.0 µg NPY suppressed ethanol consumption (ml) relative to aCSF controls (p< 0.05). A separate 2-way ANOVA revealed a significant effect of NPY, F(3,124)=3.35, p=0.021 on 24-hr ethanol ethanol consumption (ml). Bonferroni post-hoc analyses revealed that rats infused with 10.0 µg NPY consumed less ethanol (ml) than rats infused with 2.5 µg NPY (p<0.05). A separate 2-way (ethanol history × NPY dose) ANOVA of data from the second 24-hour period following infusion (24 to 48 hrs post-infusion) revealed a significant effect of NPY, F(3,124)=3.10, p=0.03, on ethanol consumption (ml).
A 2-way (ethanol history × NPY dose) ANOVA revealed a significant effect of NPY, F(3,124)=5.62, p=0.001, on 4-hr ethanol preference. Bonferroni post-hoc analyses revealed that 5.0 µg and 2.5 µg NPY suppressed ethanol preference relative to aCSF controls (p<0.05). There were no 24-hr effects on ethanol preference.
A 2-way (ethanol history × NPY dose) ANOVA revealed a significant effect of NPY, F(3,124)=15.00, p<0.001, and a significant ethanol history × NPY dose interaction effect, F(3,124)=2.87, p=0.039, on 4-hr total fluid intake (ml). Bonferroni post-hoc analyses revealed that, in VAPOR ABST rats, 5.0 µg and 2.5 µg NPY suppressed total fluid intake (ml) relative to aCSF controls (p<0.05). There was also a significant main effect of NPY, F(3,124)=6.57, p<0.001, on 24-hr total fluid intake (ml).
4-hr & 24-hr Post-Infusion Food Intake and Body Weights
Table 2 shows food intake (g/kg) and body weights for rats in the VAPOR ABST and CHOICE ABST groups during the 4 hours and 24 hours following NPY infusion. 2-way (ethanol history × NPY dose) ANOVAs revealed significant effects of NPY on 4-hour, F(3,124)=29.69, p<0.001, and 24-hr, F(3,124)=6.71, p<0.001, food intake (g/kg). Bonferroni post-hoc analyses revealed that all doses of NPY produced an increase in food intake (g/kg) relative to aCSF controls at 4 hrs and 24 hrs post-infusion (p<0.05 in all cases). There was no effect of ethanol history, nor an interaction effect of NPY dose and ethanol history on food intake (g/kg) 4 hrs or 24 hrs following infusion. A separate 2-way ANOVA revealed that CHOICE ABST rats weighed more than VAPOR ABST rats on infusion day, F(3,124)=4.53, p=0.035.
VAPOR ABST vs VAPOR CONT
The following sets of analyses include only data from rats in the VAPOR ABST and VAPOR CONT groups. The main purpose of the following analyses was to determine whether the pattern of prior ethanol exposure affects fluid and food intake and the effects of NPY on both of these when administered directly prior to the reinstatement of ethanol availability in VAPOR ABST rats and at a parallel time point in VAPOR CONT rats.
4-hr & 24-hr Post-Infusion Ethanol Intake (g/kg)
Figure 4 shows ethanol intake (g/kg) by Wistar rats in the VAPOR ABST and VAPOR CONT groups during the 4 hours following NPY infusion. A 2-way (ethanol exposure pattern × NPY dose) ANOVA revealed that rats in the VAPOR CONT group drank significantly more ethanol (g/kg) than rats in the VAPOR ABST group, F(1,135)=5.78, p=0.018. There was no effect of NPY dose, nor an interaction effect of NPY dose and ethanol exposure pattern on 4-hr ethanol intake (g/kg). Figure 4 also shows ethanol intake (g/kg) by Wistar rats in the VAPOR ABST and VAPOR CONT groups during the 24 hours following NPY infusion. A 2-way (ethanol exposure pattern × NPY dose) ANOVA of data from the 24 hours following infusion revealed no effect of NPY, nor an interaction effect of NPY dose and ethanol history on ethanol intake (g/kg). However, rats in the VAPOR CONT group drank significantly more ethanol (g/kg) than rats in the VAPOR ABST group, F(1,135)=9.41, p=0.003.
4-hr & 24-hr Post-Infusion Ethanol Consumption (ml), Preference, and Total Fluid Intake
A 2-way ANOVA revealed no significant effects of ethanol history or NPY dose on 4-hr ethanol consumption (ml). A separate 2-way ANOVA of data from the 24 hours following infusion revealed that rats in the VAPOR CONT group drank significantly more ethanol (ml) than rats in the VAPOR ABST group, F(1,135)=6.69, p=0.011.
A 2-way ANOVA revealed a significant effect of NPY dose, F(3,135)=7.37, p<0.001, on 4-hour ethanol preference. Bonferroni post-hoc analyses revealed that all doses of NPY significantly suppressed ethanol preference relative to aCSF controls (p<0.01 in all cases). There was no effect of NPY dose, nor an interaction effect of NPY dose and ethanol exposure pattern on 4-hr ethanol preference. A 2-way (ethanol history × NPY dose) ANOVA of data from the 24 hours following infusion revealed no effects on ethanol preference (Table 2).
A 2-way ANOVA revealed a significant main effect of NPY dose, F(3,135)=15.88, p<0.001, on 4-hr total fluid intake (ml). Bonferroni post-hoc analyses revealed that all NPY doses produced significant increases in total fluid intake (ml) relative to aCSF controls (p<0.05 in all cases). A separate 2-way ANOVA of data from the 24 hours following infusion revealed significant main effects of ethanol history, F(3,135)=10.77, p=0.001, and NPY dose, F(3,135)=4.48, p=0.005. Rats in the VAPOR CONT group consumed more total fluid than rats in the VAPOR ABST group (p<0.05), and rats infused with 5.0 and 2.5 µg NPY consumed more total fluid than aCSF controls (p<0.05 in both cases).
4-hr & 24-hr Post-Infusion Food Intake and Body Weights
Table 2 shows food intake (g/kg) and body weights for by Wistar rats in the VAPOR ABST and VAPOR CONT groups during the 4 hours and 24 hours following NPY infusion. A 2-way (ethanol exposure pattern × NPY dose) ANOVA revealed a significant effect of NPY, F(3,135)=33.45, p<0.001, on 4-hour food intake (g/kg). Bonferroni post-hoc analyses revealed that all doses of NPY produced an increase in 4-hr food intake (g/kg) relative to aCSF controls (p<0.001 in all cases). Also, rats in the VAPOR ABST group consumed significantly more food (g/kg) than rats in the VAPOR CONT group, F(1,135)=9.13, p=0.003. There was no interaction effect of NPY dose and ethanol exposure pattern on food intake (g/kg). A separate 2-way (ethanol history × NPY dose) ANOVA revealed a significant main effect of NPY dose on 24-hr food intake, F(3,135)=8.66, p<0.001. Bonferroni post-hoc comparisons revealed that all doses of NPY produced increases in 24-hr food intake (g/kg) relative to aCSF controls (p<0.05 in all cases). Also, rats in the VAPOR ABST group consumed significantly more food (g/kg) than rats in the VAPOR CONT group, F(1,135)=7.05, p=0.009. A separate 2-way ANOVA revealed that VAPOR CONT rats weighed less than VAPOR ABST rats on infusion day, F(3,135)=11.98, p<0.001.
DISCUSSION
Wistar rats were trained to drink ethanol in a 2-bottle choice procedure, followed by: exposure to chronic ethanol vapor with periods of ethanol abstinence (VAPOR ABST); exposure to chronic ethanol vapor without periods of ethanol abstinence (VAPOR CONT); or further access to ethanol in a 2-bottle choice situation with periods of ethanol abstinence (CHOICE ABST). Ethanol vapor-exposed rats exhibited moderate signs of physical dependence during acute withdrawal testing, representative of previously reported withdrawal severity following exposure to chronic ethanol vapor (Roberts et al., 1996) and chronic intragastric ethanol infusions (Chester et al., 2002). Also, rats that underwent cycles of chronic ethanol drinking and ethanol abstinence (CHOICE ABST group) exhibited increases in ethanol drinking and preference over baseline levels following abstinence period 1 that lasted at least two days into the reinstatement period, indicative of an alcohol deprivation effect (transient increase in ethanol intake following a period of deprivation relative to pre-deprivation intake levels; Sinclair and Senter, 1967).
NPY was effective in suppressing 2-bottle choice ethanol drinking in rats that underwent cycles of ethanol abstinence (regardless of ethanol vapor exposure), and this effect lasted at least 24 hours post-NPY infusion. These results are partly consistent with past findings that increases in brain NPY activity suppress ethanol consumption by outbred rats in a variety of access conditions following chronic exposure to high ethanol doses (Gilpin et al., 2008a; Rimondini et al., 2005; Thorsell et al., 2005a, 2005b, 2007). These results also provide support for findings in P rats following chronic access to 2-bottle choice ethanol drinking with and without periods of ethanol abstinence; in those studies, the ability of NPY to suppress ethanol drinking was greater in magnitude and duration in P rats that had undergone periods of ethanol abstinence (Gilpin et al., 2003, 2005). NPY also produced short-term decreases in ethanol preference in the VAPOR CONT group, but this effect was a result of prandial increases in water intake rather than any direct effect of NPY on ethanol intake by those rats. Therefore, NPY suppresses ethanol drinking at least 24 hours following infusion in outbred rats in a 2-bottle choice situation following cycles of chronic ethanol exposure and ethanol abstinence periods.
It is interesting that NPY effectively suppressed ethanol intake in ethanol-abstinent rats, but not in ethanol vapor-exposed non-abstinent rats. The ethanol-abstinent groups (VAPOR ABST and CHOICE ABST) endured multiple periods of forced abstinence from ethanol. During ethanol access periods, vapor-exposed animals (VAPOR ABST) were exposed to continuous ethanol vapor, while ethanol-drinking rats (CHOICE ABST), although allowed continuous 2-bottle choice access to ethanol solution, undoubtedly endured self-imposed periods of abstinence during that time. However, animals in both of the vapor-exposed groups were also allowed many weeks of 2-bottle choice ethanol access prior to vapor exposure, during which time those animals endured similar self-imposed schedules of intermittence. Therefore, self-imposed schedules of intermittence during 2-bottle choice ethanol access were an element of the ethanol exposure pattern common to all groups. These observations underscore the notion that the inclusion of forced long-term abstinence periods in ethanol exposure schedules is a critical factor in producing neural changes associated with dependence.
The ability of NPY to suppress ethanol drinking in rats was revealed by long-term abstinence periods, but not necessarily by induction of alcohol dependence. These findings raise several important points. First, these results indicate that cycles of exposure and abstinence, even in the absence of dependence, are sufficient to uncover the ability of NPY to suppress ethanol drinking in outbred rats. Second, it is difficult to discern from the present data whether abstinent are more sensitive to the effects of NPY or dependent rats are less sensitive to its effects, but when considered in the context of past findings, it appears that repeated cycles of ethanol exposure and abstinence produce a heightened sensitivity to NPY (Gilpin et al., 2003, 2005). Finally, past studies have shown that NPY does effectively suppress ethanol drinking by dependent Wistar rats during acute withdrawal, but not in non-dependent rats (Thorsell et al., 2005a,b). In those studies, animals were exposed to chronic intermittent ethanol vapor (14 hrs exposure and 10 hrs withdrawal per day), therefore it is difficult to determine whether, under those parameters, it was the dependence or the repeated withdrawal cycles that were responsible for the ability of NPY to suppress ethanol drinking by those animals. An important methodological difference between the studies is that continuous vapor-exposed rats in the current study were infused with NPY and allowed to drink ethanol shortly after removal from ethanol vapor (before BELs returned to zero), whereas animals in the studies by Thorsell et al. (2005a,b) were tested further into withdrawal (as BELs approached zero). Also, rats in this study were exposed to vapor for only 2 weeks (versus ≥ 8–9 weeks in the Thorsell et al. experiments), target BELs were lower in this study (150–200 mg% versus 200–250 mg% in the Thorsell et al. studies), and ethanol drinking session parameters differ across the studies.
Neural changes in NPY function are likely widespread because multiple behaviors exhibit heightened sensitivity to the peptide following cycles of ethanol exposure and abstinence. Past studies have shown an augmented ability of ICV NPY to suppress ethanol drinking (Gilpin et al., 2003, 2005) and increase feeding (Gilpin et al., 2005) when administered during ethanol abstinence. Further, ICV administration of BIIE0246, a Y2 autoreceptor-selective antagonist, produces stronger sedative effects and suppressive effects on ethanol drinking following chronic exposure to high doses of ethanol and periods of ethanol abstinence (Rimondini et al., 2005). Therefore, these sensitized effects of NPY during ethanol abstinence extend to multiple behavioral effects, all of which are mediated by mutually exclusive brain pathways. For example, the orexigenic effects of NPY are mediated by the paraventricular hypothalamic nucleus (PVN; Stanley et al., 1985) and the sedative effects by the posterior hypothalamic nucleus (Naveilhan et al., 2001). Therefore, cycles of chronic ethanol exposure and abstinence periods may produce a global dysregulation of NPY systems.
The anxiolytic effects of NPY may be most important in mediating the ability of the peptide to suppress ethanol drinking. It has been suggested that the negative reinforcing (i.e. anxiolytic) effects produced by ethanol when consumed during ethanol abstinence (i.e. relapse drinking), may be mediated by NPY brain systems involved in regulating anxiety-like behavior (Valdez and Koob, 2004). The anxiolytic effects of NPY are mediated by the amygdala (Heilig et al., 1993; Sajdyk et al., 2002), and recent data indicate that NPY in the amygdala also mediates the suppressive effects of ICV NPY on ethanol drinking in a variety of conditions (Gilpin et al., 2008a, 2008b; Pandey et al., 2005; Thorsell et al., 2007). Therefore, it is not surprising that ICV NPY-induced suppression of ethanol intake is amplified following a period without ethanol since NPY might be expected to alleviate the anxiogenic effects of a period of ethanol abstinence.
This notion is further supported by decreases in NPY protein levels observed in several brain regions (e.g., amygdaloid nuclei) 24 hours following removal of ethanol liquid diet (Roy and Pandey, 2002). Therefore, it is possible that abstinence-induced decreases in NPY protein expression elicit compensatory increases in the sensitivity of downstream receptor and/or intracellular signaling mechanisms, producing sensitized behavioral responses to NPY. Such changes might account for the long-term effects of a single NPY infusion observed in this and previous studies (Gilpin et al., 2003, 2005).
It is unlikely that ICV NPY-induced suppression of ethanol intake is secondary to the effects of the peptide on feeding. Initial studies in alcohol-preferring rats showed that ICV NPY suppresses ethanol intake in the absence of food and water during limited access drinking sessions (Badia-Elder et al., 2001, 2003). Furthermore, infusions of NPY directly into the PVN, the brain region that mediates feeding effects of NPY, produce increases in ethanol drinking in Wistar rats and alcohol-preferring rats (Gilpin et al., 2004; Kelley et al., 2001). These results indicate that the suppressive effects of ICV NPY on ethanol drinking are not secondary to its effects on feeding, and also that the neurocircuitry that mediates the effects of NPY on these two behaviors is dissociable.
It is worth repeating that female rats were employed in the present investigation. Most of the previous studies of NPY and ethanol drinking from our lab have also used female rats (Badia-Elder et al., 2003; Gilpin et al., 2003, 2004, 2005), except for a single study that used male rats (Badia-Elder et al., 2001). Studies of NPY function and ethanol drinking from other labs have typically used male rats (Primeaux et al., 2006; Rimondini et al., 2005; Roy and Pandey, 2002; Thorsell et al., 2005a,b). The preponderance of the literature across labs, regardless of gender, is that increased NPY activity suppresses ethanol drinking, and that this effect is augmented in vulnerable subpopulations (abstinent rats, dependent rats, “anxious” rats, and alcohol-preferring rats). However, the potential interactions between gender, chronic ethanol exposure, and NPY function can not be ignored. It is well established that female rats voluntarily consume more ethanol than male rats (Lancaster and Spiegel, 1992), and also that ethanol pharmacokinetics are different across genders (e.g., Rivier, 1993). One example of relevant gender differences in brain NPY function is provided by the observation that diet differentially affects NPY receptors in hypothalamus of male and female mice (Zamaretti et al., 2007). The regulation of other neural systems by NPY within the hypothalamus, for example vasopressin and luteinizing hormone, also differs between males and females (Kalra et al., 1988; Sato et al., 1995). Therefore, the possible interaction of ethanol history and gender differences should be further explored, particularly because cycles of chronic ethanol exposure and abstinence produce dysregulation of behaviors mediated by brain regions in which gender differences in NPY function exist.
In summary, ICV NPY effectively suppresses ethanol drinking in Wistar rats that have endured cycles of chronic ethanol exposure and abstinence, regardless of ethanol dependence history. It would be valuable to know how long ethanol abstinence-induced changes in brain NPY protein levels last, and also whether NPY receptors are alternatively regulated and/or whether intracellular signaling cascades exhibit altered sensitivity to NPY during ethanol abstinence. Also, future studies that employ chronic intermittent ethanol exposure schedules should consider the separate impacts of the chronic and intermittent aspects of that schedule on behavioral and neural endpoints.
ACKNOWLEDGEMENTS
The authors thank Drs. James Murphy, Charles Goodlett and Susan Swithers for their valuable insights and contributions to this paper. This investigation was part of the Ph.D. dissertation submitted to Purdue University by N.W. Gilpin. This investigation was supported by National Institutes of Alcohol Abuse and Alcoholism grants, AA12857, AA07611, and AA10722.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li T-K. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and –nonpreferring (NP) rats. Alcohol. Clin. Exp. Res. 2001;25:386–390. [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li TK. Effect of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol. Clin. Exp. Res. 2003;27:894–899. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Chester JA, Price CS, Froehlich JC. Inverse genetic association between alcohol preference and severity of alcohol withdrawal in two sets of rat lines selected for the same phenotype. Alcohol. Clin. Exp. Res. 2002;26:19–27. [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinol. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol. Biochem. Behav. 2008a doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y in the central nucleus of the amygdala suppresses ethanol drinking in alcohol-preferring (P) rats following imposed ethanol abstinence. Pharmacol. Biochem. Behav. 2008b doi: 10.1016/j.pbb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Neuropeptide Y (NPY) in the paraventricular nucleus of the hypothalamus (PVN) increases ethanol intake in high (HAD1) and low (LAD1)alcohol drinking rats. Alcohol. Clin. Exp. Res. 2004;28:1492–1498. doi: 10.1097/01.alc.0000141813.27875.d5. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Sensitized effects of neuropeptide Y on multiple ingestive behaviors in P rats following ethanol abstinence. Pharmacol. Biochem. Behav. 2005;81:740–749. doi: 10.1016/j.pbb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li T-K, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol. Clin. Exp. Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Heilig M, Murison R. Intracerebroventricular neuropeptide Y suppresses open field and home cage activity in the rat. Regulat. Pept. 1987;19:221–231. doi: 10.1016/0167-0115(87)90278-3. [DOI] [PubMed] [Google Scholar]
- Heilig M, Söderpalm B, Engel JA, Widerlöv E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacol. 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacol. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Allen LG, Sahu A, Kalra PS, Crowley WR. Gonadal steroids and neuropeptide Y-opioid-LHRH axis: interactions and diversities. J. Steroid. Biochem. 1988;30:185–193. doi: 10.1016/0022-4731(88)90092-1. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Nannini MA, Bratt AM, Hodge CW. Neuropeptide-Y in the paraventricular nucleus increases ethanol self-administration. Peptides. 2001;22:515–522. doi: 10.1016/s0196-9781(01)00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–470. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Canals JM, Valjakka A, Vartiainen J, Arenas E, Ernfors P. Neuropeptide Y alters sedation through a hypothalamic Y1-mediated mechanism. Eur. J. Neurosci. 2001;13:2241–2246. doi: 10.1046/j.0953-816x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J. Clin. Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self administration in “anxious” rats. Alcohol. Clin. Exp. Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci. Lett. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: Influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol. Clin. Exp. Res. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol. Clin. Exp. Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacol. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav. Neural. Bio. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol. Clin. Exp. Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacol. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sato K, Crofton JT, Wang Y-X, Share L. Effects of gender on the central actions of neuropeptide Y and norepinephrine on vasopressin and blood pressure in the rat. Brain Res. 1995;689:71–78. doi: 10.1016/0006-8993(95)00454-x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following alcohol deprivation. Psychonomic Sci. 1967;8:11. [Google Scholar]
- Slawecki CJ, Betancourt M, Walpole T, Ehlers CL. Increases in sucrose consumption, but not ethanol consumption, following ICV NPY administration. Pharmacol. Biochem. Behav. 2000;66:591–594. doi: 10.1016/s0091-3057(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res. Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in Wistar rats with a history of ethanol exposure. Alcohol. Clin. Exp. Res. 2005a;29:584–590. doi: 10.1097/01.alc.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav. Brain. Res. 2005b;161:133–140. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotrophin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol. Biochem. Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol. Clin. Exp. Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Zammaretti F, Panzica G, Eva C. Sex-dependent regulation of hypothalamic neuropeptide Y-Y1 receptor gene expression in moderate/high fat, high-energy diet-fed mice. J. Physiol. 2007;583:445–454. doi: 10.1113/jphysiol.2007.133470. [DOI] [PMC free article] [PubMed] [Google Scholar]