Abstract
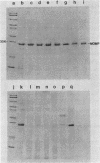
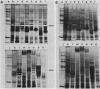
Outer membranes were isolated from eight serogroups of L. pneumophila and five other Legionella species. The protein composition of the membranes was characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A single, disulfide stabilized protein with a molecular size of 29,000 to 30,000 daltons was found to be the major outer membrane protein (MOMP) of all the serogroups. The equivalent of the L. pneumophila MOMP was not observed in any of the other Legionella species examined. Silver staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels revealed distinctive patterns for each serogroup and other Legionella species that were not observed by staining with Coomassie blue and may result from the presence of lipopolysaccharide in the membrane preparations. The MOMP from serogroup 1 was isolated by exposing crude peptidoglycan to detergent in the presence of heat and reducing agent and was found to be tightly associated with lipopolysaccharide. Antibodies to this complex were used to probe the outer membranes of the remaining, L. pneumophila serogroups and other Legionella species by Western blotting. Serogroup 1 anti-MOMP antibodies were found to react with the MOMP from the remaining seven serogroups examined, whereas antibodies directed against the lipopolysaccharide of serogroup 1 only reacted with lipopolysaccharide from two of the remaining seven serogroups.
Full text
PDF

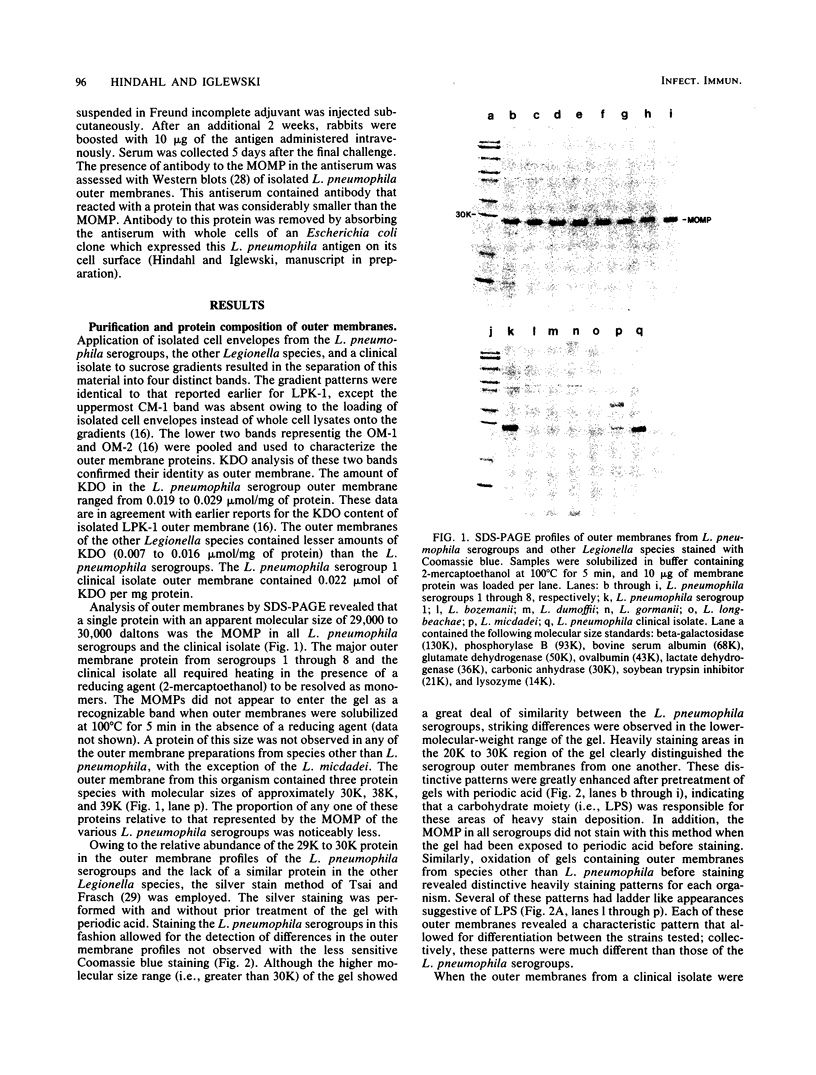
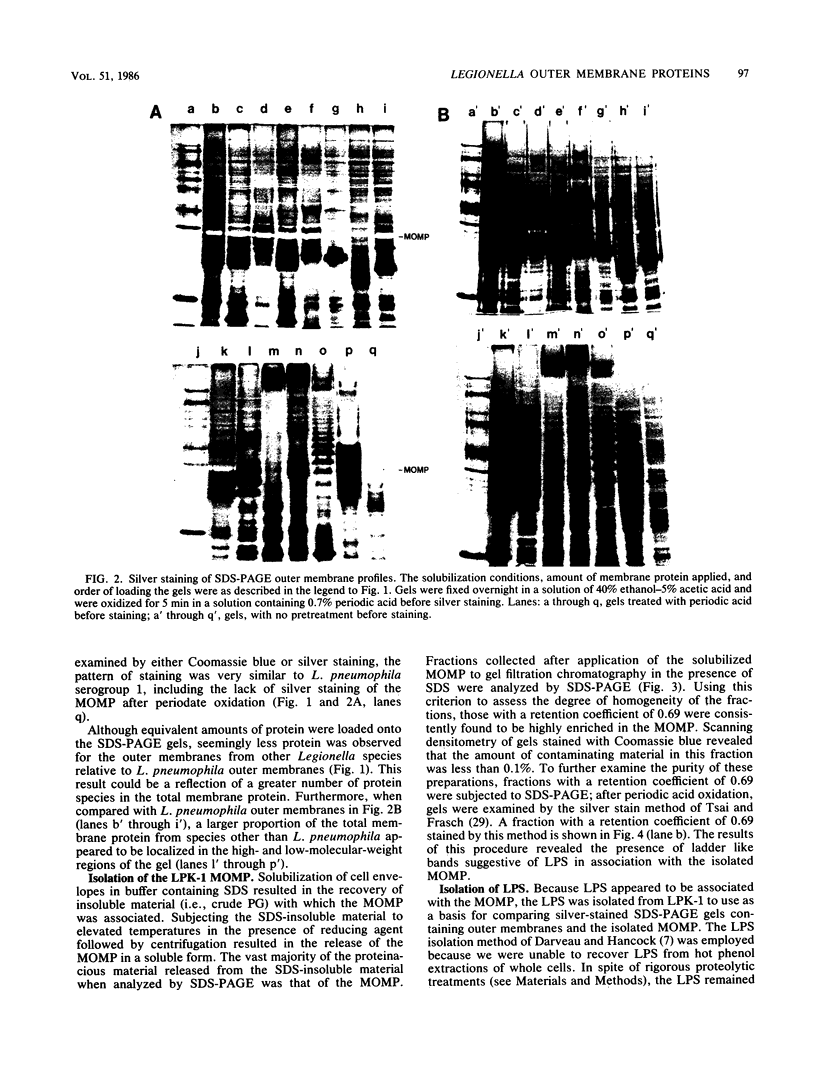
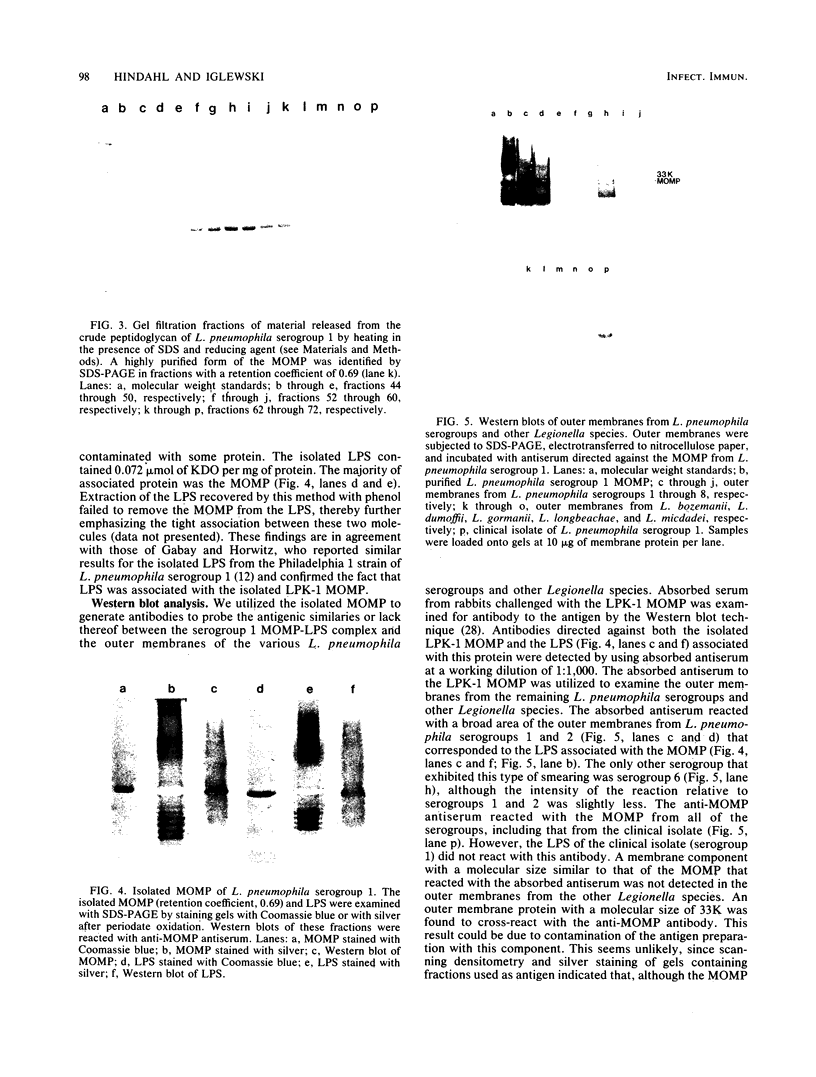



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Williams J. C., McCaul T. F., Peacock M. G. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J Bacteriol. 1984 Dec;160(3):982–988. doi: 10.1128/jb.160.3.982-988.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Williams J. C. Partial characterization of peptidoglycan-associated proteins of Legionella pneumophila. J Biochem. 1983 Aug;94(2):601–606. doi: 10.1093/oxfordjournals.jbchem.a134392. [DOI] [PubMed] [Google Scholar]
- Amano K., Williams J. C. Peptidoglycan of Legionella pneumophila: apparent resistance to lysozyme hydrolysis correlates with a high degree of peptide cross-linking. J Bacteriol. 1983 Jan;153(1):520–526. doi: 10.1128/jb.153.1.520-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Williams J. C. Sensitivity of Coxiella burnetii peptidoglycan to lysozyme hydrolysis and correlation of sacculus rigidity with peptidoglycan-associated proteins. J Bacteriol. 1984 Dec;160(3):989–993. doi: 10.1128/jb.160.3.989-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler C. A., Street E. D., Hatch T. P., Hoffman P. S. Disulfide-bonded outer membrane proteins in the genus Legionella. Infect Immun. 1985 Apr;48(1):14–18. doi: 10.1128/iai.48.1.14-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gorman G. W., Weaver R. E., Mackel D. C., Smith H. W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978 Sep;8(3):320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher A. R., Jennings H. J., Lugowski C., Kasper D. L. Isolation of a serogroup 1-specific antigen from Legionella pneumophila. J Infect Dis. 1982 Feb;145(2):224–233. doi: 10.1093/infdis/145.2.224. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Horwitz M. A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J Exp Med. 1985 Feb 1;161(2):409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosting L. H., Cabrian K., Sturge J. C., Goldstein L. C. Identification of a species-specific antigen in Legionella pneumophila by a monoclonal antibody. J Clin Microbiol. 1984 Dec;20(6):1031–1035. doi: 10.1128/jcm.20.6.1031-1035.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol. 1981 Apr;146(1):426–429. doi: 10.1128/jb.146.1.426-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindahl M. S., Iglewski B. H. Isolation and characterization of the Legionella pneumophila outer membrane. J Bacteriol. 1984 Jul;159(1):107–113. doi: 10.1128/jb.159.1.107-113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Intracellular multiplication of Legionnaires' disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J Clin Invest. 1983 Jan;71(1):15–26. doi: 10.1172/JCI110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W., Elliott J. A., Helms C. M., Renner E. D. A high molecular weight antigen in Legionnaires' disease bacterium: isolation and partial characterization. Ann Intern Med. 1979 Apr;90(4):638–641. doi: 10.7326/0003-4819-90-4-638. [DOI] [PubMed] [Google Scholar]
- Joly J. R., Winn W. C. Correlation of subtypes of Legionella pneumophila defined by monoclonal antibodies with epidemiological classification of cases and environmental sources. J Infect Dis. 1984 Nov;150(5):667–671. doi: 10.1093/infdis/150.5.667. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., Jones R. B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983 May;154(2):998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Wilkinson H. W., Fikes B. J., Cruce D. D. Indirect immunofluorescence test for serodiagnosis of Legionnaires disease: evidence for serogroup diversity of Legionnaires disease bacterial antigens and for multiple specificity of human antibodies. J Clin Microbiol. 1979 Mar;9(3):379–383. doi: 10.1128/jcm.9.3.379-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]
- Zanen-Lim O. G., van den Broek N. J., Rietra P. J., Zanen H. C. Comparison of strains of Legionella pneumophila serogroup 1 isolated in four Amsterdam hospitals from patients and hot-water supplies. J Infect Dis. 1984 Oct;150(4):508–512. doi: 10.1093/infdis/150.4.508. [DOI] [PubMed] [Google Scholar]