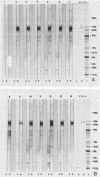
Abstract
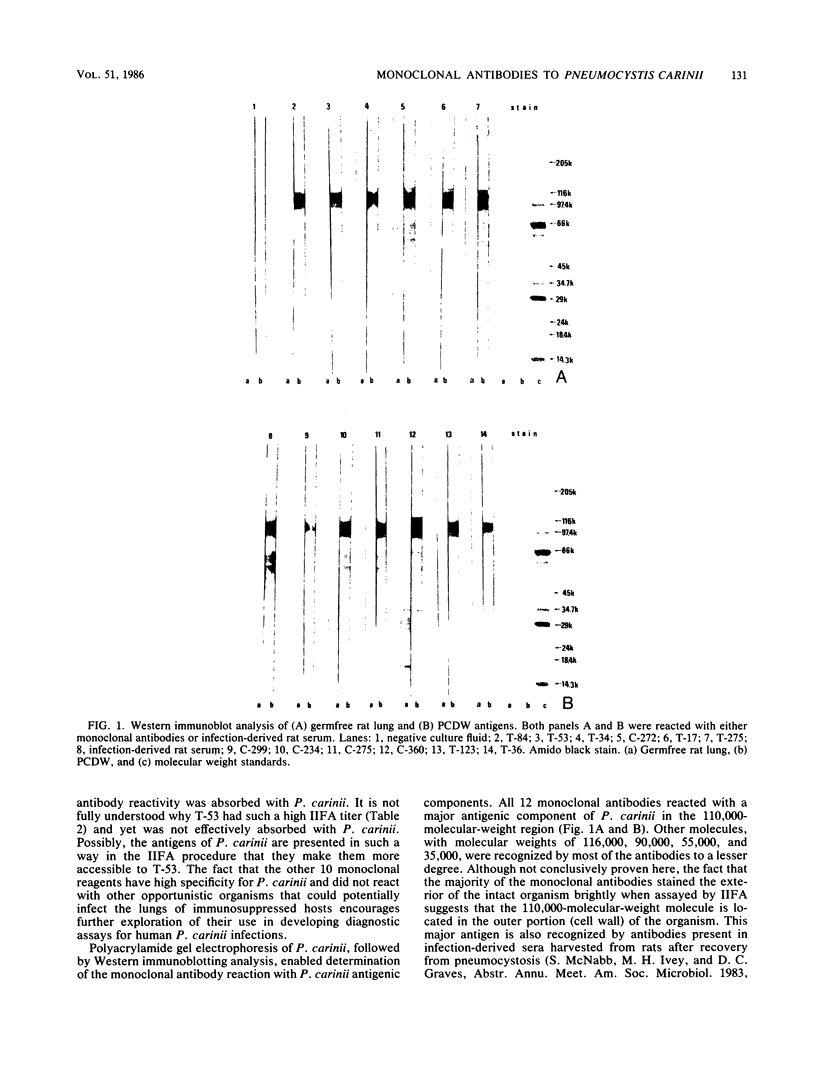
Hybridoma-producing monoclonal antibodies against Pneumocystis carinii were produced by the fusion of nonsecreting mouse myeloma cells (P3X63-Ag8.653) with splenocytes from BALB/c mice that had been immunized with partially purified preparations of P. carinii. Of 227 hybridoma clones producing antibodies against P. carinii, as measured by an enzyme-linked immunosorbent assay, 12 monoclonal antibodies showing the highest reactivity in the enzyme-linked immunosorbent assay were further characterized. The majority (11 of 12) of the monoclonal antibodies did not cross-react with Candida albicans, Cryptococcus neoformans, Histoplasma capsulatum, or Mycobacterium avium as determined by absorption experiments. By using the indirect immunofluorescence assay, serological reactivity was shown for these antibodies with titers ranging from 1:40 to 1:10,240. By using a competitive binding assay, these 12 monoclonal antibodies could be divided into seven groups, each group reacting with a different antigenic determinant of P. carinii. Sodium dodecyl sulfate-polyacrylamide gradient gel electrophoresis of P. carinii, followed by Western immunoblot analysis, allowed the identification of one major antigen with an apparent molecular weight of 110,000 by all 12 monoclonal antibodies. Other minor bands with molecular weights of approximately 116,000, 90,000, 55,000, and 35,000 were recognized by several of the monoclonal antibodies.
Full text
PDF


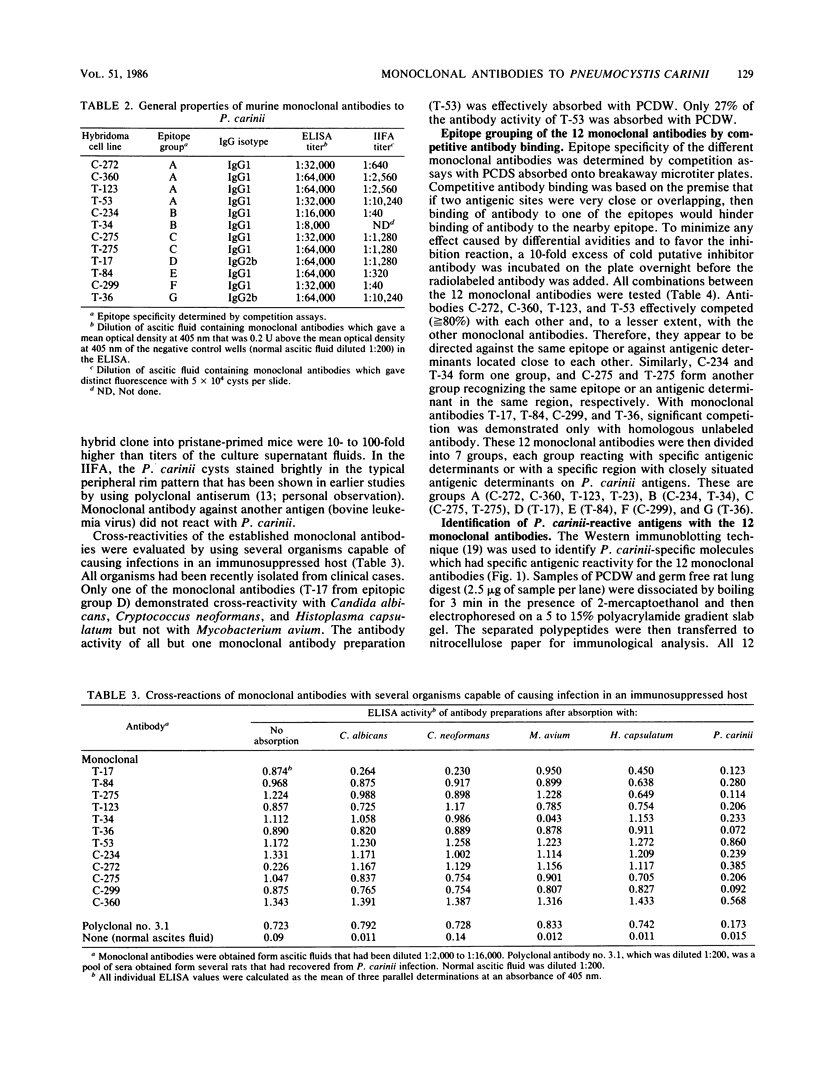
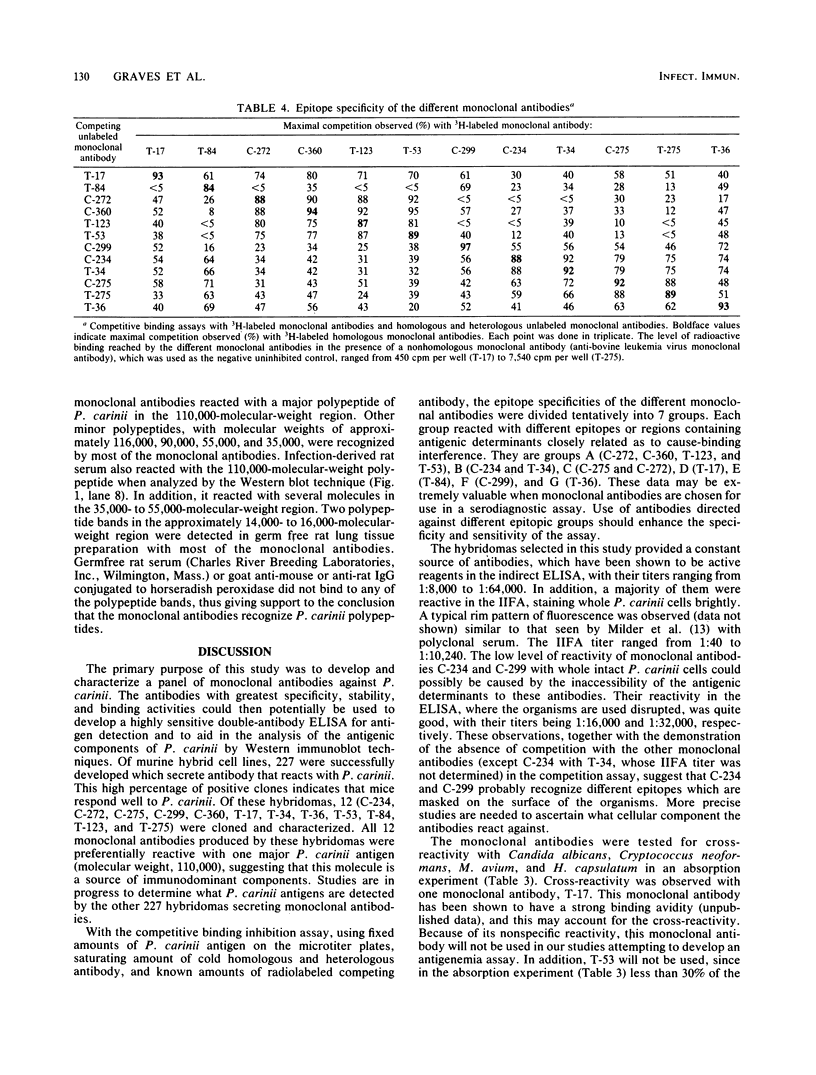


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Cunningham M. W., Krisher K., Graves D. C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect Immun. 1984 Oct;46(1):34–41. doi: 10.1128/iai.46.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. W., Choi K., Thomas P. A., Haverkos H. W., Auerbach D. M., Guinan M. E., Rogers M. F., Spira T. J., Darrow W. W., Kramer M. A. National case-control study of Kaposi's sarcoma and Pneumocystis carinii pneumonia in homosexual men: Part 1. Epidemiologic results. Ann Intern Med. 1983 Aug;99(2):145–151. doi: 10.7326/0003-4819-99-2-145. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maddison S. E., Hayes G. V., Ivey M. H., Tsang V. C., Slemenda S. B., Norman L. G. Fractionation of Pneumocystis carinii antigens used in an enzyme-linked immunosorbent assay for antibodies and in the production of antiserum for detecting Pneumocystis carinii antigenemia. J Clin Microbiol. 1982 Jun;15(6):1029–1035. doi: 10.1128/jcm.15.6.1029-1035.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison S. E., Hayes G. V., Slemenda S. B., Norman L. G., Ivey M. H. Detection of specific antibody by enzyme-linked immunosorbent assay and antigenemia by counterimmunoelectrophoresis in humans infected with Pneumocystis carinii. J Clin Microbiol. 1982 Jun;15(6):1036–1043. doi: 10.1128/jcm.15.6.1036-1043.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison S. E., Walls K. W., Haverkos H. W., Juranek D. D. Evaluation of serologic tests for Pneumocystis carinii antibody and antigenemia in patients with acquired immunodeficiency syndrome. Diagn Microbiol Infect Dis. 1984 Jan;2(1):69–73. doi: 10.1016/0732-8893(84)90025-7. [DOI] [PubMed] [Google Scholar]
- Meyers J. D., Pifer L. L., Sale G. E., Thomas E. D. The value of Pneumocystis carinii antibody and antigen detection for diagnosis of Pneumocystis carinii pneumonia after marrow transplantation. Am Rev Respir Dis. 1979 Dec;120(6):1283–1287. doi: 10.1164/arrd.1979.120.6.1283. [DOI] [PubMed] [Google Scholar]
- Milder J. E., Walzer P. D., Coonrod J. D., Rutledge M. E. Comparison of histological and immunological techniques for detection of Pneumocystis carinii in rat bronchial lavage fluid. J Clin Microbiol. 1980 Apr;11(4):409–417. doi: 10.1128/jcm.11.4.409-417.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco O., Afchain D., Dissous C., Rodriguez C., Ovlaque G., Lemesre J. L., Loyens M., Capron A. Different monoclonal antibodies against the component 5 specific for Trypanosoma cruzi. Am J Trop Med Hyg. 1984 Jul;33(4):560–568. doi: 10.4269/ajtmh.1984.33.560. [DOI] [PubMed] [Google Scholar]
- Pifer L. L., Hughes W. T., Stagno S., Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics. 1978 Jan;61(1):35–41. [PubMed] [Google Scholar]
- Pifer L. L., Lattuada C. P., Edwards C. C., Woods D. R., Owens D. R. Pneumocystis carinii infection in germ-free rats: implications for human patients. Diagn Microbiol Infect Dis. 1984 Jan;2(1):23–36. doi: 10.1016/0732-8893(84)90020-8. [DOI] [PubMed] [Google Scholar]
- Stähli C., Miggiano V., Stocker J., Staehelin T., Häring P., Takács B. Distinction of epitopes by monoclonal antibodies. Methods Enzymol. 1983;92:242–253. doi: 10.1016/0076-6879(83)92023-2. [DOI] [PubMed] [Google Scholar]
- Sugasawara R. J., Prato C. M., Sippel J. E. Enzyme-linked immunosorbent assay with a monoclonal antibody for detecting group A meningococcal antigens in cerebrospinal fluid. J Clin Microbiol. 1984 Feb;19(2):230–234. doi: 10.1128/jcm.19.2.230-234.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Comparison of rat, mouse, and human Pneumocystis carinii by immunofluorescence. J Infect Dis. 1980 Sep;142(3):449–449. doi: 10.1093/infdis/142.3.449. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E., Yoneda K., Stahr B. J. Pneumocystis carinii: new separation method from lung tissue. Exp Parasitol. 1979 Jun;47(3):356–368. doi: 10.1016/0014-4894(79)90088-2. [DOI] [PubMed] [Google Scholar]