Abstract
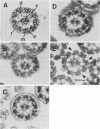
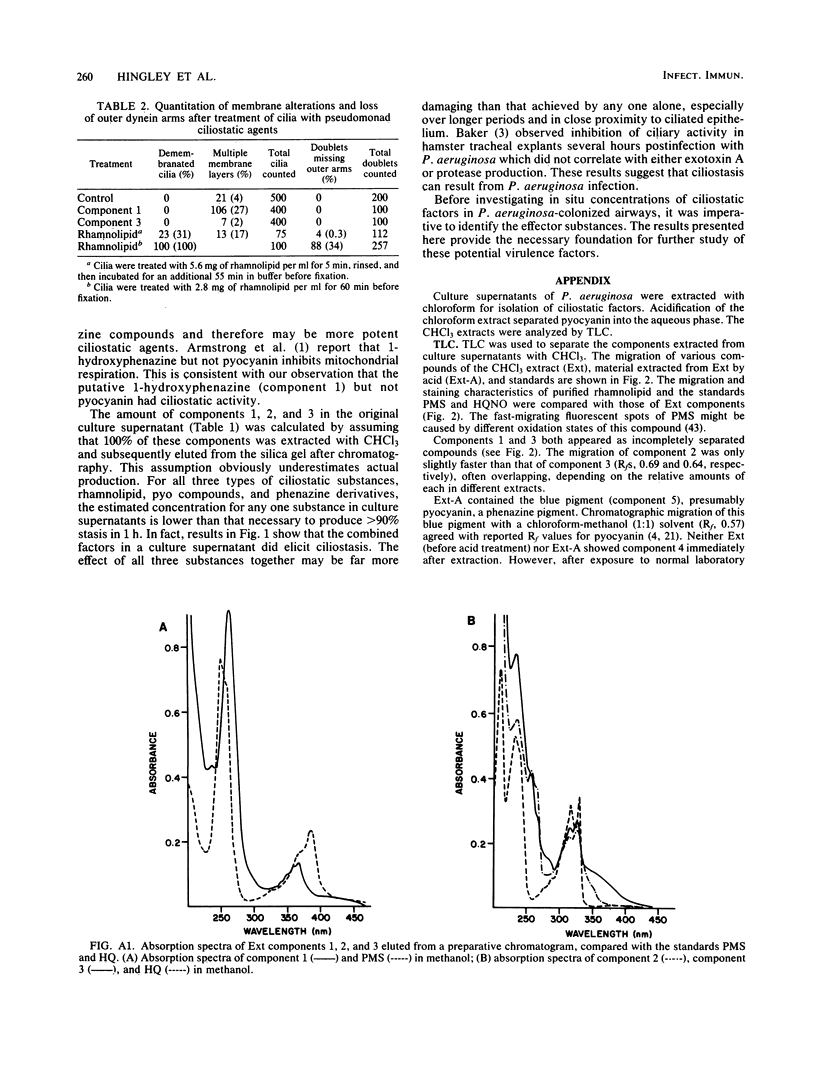
Heat-stable factors released by Pseudomonas aeruginosa in culture supernatants inhibit functional cilia of rabbit tracheal epithelium. Chloroform extraction removed heat-stable factors from stationary-phase culture supernatants. The extracts contained at least seven components separable by thin-layer chromatography (TLC). Cilioinhibitory components were identified as a phenazine derivative, pyo compounds (2-alkyl-4-hydroxyquinolines), and a rhamnolipid, also known as a hemolysin. Fluorescence and absorption spectra, relative migration on TLC, staining characteristics, and gas chromatography were the basis for identification. Inhibitory concentrations of each active component were established by quantitative measures of percent motility and beat frequency. Corresponding damage to ciliary ultrastructure was examined by electron microscopy. The pyo compounds produced ciliostasis at concentrations of 50 micrograms/ml, but without obvious ultrastructural lesions. The phenazine derivative also inhibited ciliary motility and caused some membrane disruption, although at substantially greater concentrations of 400 micrograms/ml. Limited exposure of tracheal explants to the rhamnolipid resulted in ciliostasis which was associated with altered ciliary membranes. More extensive exposure to rhamnolipid was associated with removal of dynein arms from axonemes. Pyocyanin at a concentration of 0.5 mg/ml did not inhibit ciliary beating under our conditions. The data suggest that the pyo compounds are the most effective per weight ciliostatic factors released by P. aeruginosa and rhamnolipid is the most destructive of cilia ultrastructure. By interfering with normal ciliary function, these ciliostatic factors may enable P. aeruginosa to more easily colonize the respiratory tract.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong A. V., Stewart-Tull D. E., Roberts J. S. Characterisation of the Pseudomonas aeruginosa factor that inhibits mouse-liver mitochondrial respiration. J Med Microbiol. 1971 May;4(2):249–262. doi: 10.1099/00222615-4-2-249. [DOI] [PubMed] [Google Scholar]
- Baker N. R. Role of exotoxin A and proteases of Pseudomonas aeruginosa in respiratory tract infections. Can J Microbiol. 1982 Feb;28(2):248–255. doi: 10.1139/m82-033. [DOI] [PubMed] [Google Scholar]
- Baron S. S., Rowe J. J. Antibiotic action of pyocyanin. Antimicrob Agents Chemother. 1981 Dec;20(6):814–820. doi: 10.1128/aac.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. M., Dulfano M. J. Mucus viscoelasticity and mucociliary transport rate. J Lab Clin Med. 1978 Mar;91(3):423–431. [PubMed] [Google Scholar]
- Chodosh S., Medici T. C. The bronchial epithelium in chronic bronchitis. I. Exfoliative cytology during stable, acute bacterial infection and recovery phases. Am Rev Respir Dis. 1971 Dec;104(6):888–898. doi: 10.1164/arrd.1971.104.6.888. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Dirksen E. R., Zeira M. Microtubule sliding in cilia of the rabbit trachea and oviduct. Cell Motil. 1981;1(2):247–260. doi: 10.1002/cm.970010207. [DOI] [PubMed] [Google Scholar]
- Dulfano M. J., Adler K., Philippoff W. Sputum viscoelasticity in chronic bronchitis. Am Rev Respir Dis. 1971 Jul;104(1):88–98. doi: 10.1164/arrd.1971.104.1.88. [DOI] [PubMed] [Google Scholar]
- Eckert R., Murakami A. Calcium dependence of ciliary activity in the oviduct of the salamander Necturus. J Physiol. 1972 Nov;226(3):699–711. doi: 10.1113/jphysiol.1972.sp010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Bright M. J., Agee C. C., Nickerson J. M., Henderson N. S. Development of an improved tracheal explant bioassay for the detection of the ciliary dyskinesia factor in cystic fibrosis serum. Pediatr Res. 1979 Jan;13(1):31–35. doi: 10.1203/00006450-197901000-00007. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972 Jul;54(1):75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie A. T., Hingley S. T., Kueppers F., Higgins M. L., Tannenbaum C. S., Weinbaum G. Protease production by Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Infect Immun. 1983 May;40(2):506–513. doi: 10.1128/iai.40.2.506-513.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Boese-Marrazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980 Sep;29(3):1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. R., Duckett K. E. The study of ciliary frequencies with an optical spectrum analysis system. Exp Cell Res. 1981 Sep;135(1):147–156. doi: 10.1016/0014-4827(81)90307-4. [DOI] [PubMed] [Google Scholar]
- Klinger J. D., Tandler B., Liedtke C. M., Boat T. F. Proteinases of Pseudomonas aeruginosa evoke mucin release by tracheal epithelium. J Clin Invest. 1984 Nov;74(5):1669–1678. doi: 10.1172/JCI111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Hartman P. E., Hartman Z., Young V. M. A new method of preparation of pyocyanin and demonstration of an unusual bacterial sensitivity. Anal Biochem. 1979 May;95(1):19–23. doi: 10.1016/0003-2697(79)90179-9. [DOI] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Effect of the hemolysin of Pseudomonas aeruginosa on phosphatides and on phospholipase c activity. J Bacteriol. 1967 Feb;93(2):670–674. doi: 10.1128/jb.93.2.670-674.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraya-Cuasay L. R., Cundy K. R., Huang N. N. Pseudomonas carrier rates of patients with cystic fibrosis and of members of their families. J Pediatr. 1976 Jul;89(1):23–26. doi: 10.1016/s0022-3476(76)80920-1. [DOI] [PubMed] [Google Scholar]
- Leisinger T., Margraff R. Secondary metabolites of the fluorescent pseudomonads. Microbiol Rev. 1979 Sep;43(3):422–442. doi: 10.1128/mr.43.3.422-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercke U., Hakansson C. H., Toremalm N. G. The influence of temperature on mucociliary activity. Temperature range 20 degrees C-40 degrees C. Acta Otolaryngol. 1974 Nov-Dec;78(5-6):444–450. doi: 10.3109/00016487409126378. [DOI] [PubMed] [Google Scholar]
- Propst C., Lubin L. Light-mediated changes in pigmentation of Pseudomonas aeruginosa cultures. J Gen Microbiol. 1979 Aug;113(2):261–266. doi: 10.1099/00221287-113-2-261. [DOI] [PubMed] [Google Scholar]
- Puchelle E., Zahm J. M. Influence of rheological properties of human bronchial secretions on the ciliary beat frequency. Biorheology. 1984;21(1-2):265–272. doi: 10.3233/bir-1984-211-228. [DOI] [PubMed] [Google Scholar]
- Reimer A., Klementsson K., Ursing J., Wretlind B. The mucociliary activity of the respiratory tract. I. Inhibitory effects of products of Pseudomonas aeruginosa on rabbit trachea in vitro. Acta Otolaryngol. 1980 Nov-Dec;90(5-6):462–469. doi: 10.3109/00016488009131749. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Classification of microbial, plant and animal cytolysins based on their membrane-damaging effects of human fibroblasts. Biochim Biophys Acta. 1979 Oct 19;557(1):156–169. doi: 10.1016/0005-2736(79)90098-1. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Bryan J., Bush D. J., Fujiwara K., Mooseker M. S., Murphy D. B., Snyder D. H. Microtubules: evidence for 13 protofilaments. J Cell Biol. 1973 Nov;59(2 Pt 1):267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P., Hinds T. R., Vincenzi F. F. Laser light-scattering spectroscopy: preliminary results on bioassay of cystic fibrosis factor(s). Pediatr Res. 1979 Feb;13(2):131–135. doi: 10.1203/00006450-197902000-00009. [DOI] [PubMed] [Google Scholar]
- WELLS I. C. Antibiotic substances produced by Pseudomonas aeruginosa; syntheses of Pyo Ib, Pyo Ic, and Pyo III. J Biol Chem. 1952 May;196(1):331–340. [PubMed] [Google Scholar]
- Wanner A. Clinical aspects of mucociliary transport. Am Rev Respir Dis. 1977 Jul;116(1):73–125. doi: 10.1164/arrd.1977.116.1.73. [DOI] [PubMed] [Google Scholar]
- Wasserman S. J. Ciliary function and disease. J Allergy Clin Immunol. 1984 Jan;73(1 Pt 1):17–19. doi: 10.1016/0091-6749(84)90478-0. [DOI] [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAUGG W. S. SPECTROSCOPIC CHARACTERISTICS AND SOME CHEMICAL PROPERTIES OF N-METHYLPHENAZINIUM METHYL SULFATE (PHENAZINE METHOSULFATE) AND PYOCYANINE AT THE SEMIQUIDNOID OXIDATION LEVEL. J Biol Chem. 1964 Nov;239:3964–3970. [PubMed] [Google Scholar]