Abstract
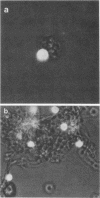
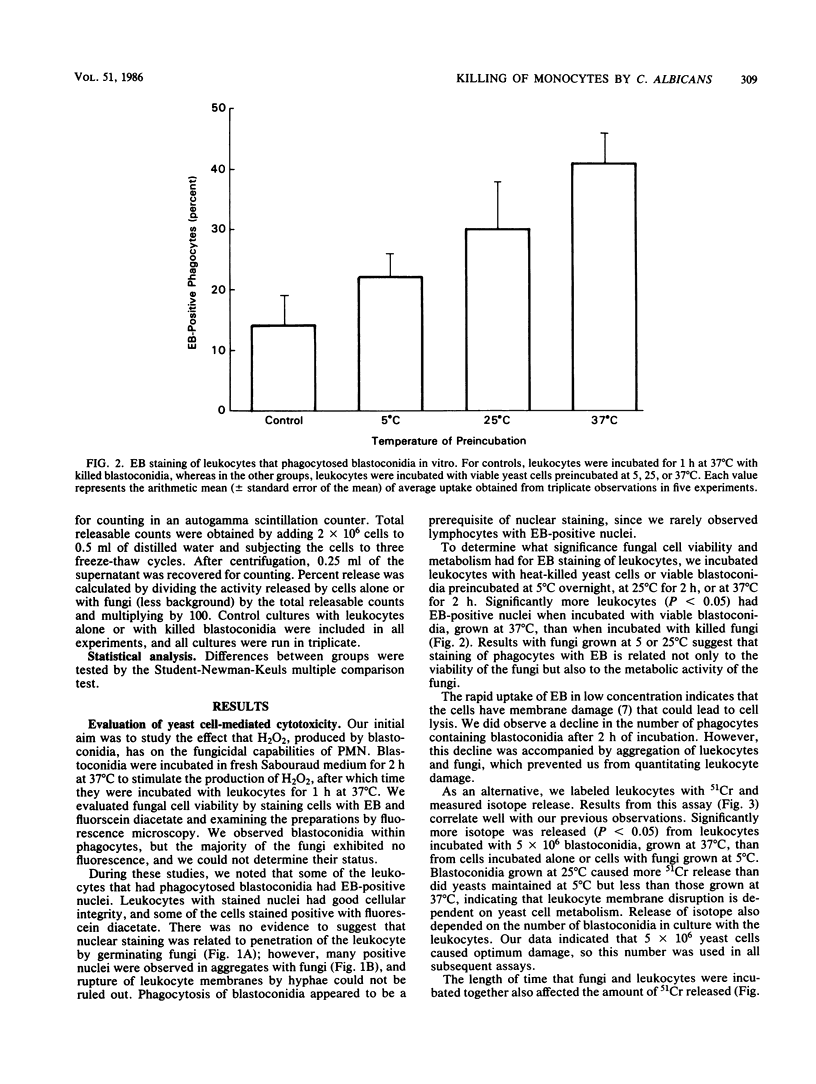
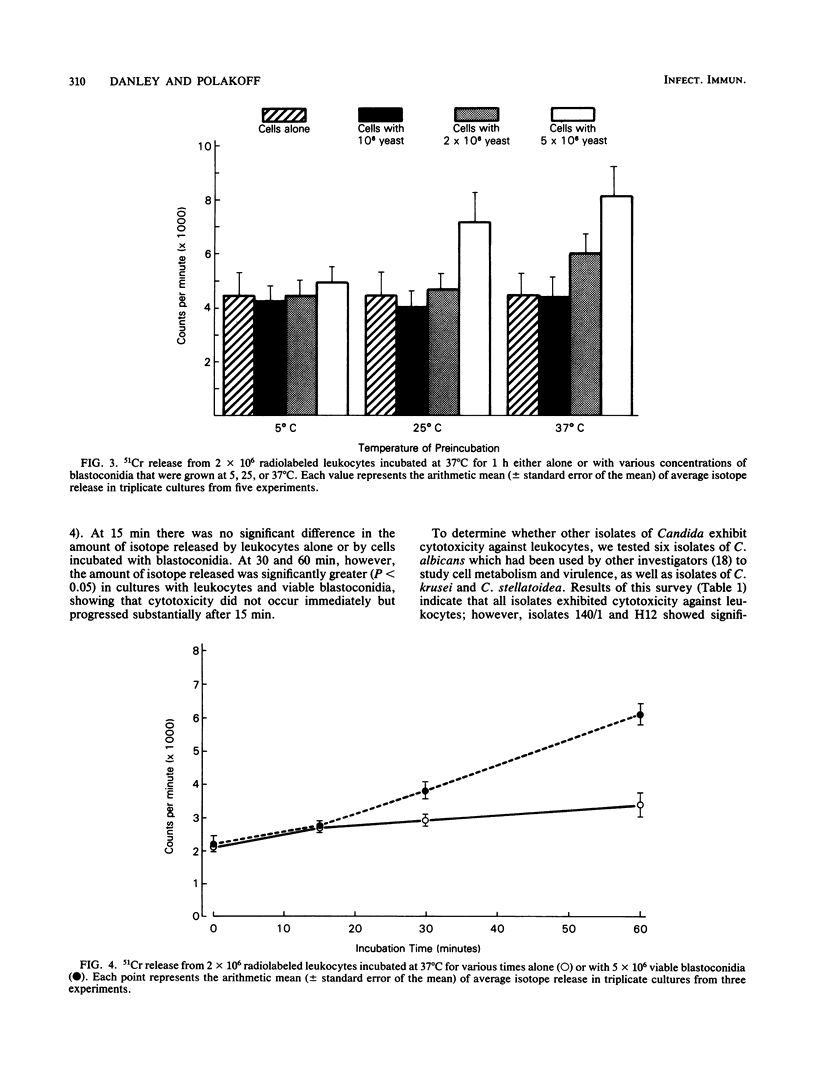
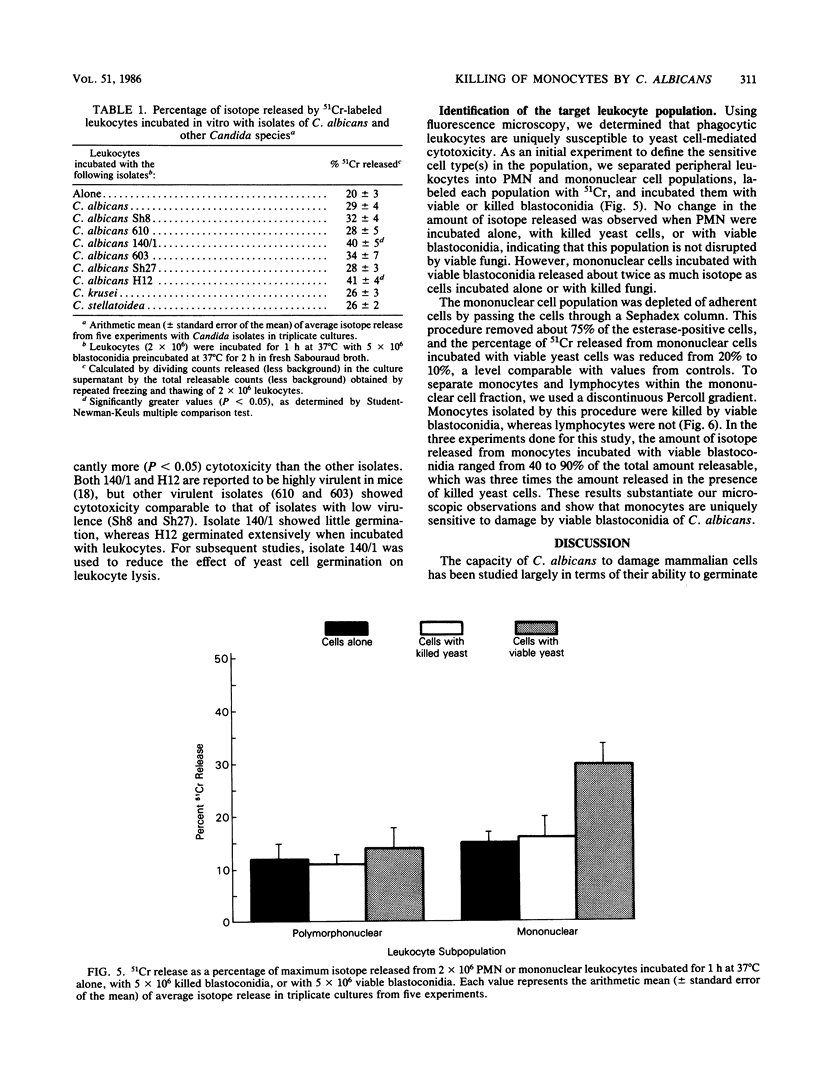
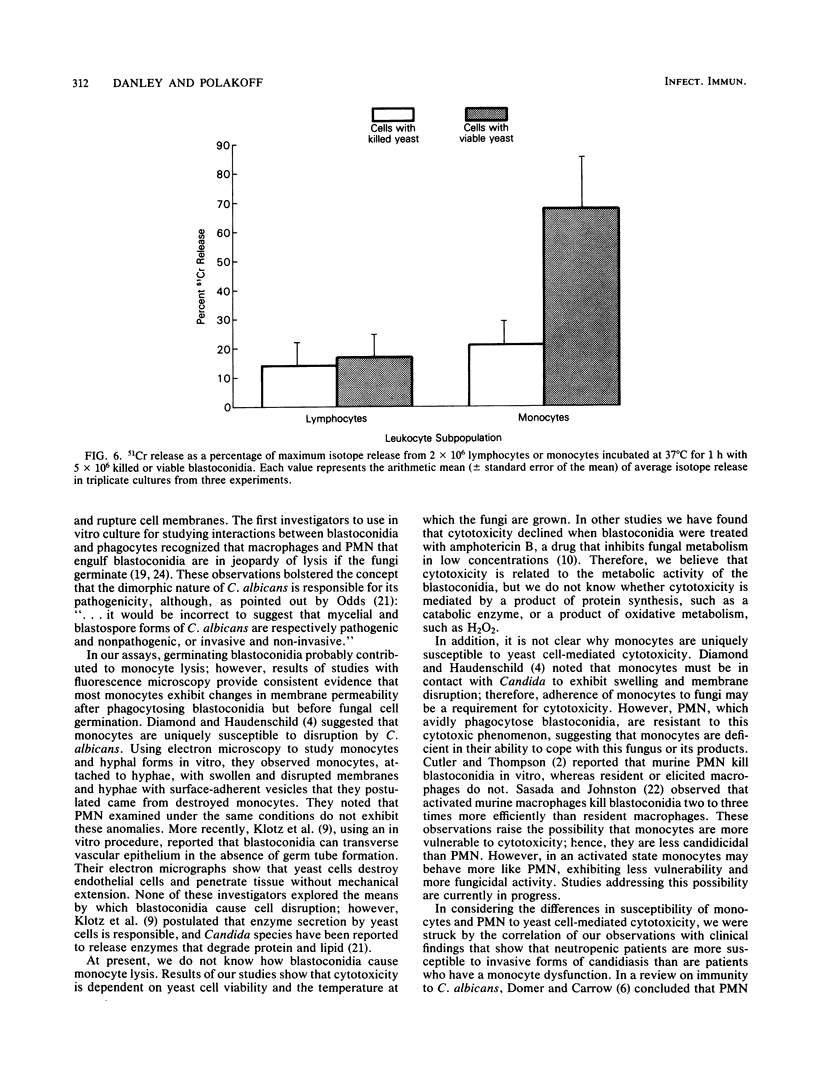
To study the interaction between Candida albicans blastoconidia and human phagocytes, we incubated peripheral leukocytes with fungi for 1 h at 37 degrees C and stained the cells with fluorescent vital stains ethidium bromide (EB) and fluorescein diacetate. Fungi that had been phagocytosed showed little staining; however, some leukocytes containing blastoconidia exhibited nuclear staining with EB, even though their cell membranes showed no signs of penetration by fungi. The number of EB-positive leukocytes was related to viability of the yeast cells and the temperature at which they were maintained before use. Because efforts to quantitate EB-positive leukocytes microscopically were frustrated by cell aggregation, we labeled the leukocytes with 51Cr and measured isotope release. We determined that leukocytes incubated with viable fungi released significantly more isotope than cells incubated alone or with killed blastoconidia. Furthermore, 51Cr release correlated directly with concentration of fungi in the assay, time of incubation, and temperature at which fungi were maintained before use. Using a number of isolates of C. albicans and several other species of Candida, we found that all exhibited cytotoxic activity against leukocytes, but the level of activity varied among organisms. Finally, we depleted or enriched peripheral leukocytes for specific cell populations and determined that only monocytes released more 51Cr after incubation with viable blastoconidia. Blastoconidia can lyse phagocytic cells through germination and penetration of cell membranes within 1 to 2 h, but the cytotoxic phenomenon we describe occurs within 15 to 30 min after yeast cells have been phagocytosed. Therefore, this capacity may represent a more immediate response by blastoconidia against phagocytosis and killing by monocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calich V. L., Purchio A., Paula C. R. A new fluorescent viability test for fungi cells. Mycopathologia. 1979 Feb 28;66(3):175–177. doi: 10.1007/BF00683967. [DOI] [PubMed] [Google Scholar]
- Cutler J. E., Thompson B. D. A simple and inexpensive method for assessing in vitro candidacidal activity of leukocytes. J Immunol Methods. 1984 Jan 20;66(1):27–33. doi: 10.1016/0022-1759(84)90244-8. [DOI] [PubMed] [Google Scholar]
- Danley D. L., Hilger A. E., Winkel C. A. Generation of hydrogen peroxide by Candida albicans and influence on murine polymorphonuclear leukocyte activity. Infect Immun. 1983 Apr;40(1):97–102. doi: 10.1128/iai.40.1.97-102.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C. Monocyte-mediated serum-independent damage to hyphal and pseudohyphal forms of Candida albicans in vitro. J Clin Invest. 1981 Jan;67(1):173–182. doi: 10.1172/JCI110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Carrow E. W. Immunity to fungal infections. Adv Exp Med Biol. 1983;162:383–408. doi: 10.1007/978-1-4684-4481-0_36. [DOI] [PubMed] [Google Scholar]
- Edidin M. A rapid, quantitative fluorescence assay for cell damage by cytotoxic antibodies. J Immunol. 1970 May;104(5):1303–1306. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Harrison J. L., Huppert M. Adherence and penetration of vascular endothelium by Candida yeasts. Infect Immun. 1983 Oct;42(1):374–384. doi: 10.1128/iai.42.1.374-384.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Boggs S., Schlessinger D., Pandey R. C., Rinehart K. L., Jr Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother. 1979 May;15(5):716–722. doi: 10.1128/aac.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforce F. M., Mills D. M., Iverson K., Cousins R., Everett E. D. Inhibition of leukocyte candidacidal activity by serum from patients with disseminated candidiasis. J Lab Clin Med. 1975 Oct;86(4):657–666. [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose D. S., Stevens D. A., Schurman D. J., Feldman D. Distribution of a corticosteroid-binding protein in Candida and other fungal genera. J Gen Microbiol. 1983 Aug;129(8):2379–2385. doi: 10.1099/00221287-129-8-2379. [DOI] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Muller G., Buckley H. R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984 Jun;44(3):576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley V. C., Hurley R. The growth of Candida species in cultures of mouse peritoneal macrophages. J Pathol. 1969 Feb;97(2):357–366. doi: 10.1002/path.1710970222. [DOI] [PubMed] [Google Scholar]
- Ulmer A. J., Flad H. D. Discontinuous density gradient separation of human mononuclear leucocytes using Percoll as gradient medium. J Immunol Methods. 1979;30(1):1–10. doi: 10.1016/0022-1759(79)90268-0. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H., Higgs J. M., Wells R. S., Yamamura M., Hobbs J. R., Holt P. J. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973 Mar;6(3):348–361. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Yasui K., Kawai H., Miyagawa Y., Komiyama A., Akabane T. A monocyte disorder in siblings with chronic candidiasis. A combined abnormality of monocyte mobility and phagocytosis-killing ability. Am J Dis Child. 1984 Feb;138(2):192–196. doi: 10.1001/archpedi.1984.02140400074018. [DOI] [PubMed] [Google Scholar]