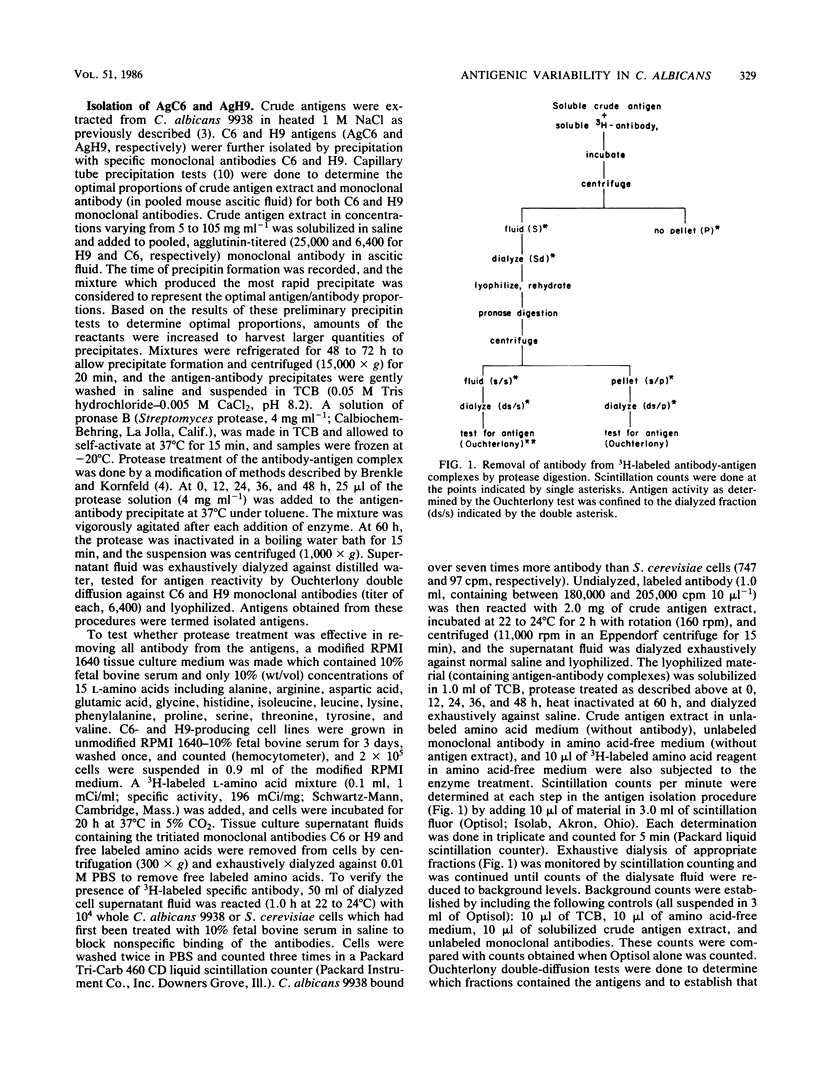
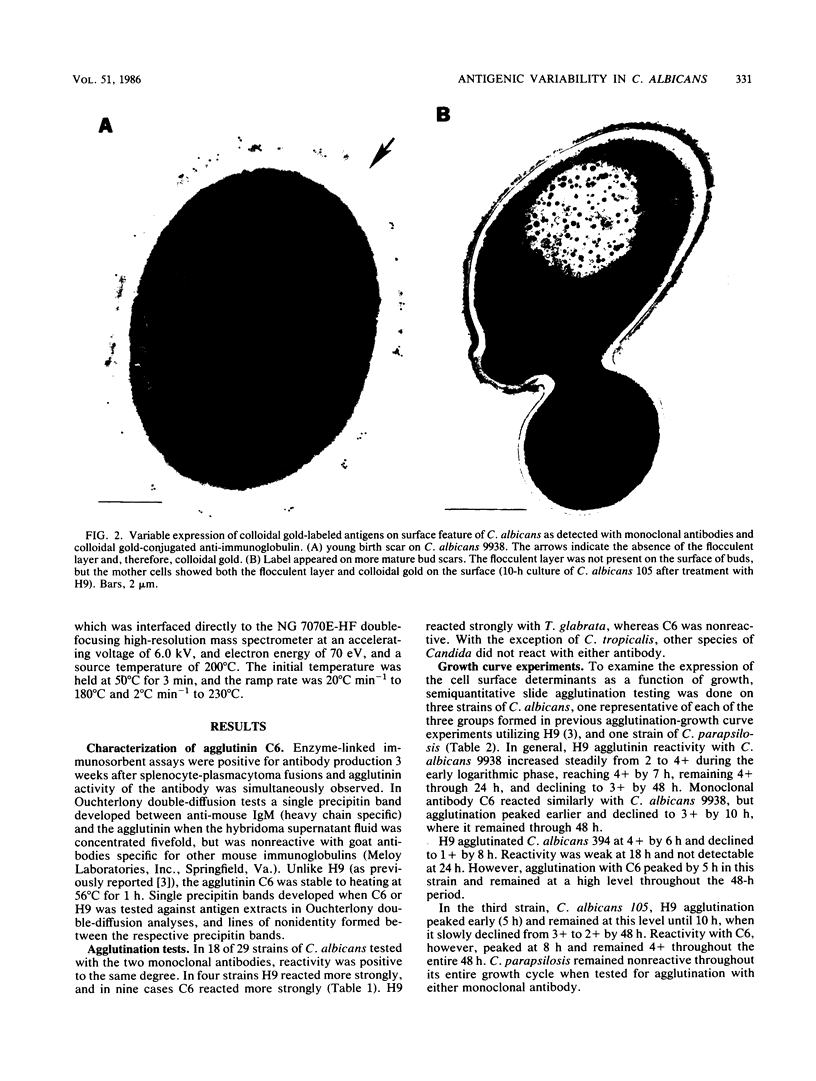
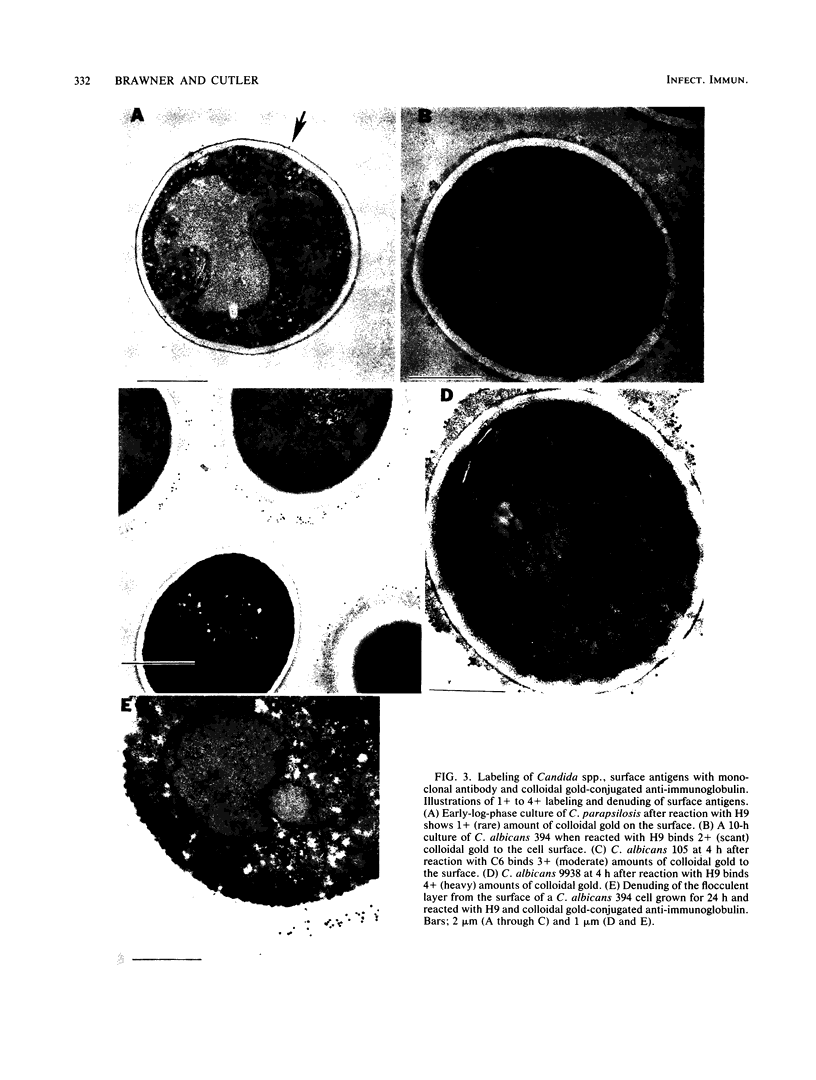
Abstract
Variability in the expression of two different cell surface carbohydrate determinants was examined with two agglutinating immunoglobulin M monoclonal antibodies (H9 and C6) and immunoelectron microscopy during growth of three strains of Candida albicans. A single strain of Candida parapsilosis did not express either antigen at any time during growth. Antigens were detected on the surface of C. albicans by agglutination tests with either H9 or C6 over a 48-h growth period. The difference in specificities of the monoclonal antibodies was demonstrated by Ouchterlony double-diffusion tests with solubilized antigens and by variabilities in the reactivity of the agglutinins among yeast strains. The antigenic determinants were isolated by specific immunoprecipitation and protease digestion and characterized by methods including high-pressure liquid chromatography, gas-liquid chromatography, and mass spectroscopy with both chemical and electron ionization. These determinants both contain mannose and glucose. In the case of antigen H9, an additional carbohydrate was detected with gas chromatography and mass spectroscopy. The location of antigens on individual cells was determined by indirect labeling of the determinants, first reacting cells with H9 or C6 followed by goat anti-mouse antibody conjugated with 20-nm colloidal gold particles. Transmission electron microscopy was used to examine cells. The antigens that were reactive with the monoclonal antibodies were associated with a flocculent surface layer. Expression of this layer and expression of the antigens is a dynamic process which is growth phase and strain dependent. The antigens were not expressed on very young cells and disappeared from the cell surface of most C. albicans strains with age. The use of monoclonal antibody to cell surface determinants may allow characterization of cell surface antigens of C. albicans and be helpful in establishing receptors which mediate adherence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee U., Mohapatra L. N., Kumar R. Role of antibody in defence against murine candidosis. Indian J Med Res. 1984 Jun;79:760–765. [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of a cell surface determinant on Candida albicans as evidenced by an agglutinating monoclonal antibody. Infect Immun. 1984 Mar;43(3):966–972. doi: 10.1128/iai.43.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenckle R., Kornfeld R. Structure of the oligosaccharides of mouse immunoglobulin M secreted by the MOPC 104E plasmacytoma. Arch Biochem Biophys. 1980 Apr 15;201(1):160–173. doi: 10.1016/0003-9861(80)90499-3. [DOI] [PubMed] [Google Scholar]
- Cassone A., Mattia E., Boldrini L. Agglutination of blastospores of Candida albicans by concanavalin A and its relationship with the distribution of mannan polymers and the ultrastructure of the cell wall. J Gen Microbiol. 1978 Apr;105(2):263–273. doi: 10.1099/00221287-105-2-263. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Djaczenko W., Cassone A. Visulization of new ultrastructural components in the cell wall of Candida albicans with fixatives containing TAPO. J Cell Biol. 1972 Jan;52(1):186–190. doi: 10.1083/jcb.52.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hether N. W., Campbell P. A., Baker L. A., Jackson L. L. Chemical composition and biological functions of Listeria monocytogenes cell wall preparations. Infect Immun. 1983 Mar;39(3):1114–1121. doi: 10.1128/iai.39.3.1114-1121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. F., Reiss E. Detection of Candida albicans mannan by immunodiffusion, counterimmunoelectrophoresis, and enzyme-linked immunoassay. Mycopathologia. 1980 Mar 17;70(2):83–88. doi: 10.1007/BF00443072. [DOI] [PubMed] [Google Scholar]
- Lehrer N., Segal E., Barr-Nea L. In vitro and in vivo adherence of Candida albicans to mucosal surfaces. Ann Microbiol (Paris) 1983 Sep-Oct;134B(2):293–306. doi: 10.1016/s0769-2609(83)80042-8. [DOI] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Role of surface mannan in the adherence of Candida albicans to fibrin-platelet clots formed in vitro. Infect Immun. 1981 Apr;32(1):92–97. doi: 10.1128/iai.32.1.92-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981 Jun;32(3):1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad S., Friedman L. Passive immunization of mice against Candida albicans. Sabouraudia. 1968 Feb;6(2):103–105. [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Dubremetz J. F., Biguet J. Ultrastructure of the cell wall of Candida albicans blastospores: study of its constitutive layers by the use of a cytochemical technique revealing polysaccharides. Ann Microbiol (Paris) 1978 Feb-Mar;129(2):141–153. [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Lefebvre B., Husson M. O. Antigenic variability between Candida albicans blastospores isolated from healthy subjects and patients with Candida infection. Sabouraudia. 1982 Sep;20(3):173–177. [PubMed] [Google Scholar]
- Rinaldi M. G. Use of potato flakes agar in clinical mycology. J Clin Microbiol. 1982 Jun;15(6):1159–1160. doi: 10.1128/jcm.15.6.1159-1160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake L. P., MacFarlane T. W. The effect of dietary carbohydrates on the in-vitro adhesion of Candida albicans to epithelial cells. J Med Microbiol. 1982 Nov;15(4):511–517. doi: 10.1099/00222615-15-4-511. [DOI] [PubMed] [Google Scholar]
- Segal E., Soroka A., Schechter A. Correlative relationship between adherence of Candida albicans to human vaginal epithelial cells in vitro and candidal vaginitis. Sabouraudia. 1984;22(3):191–200. [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Segal E., Sreevalsan T., Scheld W. M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984 Feb;30(2):221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- Smail E. H., Jones J. M. Demonstration and solubilization of antigens expressed primarily on the surfaces of Candida albicans germ tubes. Infect Immun. 1984 Jul;45(1):74–81. doi: 10.1128/iai.45.1.74-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sundstrom P. M., Kenny G. E. Characterization of antigens specific to the surface of germ tubes of Candida albicans by immunofluorescence. Infect Immun. 1984 Mar;43(3):850–855. doi: 10.1128/iai.43.3.850-855.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Poulain D., Vernes A. Cytochemical and ultrastructural studies of Candida albicans. III. Evidence for modifications of the cell wall coat during adherence to human buccal epithelial cells. Arch Microbiol. 1984 Oct;139(2-3):221–224. doi: 10.1007/BF00402004. [DOI] [PubMed] [Google Scholar]
- de Repentigny L., Kuykendall R. J., Chandler F. W., Broderson J. R., Reiss E. Comparison of serum mannan, arabinitol, and mannose in experimental disseminated candidiasis. J Clin Microbiol. 1984 Jun;19(6):804–812. doi: 10.1128/jcm.19.6.804-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]