Abstract
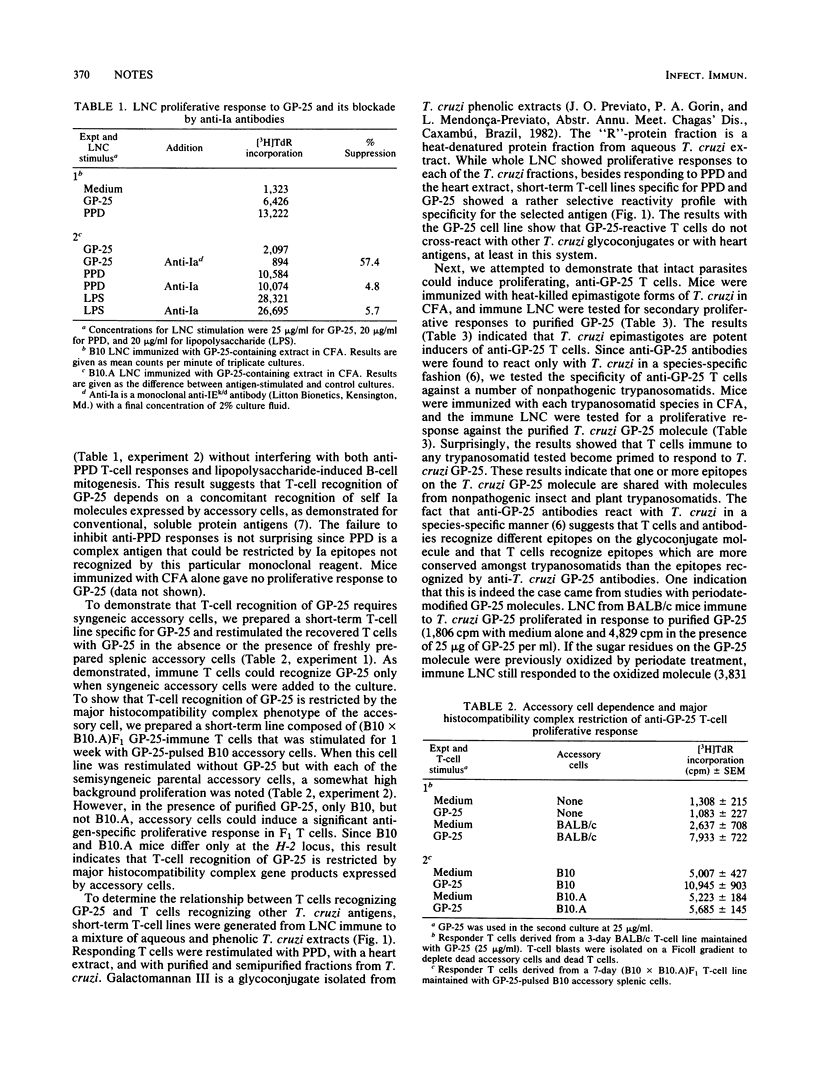
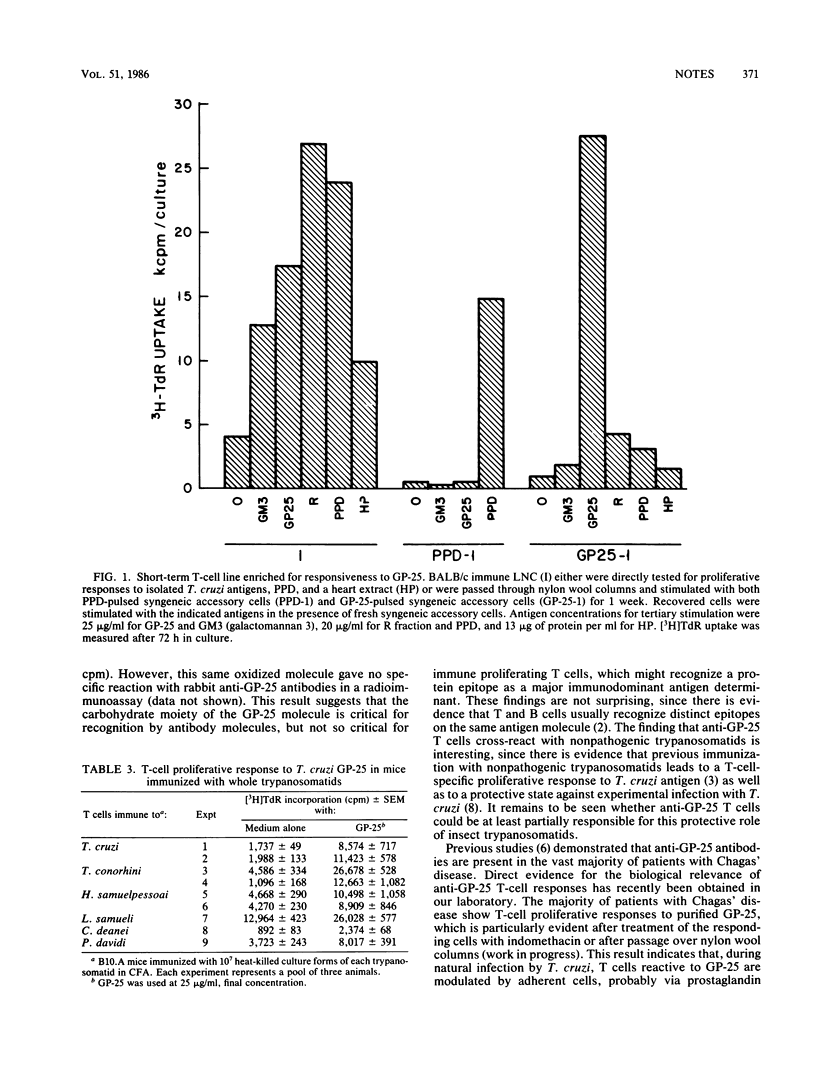
A glycoconjugate (GP-25) was previously purified from Trypanosoma cruzi and shown to be a major immunogenic constituent of the parasite cell surface, capable of inducing specific humoral responses in the vast majority of patients with Chagas' disease. In the present study, the T-cell proliferative response to GP-25 was studied in mice immunized with T. cruzi fractions or whole parasites. Recognition of GP-25 by proliferating T cells requires the participation of syngeneic, accessory spleen cells and is specifically blocked by anti-la antibodies. Furthermore, recognition of GP-25 is influenced by the MHC haplotype of accessory antigen-presenting cells. Short-term, GP-25-specific T-cell lines were used to demonstrate the specificity of anti-GP-25 T cells and to show that this glycoconjugate is not involved in T-cell cross-reactivity with heart antigens. T cells primed with nonpathogenic trypanosomatids are able to recognize the purified T. cruzi GP-25 molecule, indicating that T cells recognize a GP-25 epitope which is shared among trypanosomatids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Endres R. O., Grey H. M. Antigen recognition by T cells and B cells: recognition of cross-reactivity between native and denatured forms of globular antigens. Clin Immunol Immunopathol. 1980 Mar;15(3):397–408. doi: 10.1016/0090-1229(80)90051-3. [DOI] [PubMed] [Google Scholar]
- Fucs R., Barcinski M. A. Dependence on macrophages of the guinea pig T-cell immune response to Herpetomonas samuelpessoai. J Parasitol. 1981 Aug;67(4):463–467. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Mendonça-Previato L., Gorin P. A., Braga A. F., Scharfstein J., Previato J. O. Chemical structure and antigenic aspects of complexes obtained from epimastigotes of Trypanosoma cruzi. Biochemistry. 1983 Oct 11;22(21):4980–4987. doi: 10.1021/bi00290a016. [DOI] [PubMed] [Google Scholar]
- Scharfstein J., Rodrigues M. M., Alves C. A., de Souza W., Previato J. O., Mendonça-Previato L. Trypanosoma cruzi: description of a highly purified surface antigen defined by human antibodies. J Immunol. 1983 Aug;131(2):972–976. [PubMed] [Google Scholar]
- Schwartz R. H., Yano A., Paul W. E. Interaction between antigen-presenting cells and primed T lymphocytes: an assessment of Ir gene expression in the antigen-presenting cell. Immunol Rev. 1978;40:153–180. doi: 10.1111/j.1600-065x.1978.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Souza M. do C., Reis A. P., Da Silva W. D., Brener Z. Mechanism of acquired immunity induced by "Leptomonas pessoai" against Trypanosoma cruzi in mice. J Protozool. 1974 Oct;21(4):579–584. doi: 10.1111/j.1550-7408.1974.tb03705.x. [DOI] [PubMed] [Google Scholar]


