Abstract
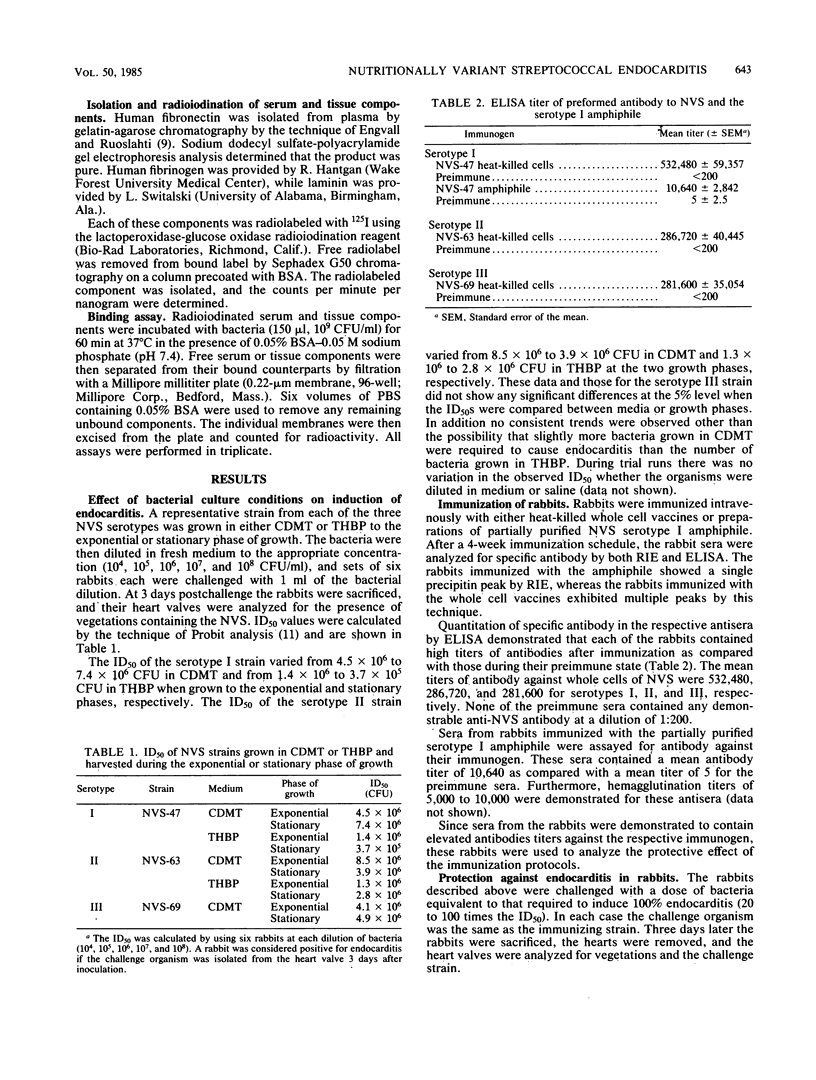
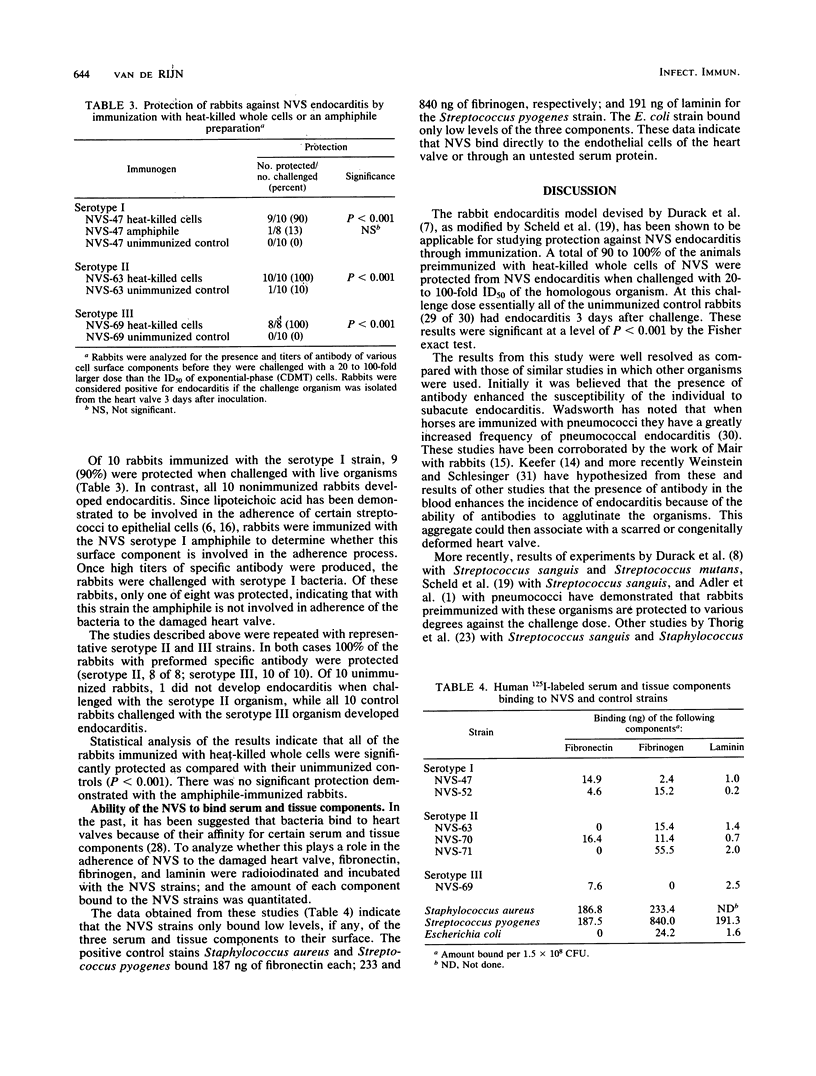
The nutritionally variant streptococci (NVS) are usually isolated from patients with NVS endocarditis and recently have been serotyped into three groups. In the past, studies on microbial endocarditis have not analyzed the effect of the growth medium or growth phase on the bacteria used to induce the disease in the experimental rabbit model. Therefore, in this study various bacterial growth parameters were examined, including growth in semisynthetic or complex medium to the exponential or stationary phase of growth. The 50% infective dose ranged from 3.7 X 10(5) to 8.5 X 10(6) CFU for representative strains from each of the three serotypes grown under these conditions, indicating that there was no significant difference. The role of immunization was also examined in this model using organisms grown to the exponential phase in semisynthetic medium. Rabbits were immunized with heat-killed whole cells, high titres of specific antibody were produced as demonstrated by enzyme-linked immunosorbent assay, and then the rabbits were challenged with 20- to 100-fold 50% infective dose of the homologous strain. A total of 90 to 100% of the rabbits were protected from the disease process, as shown by the absence of the organisms from the heart valve 3 days after the challenge. Rabbits immunized with the amphiphile that replaces lipoteichoic acid in these organisms were not protected from challenge, demonstrating that another surface component is responsible for adherence or colonization or both. Finally NVS were incubated with radioiodinated fibronectin, fibrinogen, or laminin to determine whether these molecules aided in the adherence of the organisms to the heart valve. Only minor amounts of these components were bound to NVS as compared with controls. Therefore, NVS bind directly to the damaged heart valve or through an unknown mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. W., 2nd, Selinger D. S., Reed W. P. Effect of immunization on the genesis of pneumococcal endocarditis in rabbits. Infect Immun. 1981 Oct;34(1):55–61. doi: 10.1128/iai.34.1.55-61.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Effect of type-specific active immunization on the development and progression of experimental Pseudomonas aeruginosa endocarditis. Infect Immun. 1979 Apr;24(1):167–173. doi: 10.1128/iai.24.1.167-173.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet A., van de Rijn I., McCarty M. Nutritionally variant streptococci from patients with endocarditis: growth parameters in a semisynthetic medium and demonstration of a chromophore. J Bacteriol. 1981 Jun;146(3):1075–1082. doi: 10.1128/jb.146.3.1075-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox F. Prevention of group B streptococcal colonization with topically applied lipoteichoic acid in a maternal-newborn mouse model. Pediatr Res. 1982 Oct;16(10):816–819. doi: 10.1203/00006450-198210000-00003. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Gilliland B. C., Petersdorf R. G. Effect of immunization on susceptibility to experimental Streptococcus mutans and Streptococcus sanguis endocarditis. Infect Immun. 1978 Oct;22(1):52–56. doi: 10.1128/iai.22.1.52-56.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- FRENKEL A., HIRSCH W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature. 1961 Aug 12;191:728–730. doi: 10.1038/191728a0. [DOI] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Immunogenicity of a purified and carrier-complexed streptococcal lipoteichoic acid. Infect Immun. 1976 Jun;13(6):1585–1590. doi: 10.1128/iai.13.6.1585-1590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison P. K., Freedman L. R. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970 Jun;42(6):394–410. [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J Clin Invest. 1978 Oct;62(4):805–814. doi: 10.1172/JCI109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Thomas J. H., Sande M. A. Influence of preformed antibody on experimental Streptococcus sanguis endocarditis. Infect Immun. 1979 Sep;25(3):781–785. doi: 10.1128/iai.25.3.781-785.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thörig L., Thompson J., van Furth R. Effects of immunization and anticoagulation on the development of experimental Escherichia coli endocarditis. Infect Immun. 1980 May;28(2):325–330. doi: 10.1128/iai.28.2.325-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Weinstein L., Schlesinger J. J. Pathoanatomic, pathophysiologic and clinical correlations in endocarditis (first of two parts). N Engl J Med. 1974 Oct 17;291(16):832–837. doi: 10.1056/NEJM197410172911609. [DOI] [PubMed] [Google Scholar]
- van de Rijn I., Bouvet A. Characterization of a pH-dependent chromophore from nutritionally variant streptococci. Infect Immun. 1984 Jan;43(1):28–31. doi: 10.1128/iai.43.1.28-31.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., George M. Immunochemical study of nutritionally variant streptococci. J Immunol. 1984 Oct;133(4):2220–2225. [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I. Quantitative analysis of cell walls of nutritionally variant streptococci grown under various growth conditions. Infect Immun. 1985 Sep;49(3):518–522. doi: 10.1128/iai.49.3.518-522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


