Abstract
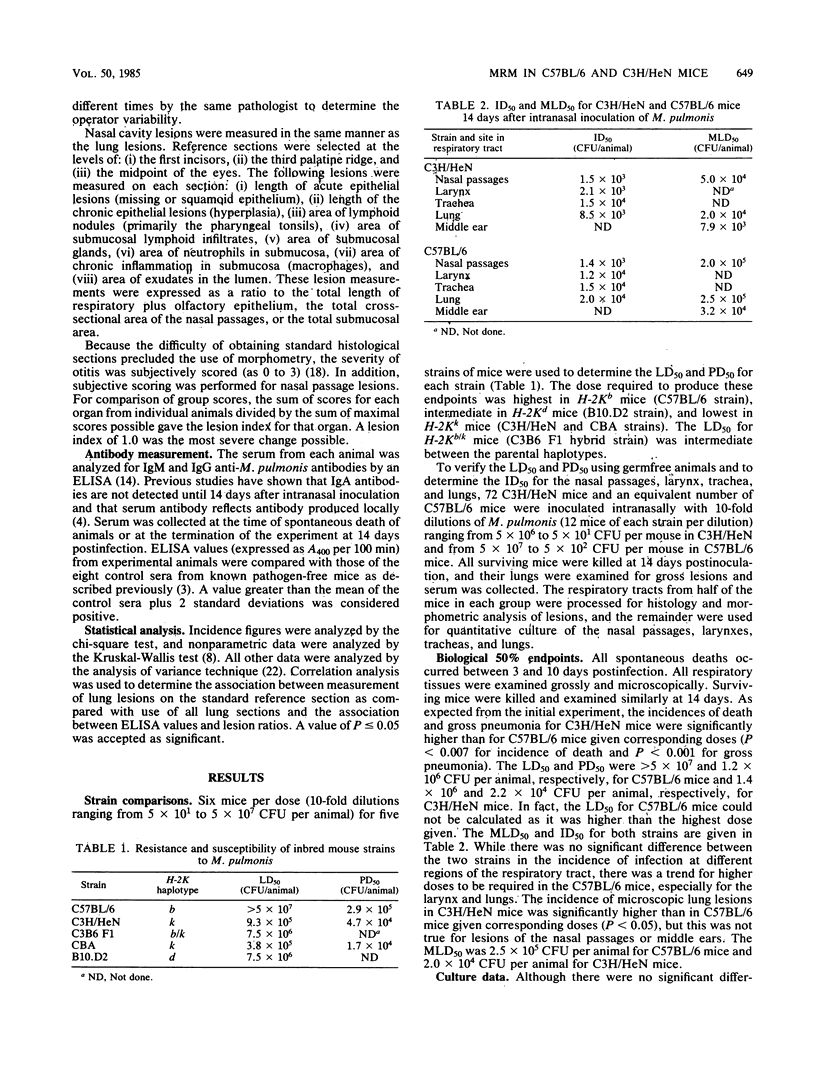
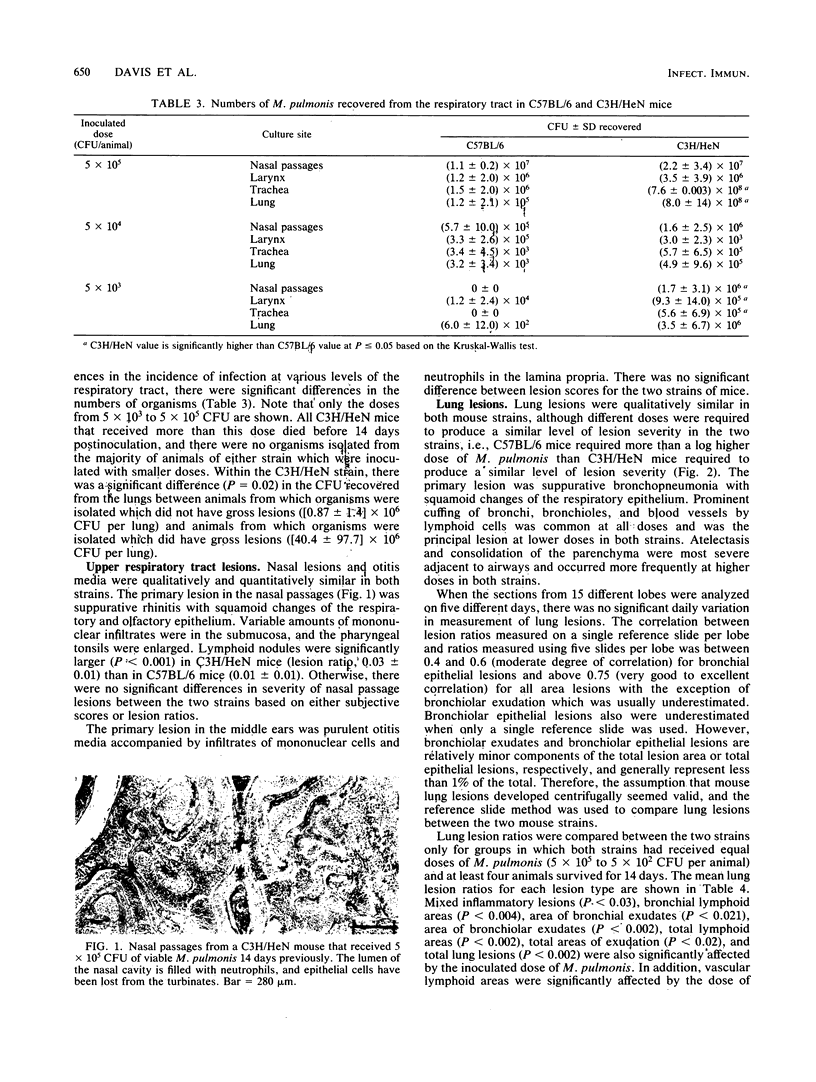
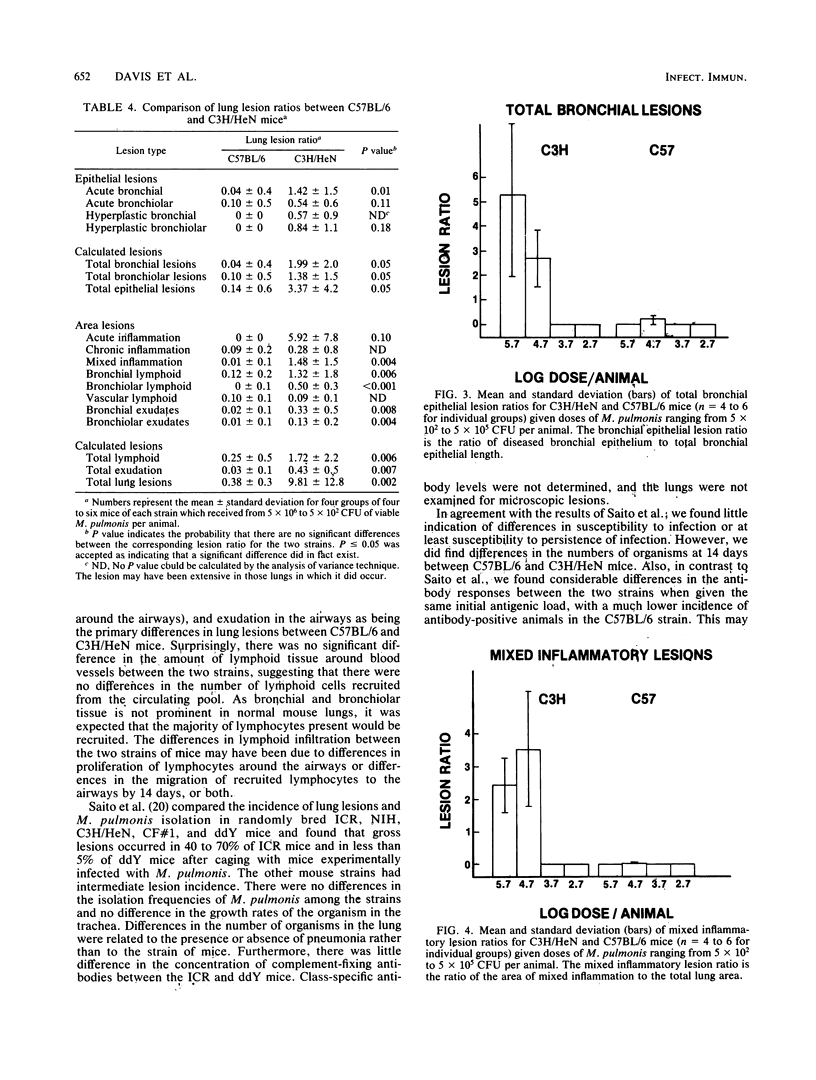
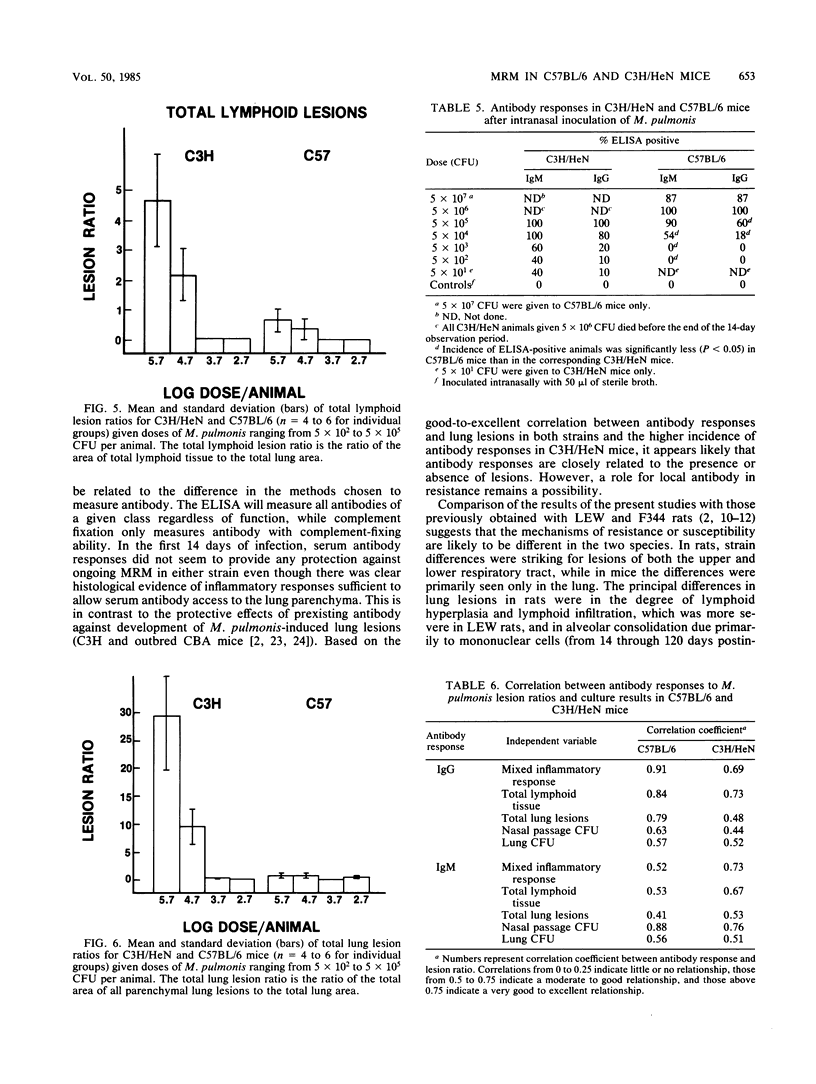
Not only is murine respiratory mycoplasmosis, due to Mycoplasma pulmonis, a complication of biomedical research, it provides excellent animal models to study the development of a naturally occurring respiratory disease induced by an infectious agent. The understanding of pathogenic mechanisms of disease can be greatly facilitated by studying genetic differences in susceptibility. Five strains of mice with various H-2K haplotypes were examined for their susceptibility to murine respiratory mycoplasmosis; of these, C57BL/6 and C3H/HeN mice were chosen for additional study. There were no significant differences in the incidence of infection in either the upper or lower respiratory tract or in the severity of upper respiratory tract lesions in the two strains as determined at 14 days postinfection. In striking contrast, the C57BL/6 mice were significantly more resistant to the development of gross and microscopic lung lesions and to death due to pneumonia as shown by an almost 100-fold difference in the 50% lethal dose, 50% gross pneumonia dose, and 50% microscopic lesion dose. The most apparent differences in lung lesions between the two strains were in the severity of acute lesions of the bronchial epithelium, the amount of mixed inflammatory response in the alveoli, and the amount of lymphoid infiltrates. All were significantly more severe in C3H/HeN mice. In addition, more C3H/HeN mice developed antibody responses to M. pulmonis. The amount of antibody correlated with lesion severity in both strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broderson J. R., Lindsey J. R., Crawford J. E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976 Oct;85(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H. Derrick Edward Award Lecture. The pathogenic potential of mycoplasmas: Mycoplasma pulmonis as a model. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S18–S34. doi: 10.1093/clinids/4.supplement_1.s18. [DOI] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Baker H. J. Immune response of pathogen-free mice inoculated intranasally with Mycoplasma pulmonis. J Immunol. 1974 Jan;112(1):124–136. [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K., Davidson M. K., Brown M. B., Mayo J. G. Detection of natural Mycoplasma pulmonis infection in rats and mice by an enzyme linked immunosorbent assay (ELISA). Lab Anim Sci. 1981 Dec;31(6):676–682. [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N., Hassell L. A., Washburn L. R., Ward J. R. Toxicity but not arthritogenicity of Mycoplasma arthritidis for mice associates with the haplotype expressed at the major histocompatibility complex. Infect Immun. 1983 Sep;41(3):1010–1015. doi: 10.1128/iai.41.3.1010-1015.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L. S., Duthie J. J., Sugar M. Focal Infection in Rheumatoid Arthritis: A Comparison of the Incidence of Foci of Infections in the Upper Respiratory Tract in One Hundred Cases of Rheumatoid Arthritis and One Hundred Controls. Ann Rheum Dis. 1949 Sep;8(3):205–208. doi: 10.1136/ard.8.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Cassell G. H. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol. 1982 May;19(3):280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Delozier K. M., Asa D. K., Minion F. C., Cassell G. H. Interactions between murine alveolar macrophages and Mycoplasma pulmonis in vitro. Infect Immun. 1980 Aug;29(2):590–599. doi: 10.1128/iai.29.2.590-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice R. A., Robillard N. F., Carski T. R. Immunofluorescence identification of Mycoplasma on agar by use of incident illumination. J Bacteriol. 1967 Apr;93(4):1205–1209. doi: 10.1128/jb.93.4.1205-1209.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Stott E. J., Taylor G. The effect of pneumonia induced in mice with Mycoplasma pulmonis on resistance to subsequent bacterial infection and the effect of a respiratory infection with Sendai virus on the resistance of mice to Mycoplasma pulmonis. J Gen Microbiol. 1978 Nov;109(1):79–87. doi: 10.1099/00221287-109-1-79. [DOI] [PubMed] [Google Scholar]
- Korotzer T. I., Weiss H. S., Hamparian V. V., Somerson N. L. Oxygen uptake and lung function in mice infected with Streptococcus pneumoniae, influenza virus, or Mycoplasma pulmonis. J Lab Clin Med. 1978 Feb;91(2):280–294. [PubMed] [Google Scholar]
- Lindsey J. R., Cassell H. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Models for studying mycoplasmosis of the respiratory tract. Am J Pathol. 1973 Jul;72(1):63–90. [PMC free article] [PubMed] [Google Scholar]
- Saito M., Nakagawa M., Muto T., Imaizumi K. Strain difference of mouse in susceptibility to Mycoplasma pulmonis infection. Nihon Juigaku Zasshi. 1978 Dec;40(6):697–705. doi: 10.1292/jvms1939.40.697. [DOI] [PubMed] [Google Scholar]
- Schreiber H., Nettesheim P., Lijinsky W., Richter C. B., Walburg H. E., Jr Induction of lung cancer in germfree, specific-pathogen-free, and infected rats by N-nitrosoheptamethyleneimine: enhancement by respiratory infection. J Natl Cancer Inst. 1972 Oct;49(4):1107–1114. [PubMed] [Google Scholar]
- Taylor G., Howard C. J. Protection of mice against Mycoplasma pulmonis infection using purified mouse immunoglobulins: comparison between protective effect and biological properties of immunoglobulin classes. Immunology. 1981 Jul;43(3):519–525. [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Taylor-Robinson D. Effects of active and passive immunization on Mycoplasma pulmonis-induced pneumonia in mice. Immunology. 1976 May;30(5):611–618. [PMC free article] [PubMed] [Google Scholar]
- Wells A. B. The kinetics of cell proliferation in the tracheobronchial epithelia of rats with and without chronic respiratory disease. Cell Tissue Kinet. 1970 Apr;3(2):185–206. doi: 10.1111/j.1365-2184.1970.tb00265.x. [DOI] [PubMed] [Google Scholar]




