Abstract
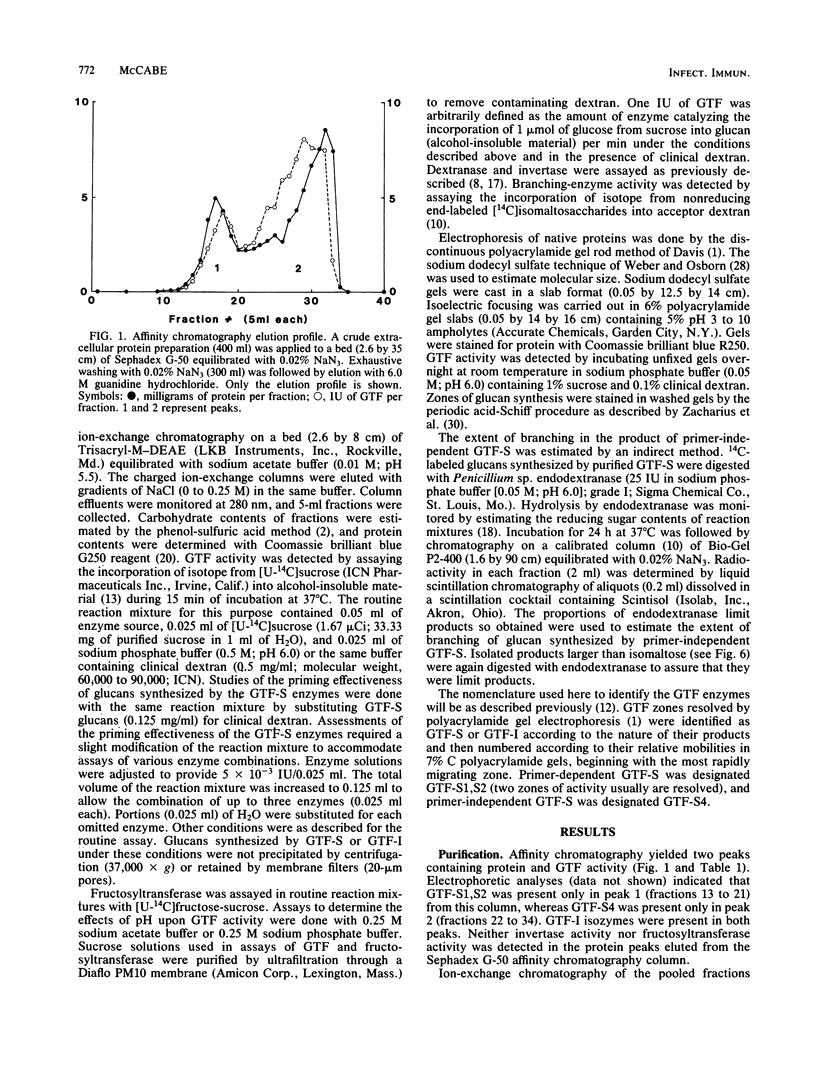
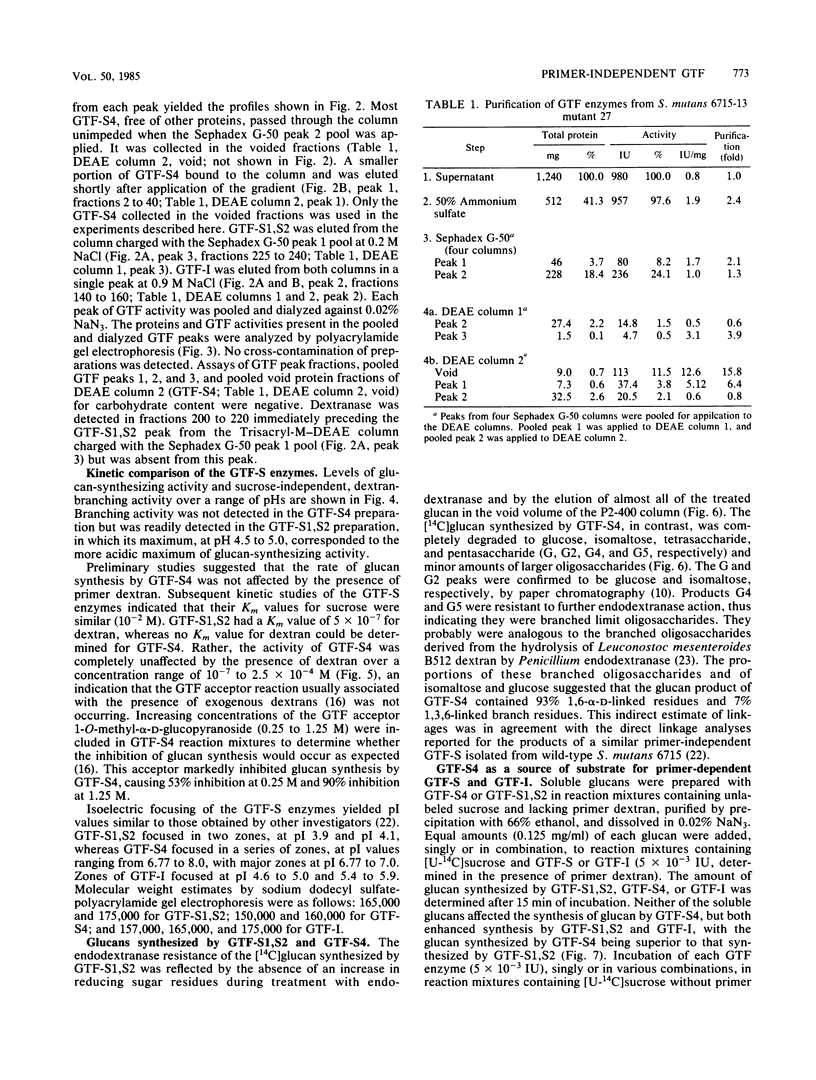
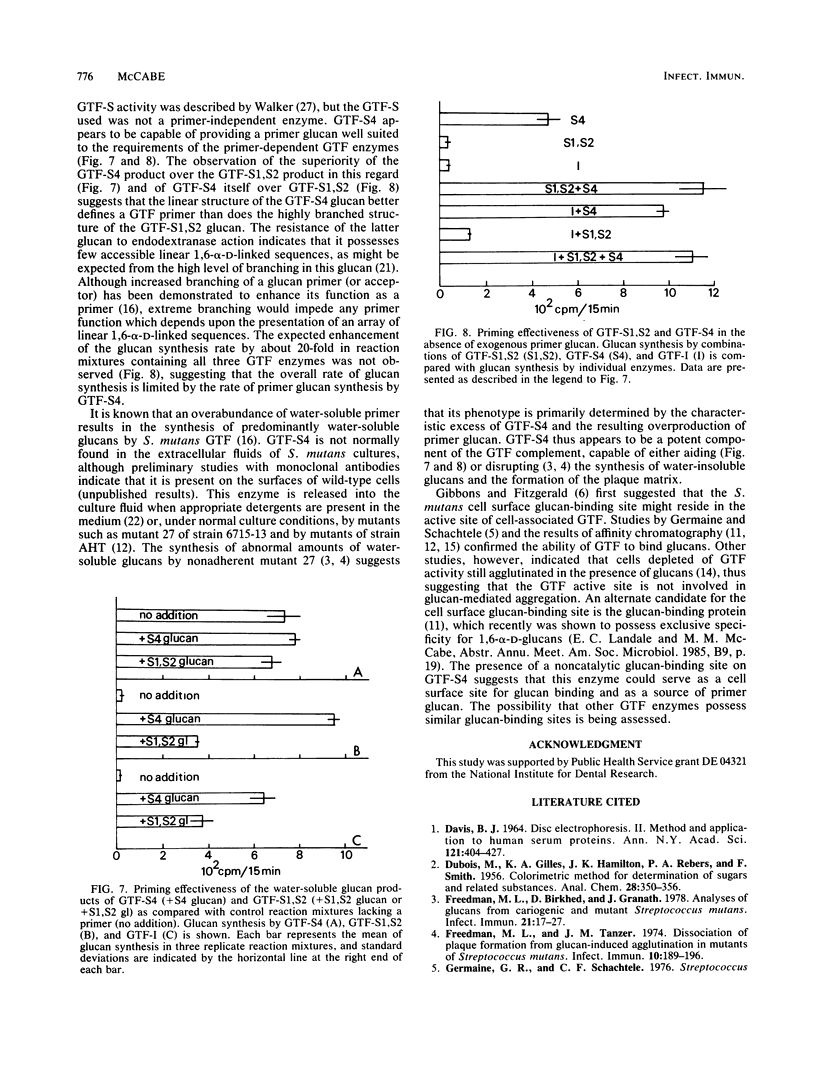
Affinity chromatography on Sephadex G-50 and subsequent ion-exchange chromatography on Trisacryl-M-DEAE were used to purify the glucosyltransferase (GTF) enzymes produced by mutant 27 of Streptococcus mutans 6715-13. Complete separation of three types of GTF, including a primer-independent GTF capable of synthesizing a slightly branched, water-soluble glucan (GTF-S), was obtained. The characteristics of this primer-independent GTF-S were compared with those of the normally occurring primer-dependent GTF-S. The Km for sucrose was easily obtained for each enzyme (10(-2) M), but the Km for dextran could only be determined for the primer-dependent GTF-S (5 X 10(-7) M for clinical dextran of molecular weight 60,000 to 90,000). The primer-independent GTF-S did not respond catalytically to the presence of either clinical dextran or the highly branched, water-soluble glucan produced by primer-dependent GTF-S, although it was capable of binding these polysaccharides at a noncatalytic site and of responding to the low-molecular-weight acceptor 1-O-methyl-alpha-D-glucopyranoside. The water-soluble glucan product of primer-independent GTF-S was a superior priming glucan for primer-dependent GTF enzymes as compared with the glucan product of primer-dependent GTF-S. The presence of primer-independent GTF-S in reaction mixtures stimulated glucan synthesis by primer-dependent GTF-S and by GTF synthesizing water-insoluble glucan by at least 10-fold, whereas the presence of similar amounts of primer-dependent GTF-S had no effect on synthesis by GTF synthesizing water-insoluble glucan. Primer-independent GTF-S appears to be a potent source of priming glucan for the primer-dependent GTF enzymes. Its possession of a noncatalytic binding site for glucan, the first observed for the GTF of S. mutans, suggests that it may also serve as a glucan receptor on the S. mutans cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Birked D., Granath K. Analyses of glucans from cariogenic and mutant Streptococcus mutans. Infect Immun. 1978 Jul;21(1):17–27. doi: 10.1128/iai.21.1.17-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelik R. M., McCabe M. M. An endodextranase inhibitor from batch cultures of streptococcus mutans. Biochem Biophys Res Commun. 1982 Jun 15;106(3):875–880. doi: 10.1016/0006-291x(82)91792-2. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M. An enzyme from Streptococcus mutans forms branches on dextran in the absence of sucrose. Biochem Biophys Res Commun. 1983 Aug 30;115(1):287–294. doi: 10.1016/0006-291x(83)91002-1. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E., Cowman R. A. Invertase activity in Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1973 Apr;18(4):525–531. doi: 10.1016/0003-9969(73)90073-3. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Relationship between cell-bound dextransucrase and the agglutination of Streptococcus mutans. Infect Immun. 1975 Sep;12(3):512–520. doi: 10.1128/iai.12.3.512-520.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Specific method for the purification of Streptococcus mutans dextransucrase. Infect Immun. 1977 Jun;16(3):760–765. doi: 10.1128/iai.16.3.760-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. The dextran acceptor reaction of dextransucrase from Streptococcus mutans K1-R. Carbohydr Res. 1978 Jun;63:223–239. doi: 10.1016/s0008-6215(00)80946-5. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Purification and properties of Streptococcus mutans extracellular glucosyltransferase. Biochim Biophys Acta. 1982 Mar 18;702(1):72–80. doi: 10.1016/0167-4838(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Three kinds of extracellular glucosyltransferases from Streptococcus mutans 6715 (serotype g). FEBS Lett. 1983 Jun 27;157(1):79–84. doi: 10.1016/0014-5793(83)81120-x. [DOI] [PubMed] [Google Scholar]
- Sidebotham R. L. Dextrans. Adv Carbohydr Chem Biochem. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- Takeo K., Kabat E. A. Binding constants of dextrans and isomaltose oligosaccharides to dextran-specific myeloma proteins determined by affinity electrophoresis. J Immunol. 1978 Dec;121(6):2305–2310. [PubMed] [Google Scholar]
- Tsumori H., Shimamura A., Mukasa H. Comparative study of Streptococcus mutans extracellular glycosyltransferases by isoelectric focusing. J Gen Microbiol. 1983 Oct;129(10):3261–3269. doi: 10.1099/00221287-129-10-3261. [DOI] [PubMed] [Google Scholar]
- Tsumori H., Shimamura A., Mukasa H. Purification and properties of extracellular glucosyltransferases from Streptococcus mutans serotype a. J Gen Microbiol. 1983 Oct;129(10):3251–3259. doi: 10.1099/00221287-129-10-3251. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wittenberger C. L., Beaman A. J., Lee L. N. Tween 80 effect on glucosyltransferase synthesis by Streptococcus salivarius. J Bacteriol. 1978 Jan;133(1):231–239. doi: 10.1128/jb.133.1.231-239.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]



