Abstract
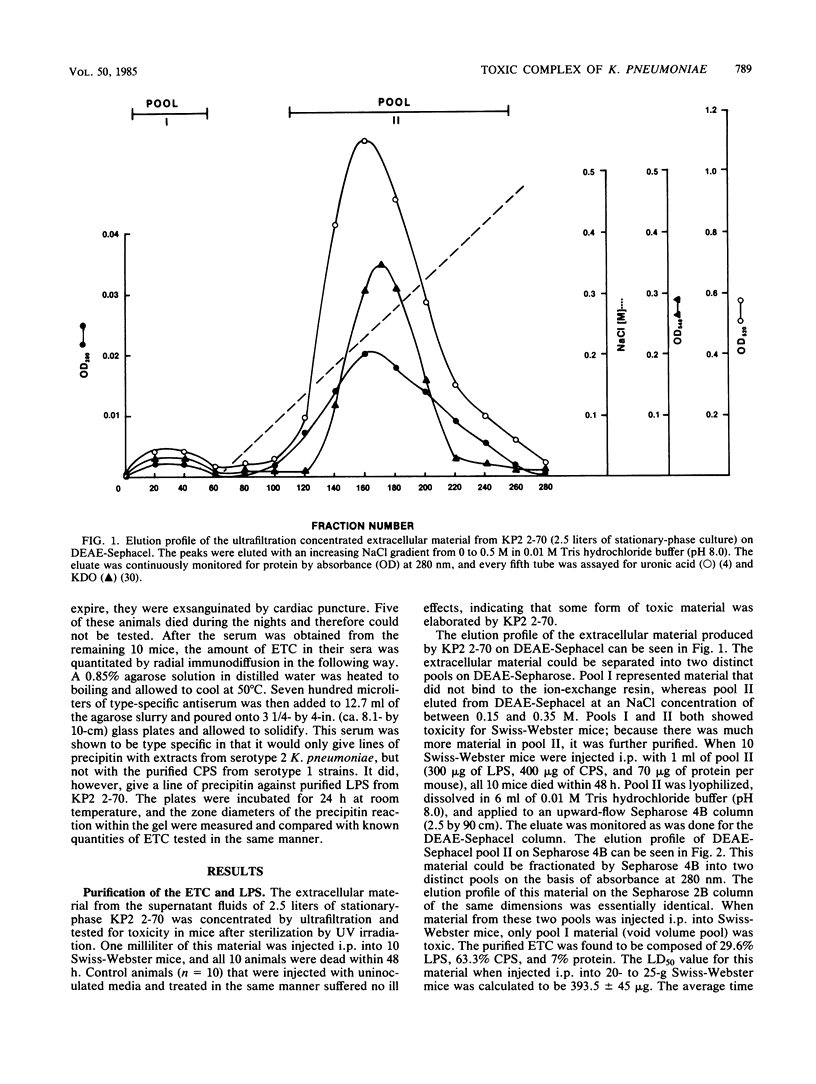
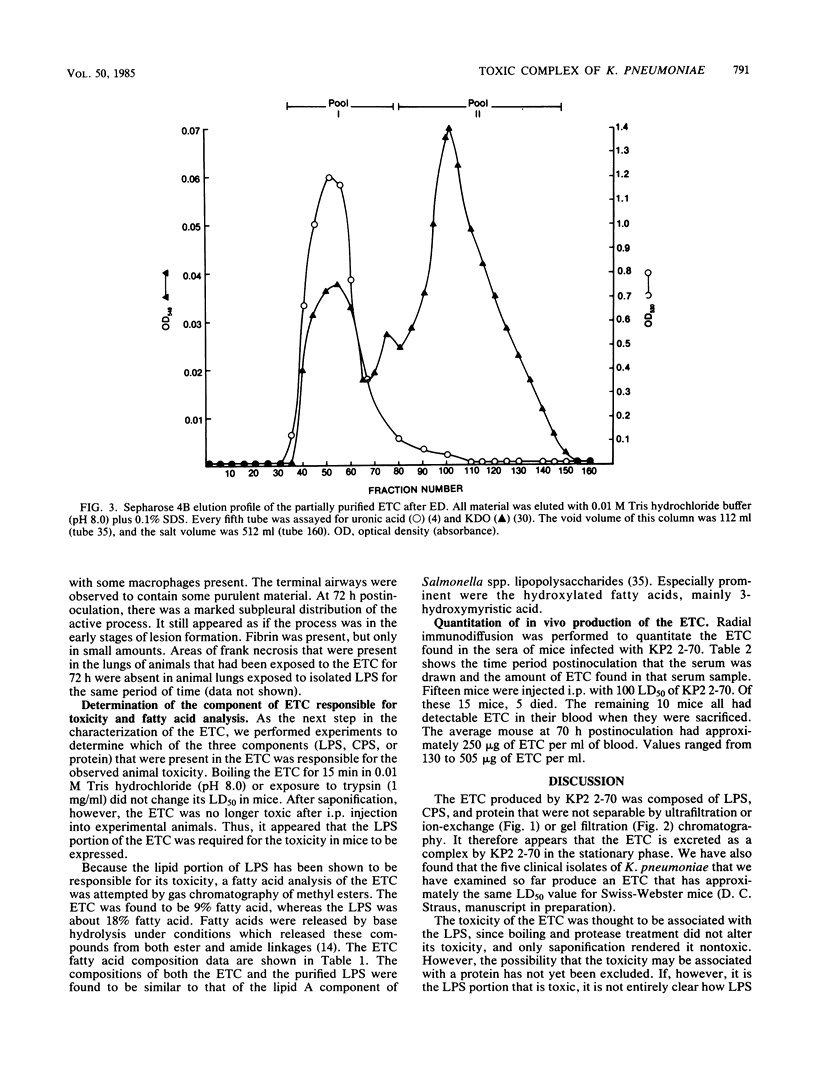
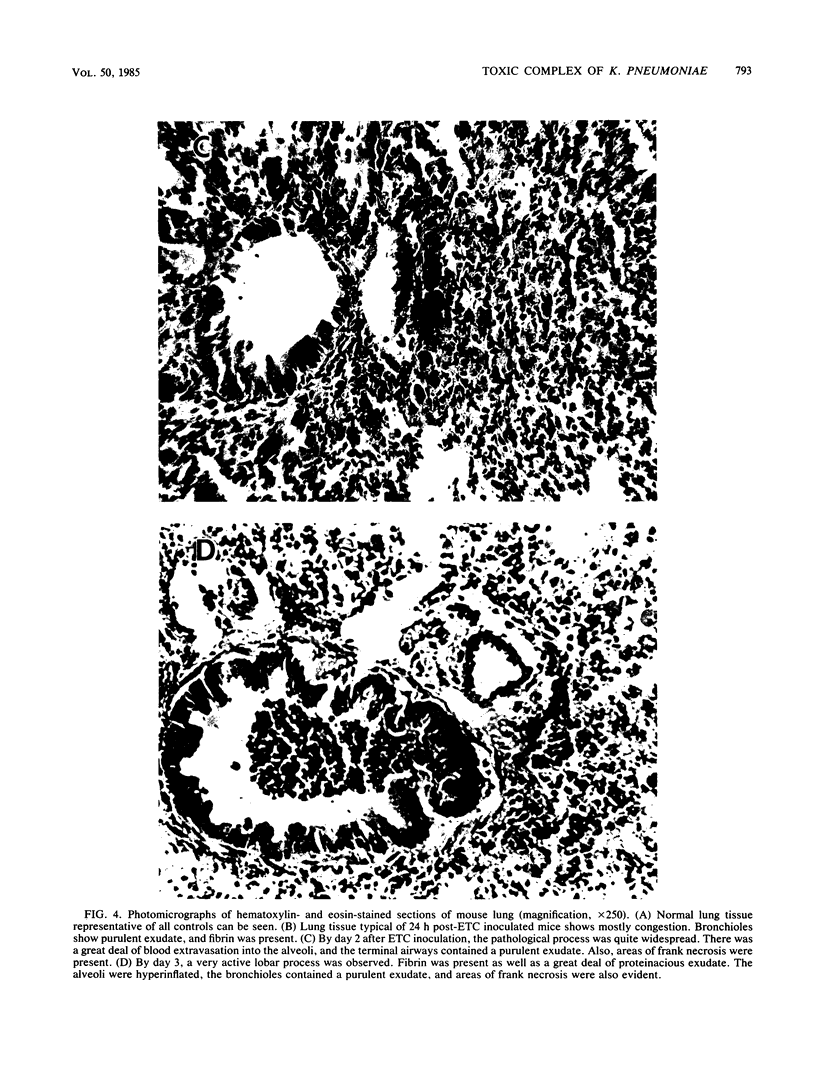
A Klebsiella pneumoniae serotype 2 strain was examined for its ability to produce extracellular toxic material. The organism was grown to the stationary phase in a defined medium, and the toxic material was isolated by ultrafiltration-ion-exchange chromatography on DEAE-Sephacel and gel filtration chromatography on Sepharose 4B or 2B. It was found to be comprised of 63% capsular polysaccharide, 30% lipopolysaccharide, and 7% protein and possessed a 50% lethal dose (when injected intraperitoneally into mice) of 393 +/- 45 micrograms. The toxicity appeared to be associated with the endotoxin portion of the compound, because boiling for 15 min and exposure to proteolytic enzymes had no effect on the toxicity. However, saponification destroyed the toxicity of the compound. Studies employing radial immunodiffusion examining the sera of mice infected with this organism demonstrated in vivo production of the complex at levels sufficiently high to produce death. When sublethal amounts of this complex were placed in the lungs of specific-pathogen-free mice, the lung pathology observed after 24, 48, and 72 h was similar to the damage caused by an active K. pneumoniae lobar pneumonia. These data indicate that this extracellular toxic compound produced by K. pneumoniae may be responsible for the lethality and lung tissue destruction normally associated with an active lobar pneumonia caused by this organism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. R., Hightower A. W., Martin S. M., Dixon R. E. Secular trends in nosocomial infections: 1970-1979. Am J Med. 1981 Feb;70(2):389–392. doi: 10.1016/0002-9343(81)90777-4. [DOI] [PubMed] [Google Scholar]
- BAER H., BRINGAZE J. K., McNAMEE M. The effect of the dose of bacterial polysaccharide antigen on antibody production in mice. J Bacteriol. 1954 Jan;67(1):123–124. doi: 10.1128/jb.67.1.123-124.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Cash H. A., Straus D. C., Bass J. A. Pseudomonas aeruginosa exoproducts as pulmonary virulence factors. Can J Microbiol. 1983 Apr;29(4):448–456. doi: 10.1139/m83-072. [DOI] [PubMed] [Google Scholar]
- Christensen S. C., Korner B. An endemic caused by multiresistant klebsiella in an urological unit. Scand J Urol Nephrol. 1972;6(3):232–238. doi: 10.3109/00365597209132093. [DOI] [PubMed] [Google Scholar]
- Cross A., Allen J. R., Burke J., Ducel G., Harris A., John J., Johnson D., Lew M., MacMillan B., Meers P. Nosocomial infections due to Pseudomonas aeruginosa: review of recent trends. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S837–S845. doi: 10.1093/clinids/5.supplement_5.s837. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., WILKINSON J. F. The influence of cultural conditions on polysaccharide production by Aerobacter aerogenes. J Gen Microbiol. 1953 Oct;9(2):174–189. doi: 10.1099/00221287-9-2-174. [DOI] [PubMed] [Google Scholar]
- Domenico P., Diedrich D. L., Straus D. C. Extracellular polysaccharide production by Klebsiella pneumoniae and its relationship to virulence. Can J Microbiol. 1985 May;31(5):472–478. doi: 10.1139/m85-088. [DOI] [PubMed] [Google Scholar]
- Domenico P., Johanson W. G., Jr, Straus D. C. Lobar pneumonia in rats produced by clinical isolates of Klebsiella pneumoniae. Infect Immun. 1982 Jul;37(1):327–335. doi: 10.1128/iai.37.1.327-335.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Avots-Avotins A. E., Davis C. P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979 Aug;25(2):729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Marshall L. W., Charache P., Wallace C. K., Melvin V. B. Nosocomial pneumonia. A continuing major problem. Am Rev Respir Dis. 1973 Nov;108(5):1130–1140. doi: 10.1164/arrd.1973.108.5.1130. [DOI] [PubMed] [Google Scholar]
- Hase S., Rietschel E. T. Isolation and analysis of the lipid A backbone. Lipid A structure of lipopolysaccharides from various bacterial groups. Eur J Biochem. 1976 Mar 16;63(1):101–107. doi: 10.1111/j.1432-1033.1976.tb10212.x. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Hunt C. E., Matsen J. M. Nosocomial colonization with Klebsiella, type 26, in a neonatal intensive-care unit associated with an outbreak of sepsis, meningitis, and necrotizing enterocolitis. J Pediatr. 1974 Sep;85(3):415–419. doi: 10.1016/s0022-3476(74)80133-2. [DOI] [PubMed] [Google Scholar]
- Hoffman N. R., Preston F. S., Jr Friedlander's pneumonia. A report of 11 cases and appraisal of antibiotic therapy. Dis Chest. 1968 Apr;53(4):481–486. doi: 10.1378/chest.53.4.481. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Pierce A. K., Sanford J. P., Thomas G. D. Nosocomial respiratory infections with gram-negative bacilli. The significance of colonization of the respiratory tract. Ann Intern Med. 1972 Nov;77(5):701–706. doi: 10.7326/0003-4819-77-5-701. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Roe E. A., Gupta J. L. Controlled trials of a polyvalent pseudomonas vaccine in burns. Lancet. 1979 Nov 10;2(8150):977–982. doi: 10.1016/s0140-6736(79)92559-5. [DOI] [PubMed] [Google Scholar]
- Kato N., Kato O., Nakashima I. Effect of capsular polysaccharide of Klebsiella pneumoniae on host resistance to bacterial infections. I. Induction of increased susceptibility to infections in mice. Jpn J Microbiol. 1976 Jun;20(3):163–172. doi: 10.1111/j.1348-0421.1976.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Kato N., Kato O., Nakashima I., Naito S., Asai J. Effect of capsular polysaccharide of Klebsiella pneumoniae on host resistance to bacterial infections. III. Further study of its effects on interactions between peritoneal leukocytes and virulent Salmonella enteritidis. Microbiol Immunol. 1979;23(5):369–382. doi: 10.1111/j.1348-0421.1979.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Houghten R. A. Immunological properties of purified Klebsiella pneumoniae heat-stable enterotoxin. Infect Immun. 1983 Nov;42(2):838–841. doi: 10.1128/iai.42.2.838-841.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Lorian V., Topf B. Microbiology of nosocomial infections. Arch Intern Med. 1972 Jul;130(1):104–110. [PubMed] [Google Scholar]
- Mertz J. J., Scharer L., McClement J. H. A hospital outbreak of Klebsiella pneumonia from inhalation therapy with contaminated aerosol solutions. Am Rev Respir Dis. 1967 Mar;95(3):454–460. doi: 10.1164/arrd.1967.95.3.454. [DOI] [PubMed] [Google Scholar]
- Mizuta K., Ohta M., Mori M., Hasegawa T., Nakashima I., Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983 Apr;40(1):56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan P., Holder I. A., MacMillan B. G. Burn wounds: microbiology, local host defenses, and current therapy. CRC Crit Rev Clin Lab Sci. 1973 Jul;4(1):61–100. doi: 10.3109/10408367309151684. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M. Significance of circulating capsular antigen in Klebsiella infections. Infect Immun. 1976 Jun;13(6):1543–1548. doi: 10.1128/iai.13.6.1543-1548.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt B. A., Jr Infections caused by Pseudomonas species in patients with burns and in other surgical patients. J Infect Dis. 1974 Nov;130 (Suppl)(0):S8–13. doi: 10.1093/infdis/130.supplement.s8. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N. Effect of capsular polysaccharide of Klebsiella pneumoniae on the differentiation and functional capacity of macrophages cultured in vitro. Microbiol Immunol. 1977 Oct 20;21(10):601–610. doi: 10.1111/j.1348-0421.1977.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N. Further studies on generation of macrophages in in vitro cultures of mouse spleen cells and its inhibition by the capsular polysaccharide of Klebsiella pneumoniae. Microbiol Immunol. 1979;23(6):487–499. doi: 10.1111/j.1348-0421.1979.tb00488.x. [DOI] [PubMed] [Google Scholar]




