Abstract
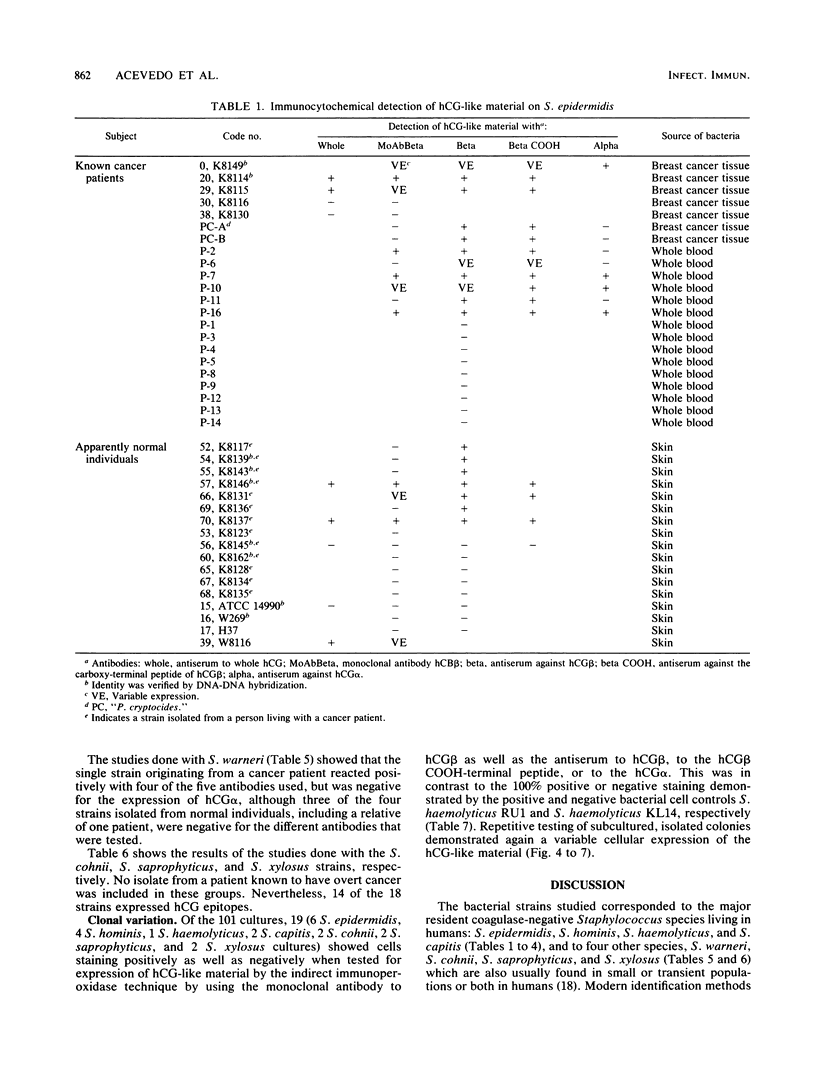
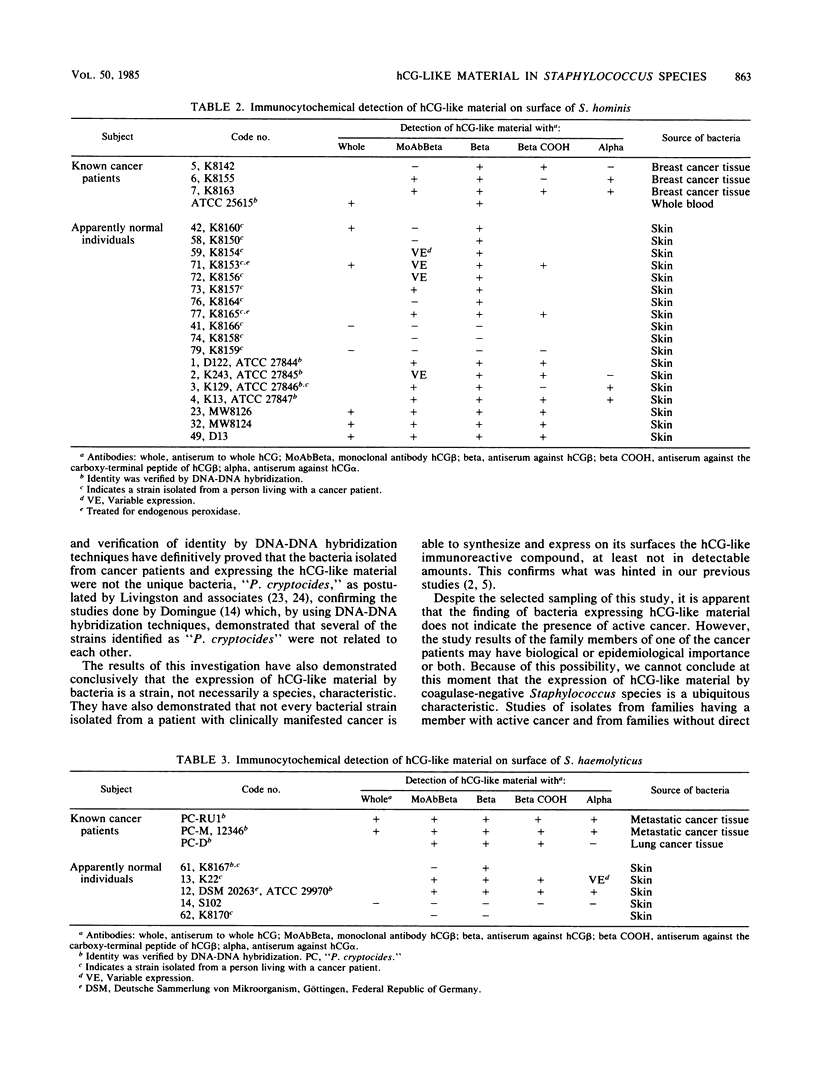
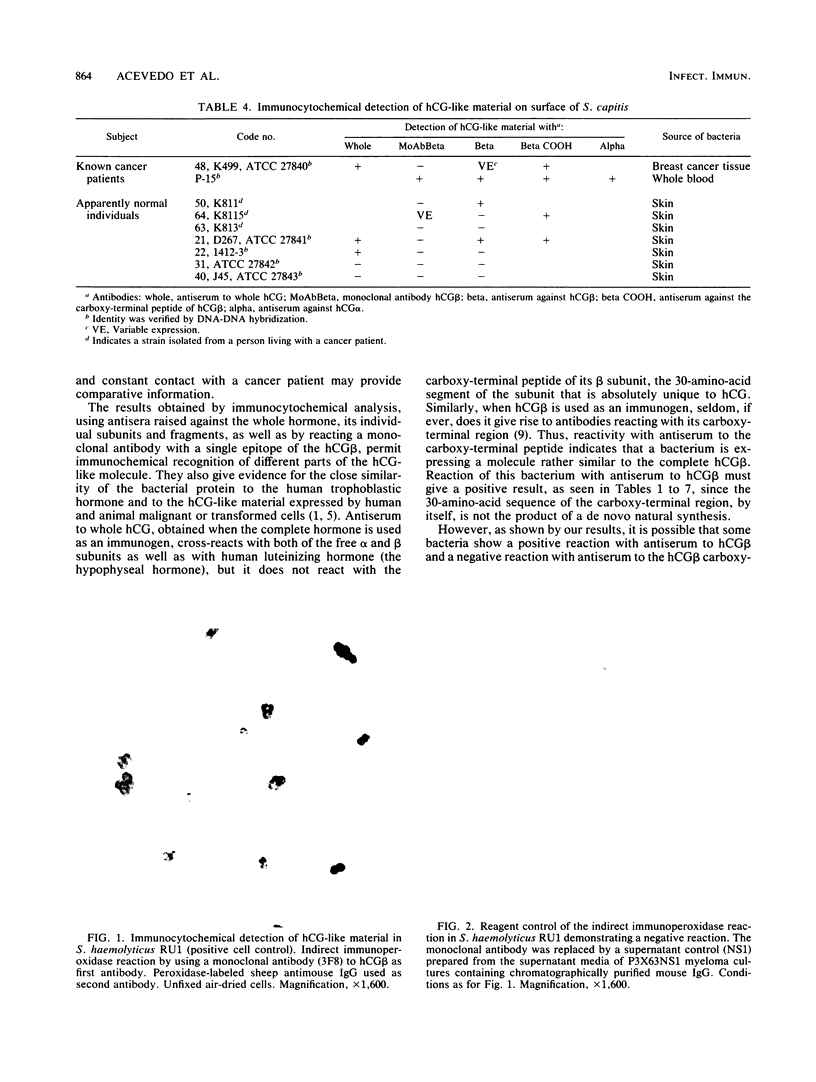
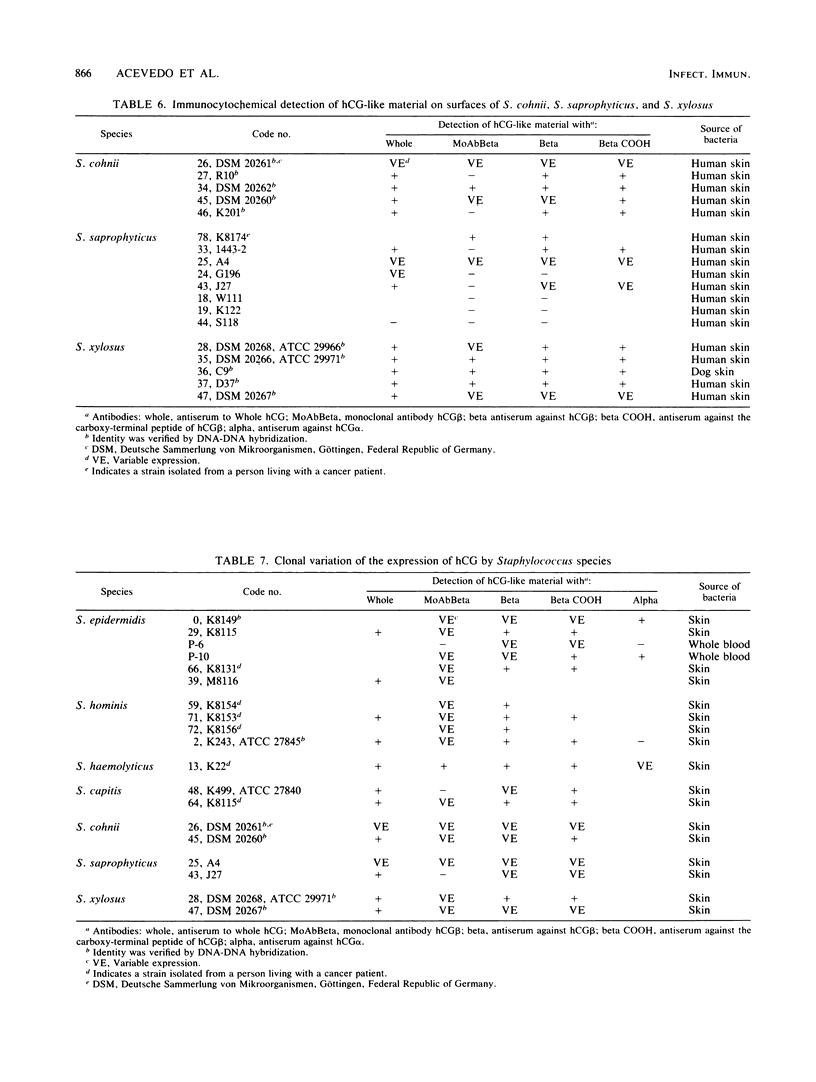
We identified 101 coagulase-negative Staphylococcus strains obtained from different laboratories, the American Type Culture Collection, and our collection, isolated from 23 patients with overt cancer and 34 normal individuals through Kloos and Schleifer conventional methods and the Staph-Ident staphylococcal system (Analytab Products, Plainview, N.Y.). In 40 strains, identity was further verified by DNA-DNA hybridization techniques. Identification revealed 39 S. epidermidis, 22 S. hominis, 8 S. haemolyticus, 9 S. capitis, 5 S. warneri, 5 S. cohnii, 8 S. saprophyticus, and 5 S. xylosus strains, all resident species found in humans. All bacteria were tested for the expression of human choriogonadotropin (hCG)-like material by the indirect fluorescein and peroxidase immunocytochemical labeling techniques by using specific antisera to the whole hormone, to its alpha and beta subunits, to the hCG beta COOH-terminal peptide, and to a monoclonal antibody to the hCG beta. The results demonstrated that the isolates from cancer patients were not unique bacteria, as has been postulated by others; the expression of immunoreactive hCG-like material is a strain, not a species, characteristic; not every bacterial strain isolated from a cancer patient is able to express the material; hCG-producing bacteria do not necessarily indicate the presence of active disease; 20% of the strains that we studied revealed a clonal variation of the expression of hCG-like material or its subunits or both as well as a variable expression of a single hCG epitope, an observation similar to that described for malignant cells; and a specific antiserum to the whole hormone with a high affinity and high sensitivity for immunocytochemistry can be a reliable reagent for screening purposes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo H. F., Koide S. S., Slifkin M., Maruo T., Campbell-Acevedo E. A. Choriogonadotropin-like antigen in a strain of Streptococcus faecalis and a strain of Staphylococcus simulans: detection, identification, and characterization. Infect Immun. 1981 Jan;31(1):487–494. doi: 10.1128/iai.31.1.487-494.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo H. F., Slifkin M., Pouchet-Melvin G. R., Campbell-Acevedo E. A. Choriogonadotropin-like antigen in an anaerobic bacterium, Eubacterium lentum, isolated from a rectal tumor. Infect Immun. 1979 Jun;24(3):920–924. doi: 10.1128/iai.24.3.920-924.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo H. F., Slifkin M., Pouchet G. R., Pardo M. Immunohistochemical localization of a choriogonadotropin-like protein in bacteria isolated from cancer patients. Cancer. 1978 Apr;41(4):1217–1229. doi: 10.1002/1097-0142(197804)41:4<1217::aid-cncr2820410401>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Backus B. T., Affronti L. F. Tumor-associated bacteria capable of producing a human choriogonadotropin-like substance. Infect Immun. 1981 Jun;32(3):1211–1215. doi: 10.1128/iai.32.3.1211-1215.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar Y. M., Carraway R. Bacterial peptides with C-terminal similarities to bovine neurotensin. Peptides. 1981 Spring;2(1):51–59. doi: 10.1016/s0196-9781(81)80011-3. [DOI] [PubMed] [Google Scholar]
- Birken S., Canfield R., Agosto G., Lewis J. Preparation and characterization of an improved beta-COOH-terminal immunogen for generation of specific and sensitive antisera to human chorionic gonadotropin. Endocrinology. 1982 May;110(5):1555–1563. doi: 10.1210/endo-110-5-1555. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Rake A. V., Johnson K. E. Batch procedure for thermal elution of DNA from hydroxyapatite. Anal Biochem. 1969 Apr 4;28(1):447–459. doi: 10.1016/0003-2697(69)90199-7. [DOI] [PubMed] [Google Scholar]
- Cohen H., Strampp A. Bacterial synthesis of substance similar to human chorionic gonadotrophin. Proc Soc Exp Biol Med. 1976 Jul;152(3):408–410. doi: 10.3181/00379727-152-39407. [DOI] [PubMed] [Google Scholar]
- Cole L. A., Birken S., Sutphen S., Hussa R. O., Pattillo R. A. Absence of the COOH-terminal peptide on ectopic human chorionic gonadotropin beta-subunit (hCG beta). Endocrinology. 1982 Jun;110(6):2198–2200. doi: 10.1210/endo-110-6-2198. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Tin A. W., Sussman H. H., Weintraub B. D., Rosen S. W. Independent expression of alpha-HCG and placental alkaline phosphatase in clonal human cell lines. Oncodev Biol Med. 1980;1(3):161–167. [PubMed] [Google Scholar]
- Hussa R. O. Biosynthesis of human chorionic gonadotropin. Endocr Rev. 1980 Summer;1(3):268–294. doi: 10.1210/edrv-1-3-268. [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Yonemitsu K., Matsui K., Fukunaga K., Miyamoto E. Calmodulin-like activity in the soluble fraction of Escherichia coli. Biochem Biophys Res Commun. 1981 Feb 12;98(3):656–660. doi: 10.1016/0006-291x(81)91164-5. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E., Wolfshohl J. F. Identification of Staphylococcus species with the API STAPH-IDENT system. J Clin Microbiol. 1982 Sep;16(3):509–516. doi: 10.1128/jcm.16.3.509-516.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D., Shiloach J., Roth J., Lesniak M. A. Insulin or a closely related molecule is native to Escherichia coli. J Biol Chem. 1981 Jul 10;256(13):6533–6536. [PubMed] [Google Scholar]
- Livingston V. W., Alexander-Jackson E. A specific type of organism cultivated from malignancy: bacteriology and proposed classification. Ann N Y Acad Sci. 1970 Oct 30;174(2):636–654. doi: 10.1111/j.1749-6632.1970.tb45588.x. [DOI] [PubMed] [Google Scholar]
- Livingston V. W., Livingston A. M. Some cultural, immunological, and biochemical properties of Progenitor cryptocides. Trans N Y Acad Sci. 1974 Jun;36(6):569–582. doi: 10.1111/j.2164-0947.1974.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Macchia V., Bates R. W., Pastan I. The purification and properties of a thyroid-stimulating factor isolated from Clostridium perfringens. J Biol Chem. 1967 Aug 25;242(16):3726–3730. [PubMed] [Google Scholar]
- Maruo T., Cohen H., Segal S. J., Koide S. S. Production of choriogonadotropin-like factor by a microorganism. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6622–6626. doi: 10.1073/pnas.76.12.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi J. T., Hecht D. W. Plasmid profiles in epidemiologic studies of infections by Staphylococcus epidermidis. J Infect Dis. 1980 May;141(5):637–643. doi: 10.1093/infdis/141.5.637. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Van de Putte P. Genetic switches by DNA inversions in prokaryotes. Biochim Biophys Acta. 1984 Jun 16;782(2):111–119. doi: 10.1016/0167-4781(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Slifkin M., Pardo M., Pouchet-Melvin G. R., Acevedo H. F. Immuno-electron microscopic localization of a choriogonadotropin-like antigen in cancer-associated bacteria. Oncology. 1979;36(5):208–210. doi: 10.1159/000225343. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Rosen S. W., Sussman H. H. Expression of the alpha subunit of human chorionic gonadotropin is specifically correlated with tumorigenic expression in human cell hybrids. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6242–6245. doi: 10.1073/pnas.79.20.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]