Abstract
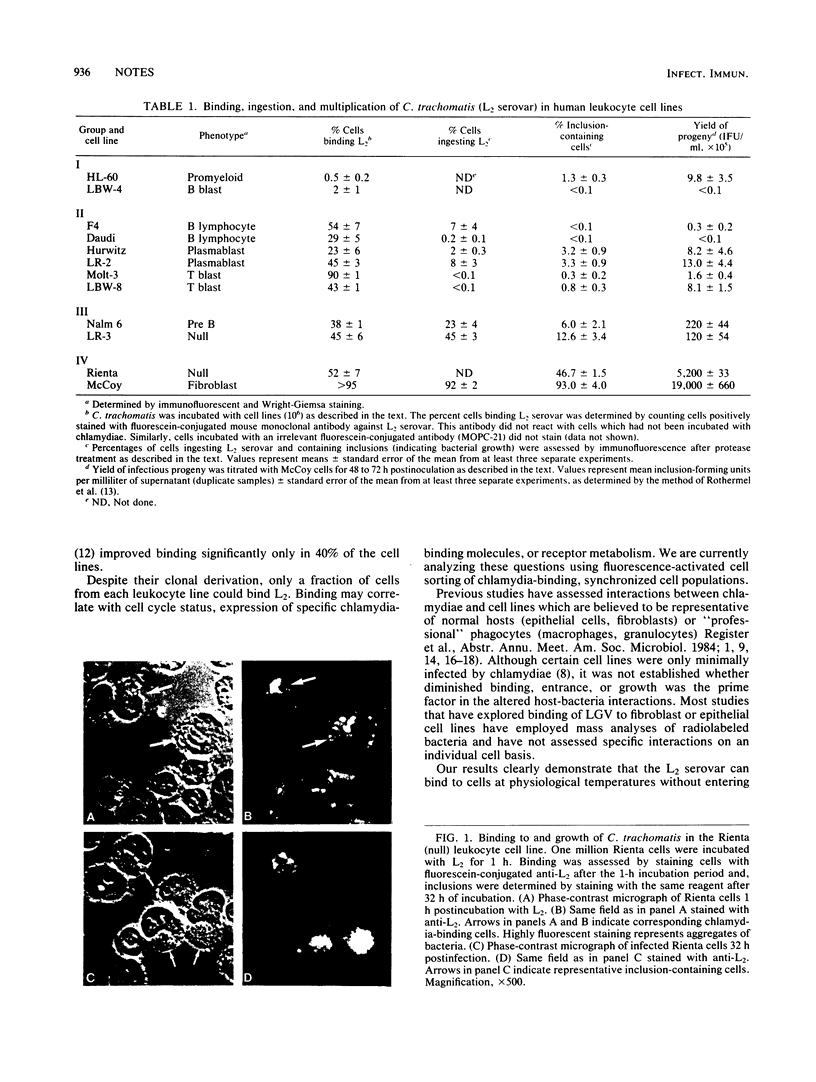
We examined the ability of lymphoblastoid-myeloid cell lines to bind, ingest, and permit multiplication of Chlamydia trachomatis (L2 serovar). Four types of chlamydia-cell line interactions were observed: minimal bacterial binding; bacterial binding, followed by ingestion and high-level multiplication; bacterial binding, followed by ingestion but minimal multiplication; and bacterial binding, but minimal ingestion or replication. Our data demonstrate that at 37 degrees C in vitro the L2 serovar can both bind avidly to a cell without entering it and enter nonphagocytic cells but not grow.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Pearce J. H. Modulation by centrifugation of cell susceptibility to chlamydial infection. J Gen Microbiol. 1979 Mar;111(1):87–92. doi: 10.1099/00221287-111-1-87. [DOI] [PubMed] [Google Scholar]
- Bard J., Levitt D. Chlamydia trachomatis stimulates human peripheral blood B lymphocytes to proliferate and secrete polyclonal immunoglobulins in vitro. Infect Immun. 1984 Jan;43(1):84–92. doi: 10.1128/iai.43.1.84-92.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S. K., Smith G. B., Paul R. G. Influence of lectins, hexoses, and neuraminidase on the association of purified elementary bodies of Chlamydia trachomatis UW-31 with HeLa cells. Infect Immun. 1983 Jun;40(3):1060–1067. doi: 10.1128/iai.40.3.1060-1067.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978 Feb;19(2):607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Croy T. R., Kuo C. C., Wang S. P. Comparative susceptibility of eleven mammalian cell lines to infection with trachoma organisms. J Clin Microbiol. 1975 May;1(5):434–439. doi: 10.1128/jcm.1.5.434-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A., Ward M. E. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: the role of calmodulin. J Gen Microbiol. 1984 Jan;130(1):193–201. doi: 10.1099/00221287-130-1-193. [DOI] [PubMed] [Google Scholar]
- Pearce J. H., Allan I., Ainsworth S. Interaction of chlamydiae with host cells and mucous surfaces. Ciba Found Symp. 1981;80:234–249. doi: 10.1002/9780470720639.ch15. [DOI] [PubMed] [Google Scholar]
- Rothermel C. D., Byrne G. I., Havell E. A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect Immun. 1983 Jan;39(1):362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984 Jul;130(7):1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A., Ivins B. E. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect Immun. 1978 Mar;19(3):1061–1067. doi: 10.1128/iai.19.3.1061-1067.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Klebanoff S. J., Kuo C. C. Toxic effect of human polymorphonuclear leukocytes on Chlamydia trachomatis. Infect Immun. 1982 Aug;37(2):422–426. doi: 10.1128/iai.37.2.422-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]