Abstract
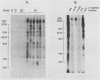
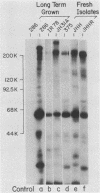
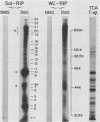
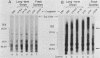
The antibody response in trichomoniasis patients was examined with a variety of methodologies including enzyme-linked immunosorbent assays, indirect immunofluorescence, immunoblotting, and radioimmunoprecipitation-electrophoresis-autoradiography. Based on enzyme-linked immunosorbent assay recognition of trichomonal isolates, sera from patients with trichomoniasis were categorized into reactive class I (IA, IB, and IC) and nonreactive class II sera. A diminished ability to precipitate antibody-binding trichomonad membrane proteins by the whole cell radioimmunoprecipitation assay was noted from class IA to class II sera. The antigenic distinctions among various Trichomonas vaginalis isolates appeared due to high-molecular-weight protein antigens detected by class IA sera in a whole cell radioimmunoprecipitation assay. The heterogeneity in antigenic patterns was confirmed among the isolates with sera from experimental animals. Also, live T. vaginalis cells appear to have only a few of the entire repertoire of major immunogenic surface proteins accessible to antibody binding. Immunoblotting demonstrated that the high-molecular-weight proteins responsible for trichomonal isolate heterogeneity are present in all isolates. The data suggest that trichomonads of a given isolate express only a subset of internally synthesized protein antigens on their surface. Importantly, the presence of these protein antigens on T. vaginalis membranes correlated with antibody production in subcutaneously challenged mice. Finally, indirect immunofluorescence studies with highly reactive, pooled sera from either patients or mice revealed a subpopulation of nonstaining trichomonads. These data support the view that heterogeneity among T. vaginalis is dependent upon the surface disposition of highly immunogenic protein antigens. Strategies may now be developed not only for studying potential vaccine reagents, but also for examining possible antigenic phenotypic variations in this experimental model.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Enzyme linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984 Jun;60(3):164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G. E. Soluble Trichomonas vaginalis antigens in cell-free culture supernatants. Mol Biochem Parasitol. 1984 Oct;13(2):147–158. doi: 10.1016/0166-6851(84)90109-9. [DOI] [PubMed] [Google Scholar]
- Alderete J. F. Identification of immunogenic and antibody-binding membrane proteins of pathogenic Trichomonas vaginalis. Infect Immun. 1983 Apr;40(1):284–291. doi: 10.1128/iai.40.1.284-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):55–72. doi: 10.1098/rspb.1978.0057. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Delachambre D. Difficultés d'obtention d'un test fiable pour déterminer la virulence d'un Flagellé parasite (Trichomonas vaginalis). C R Seances Acad Sci III. 1981 Mar 2;292(9):613–618. [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- Gillin F. D., Sher A. Activation of the alternative complement pathway by Trichomonas vaginalis. Infect Immun. 1981 Oct;34(1):268–273. doi: 10.1128/iai.34.1.268-273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- Howard R. J., Barnwell J. W., Kao V. Antigenic variation of Plasmodium knowlesi malaria: identification of the variant antigen on infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4129–4133. doi: 10.1073/pnas.80.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. N., Poisson M. A., Rein M. F. Beta-hemolytic activity of Trichomonas vaginalis correlates with virulence. Infect Immun. 1983 Sep;41(3):1291–1295. doi: 10.1128/iai.41.3.1291-1295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. N., Rein M. F. Canine prostatic secretions kill Trichomonas vaginalis. Infect Immun. 1982 Jul;37(1):77–81. doi: 10.1128/iai.37.1.77-81.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M. H. The ultrastructure of Trichomonas vaginalis donné before and after transfer from vaginal secretion to Diamonds medium. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):581–589. doi: 10.1111/j.1699-0463.1975.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Acquisition of alpha 1-Antitrypsin by a pathogenic strain of Trichomonas vaginalis. Infect Immun. 1983 May;40(2):640–646. doi: 10.1128/iai.40.2.640-646.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J Exp Med. 1984 Aug 1;160(2):398–410. doi: 10.1084/jem.160.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement for this species. Mol Biochem Parasitol. 1984 May;12(1):37–48. doi: 10.1016/0166-6851(84)90042-2. [DOI] [PubMed] [Google Scholar]
- Rein M. F., Sullivan J. A., Mandell G. L. Trichomonacidal activity of human polymorphonuclear neutrophils: killing by disruption and fragmentation. J Infect Dis. 1980 Oct;142(4):575–585. doi: 10.1093/infdis/142.4.575. [DOI] [PubMed] [Google Scholar]
- Street D. A., Taylor-Robinson D., Ackers J. P., Hanna N. F., McMillan A. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibody to Trichomonas vaginalis in sera and vaginal secretions. Br J Vener Dis. 1982 Oct;58(5):330–333. doi: 10.1136/sti.58.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su-Lin K. E., Honigberg B. M. Antigenic analysis of Trichomonas vaginalis strains by quantitative fluorescent antibody methods. Z Parasitenkd. 1983;69(2):161–181. doi: 10.1007/BF00926952. [DOI] [PubMed] [Google Scholar]
- Su K. E. Antibody to Trichomonas vaginalis in human cervicovaginal secretions. Infect Immun. 1982 Sep;37(3):852–857. doi: 10.1128/iai.37.3.852-857.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartoń A., Honigberg B. M. Analysis of surface saccharides in Trichomonas vaginalis strains with various pathogenicity levels by fluorescein-conjugated plant lectins. Z Parasitenkd. 1983;69(2):149–159. doi: 10.1007/BF00926951. [DOI] [PubMed] [Google Scholar]
- Zieg J., Simon M. Analysis of the nucleotide sequence of an invertible controlling element. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4196–4200. doi: 10.1073/pnas.77.7.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]