Abstract
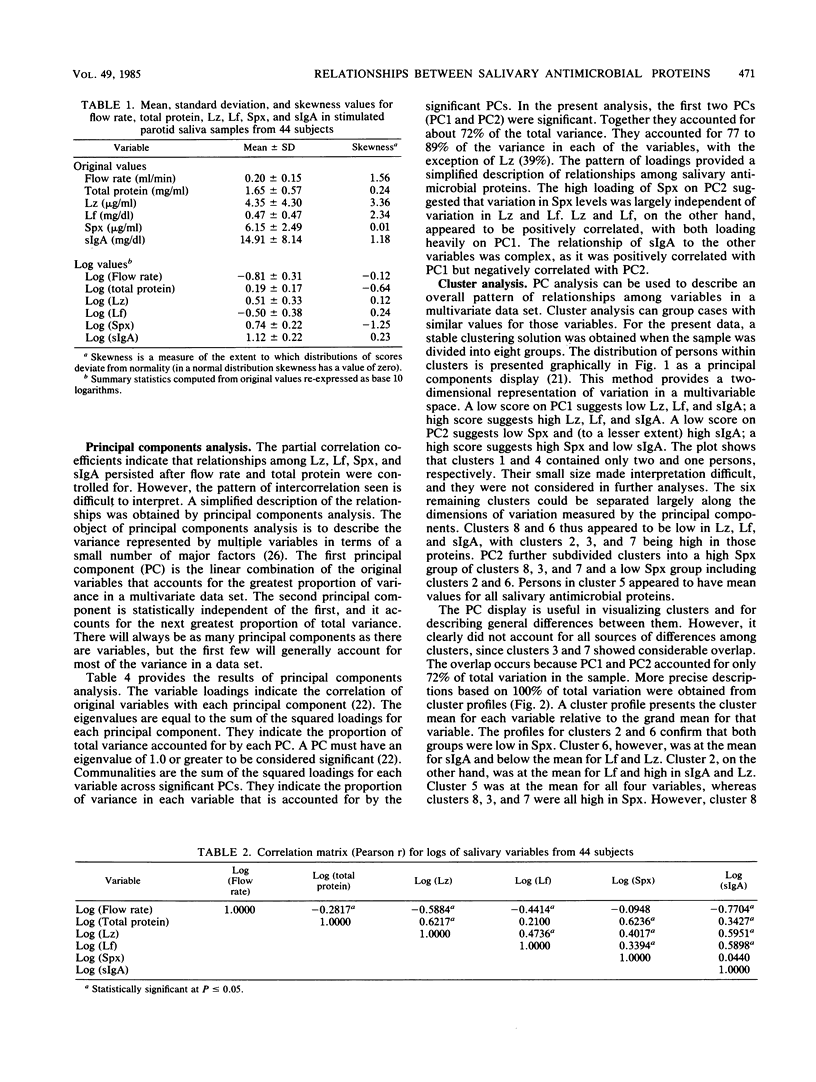
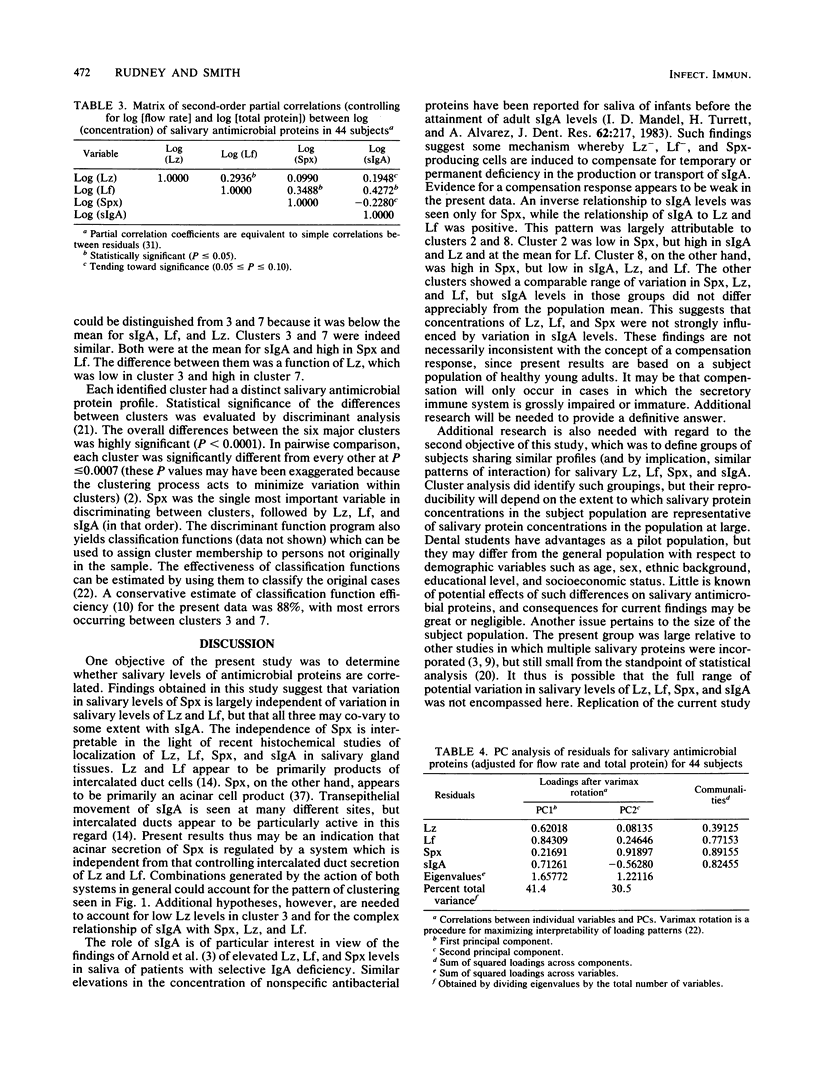
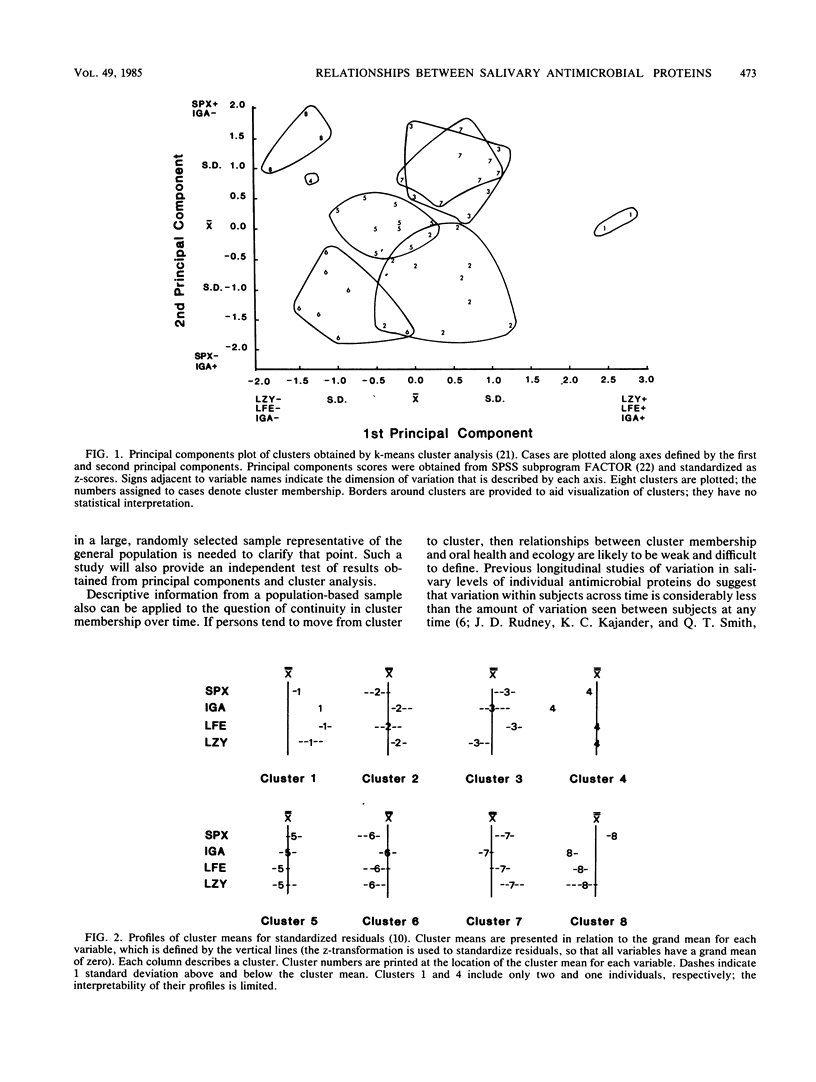
Recent studies suggest that salivary lysozyme (Lz), lactoferrin (Lf), peroxidase (Spx), and secretory immunoglobulin A (sIgA) may interact in a common antimicrobial system. A multiple protein approach therefore may be needed to determine the role of this system in oral health and ecology. In the present study we investigate the relationships between levels of Lz, Lf, Spx, and sIgA (adjusted for flow rate and total protein) in stimulated parotid saliva from 44 dental students. Principal components analysis was used to determine major patterns of intercorrelation between variables; cluster analysis was used to identify groups of subjects with similar salivary profiles for Lz, Lf, Spx, and sIgA. Spx tended to vary independently of Lz and Lf, which, in turn, tended to vary together. sIgA showed a weak negative relationship with Spx and a weak positive relationship with Lz and Lf. Six major clusters of subjects with similar antimicrobial protein profiles were found. These were significantly different at P less than 0.0001. Spx was the most important determinant of cluster membership followed (in order of importance) by Lz, Lf, and sIgA. Cluster profiles were Spx-, sIgAmu, Lf-, Lz-; Spx-, sIgA+, Lfmu, Lz+; Spxmu, sIgAmu, Lfmu, Lzmu; Spx+, sIgA-, Lf-, Lz-; Spx+, sIgAmu, Lf+, Lz-; and Spx+, sIgAmu, Lf+, Lz+ (-, mu, and + refer to the position of the cluster mean each variable relative to the overall mean for that variable). Results suggest that clusters may be a product of independent variation in the secretory activity of acinar and intercalated duct cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M. R., Burns C. A., Arnold R. R. Comparison fo agglutinin titers for Streptococcus mutans in tears, saliva, and serum. Infect Immun. 1982 Jan;35(1):202–204. doi: 10.1128/iai.35.1.202-204.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Russell J. E., Champion W. J., Brewer M., Gauthier J. J. Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect Immun. 1982 Mar;35(3):792–799. doi: 10.1128/iai.35.3.792-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azen E. A. Salivary peroxidase activity and thiocyanate concentration in human subjects with genetic variants of salivary peroxidase. Arch Oral Biol. 1978;23(9):801–805. doi: 10.1016/0003-9969(78)90158-9. [DOI] [PubMed] [Google Scholar]
- Burgio G. R., Lanzavecchia A., Plebani A., Jayakar S., Ugazio A. G. Ontogeny of secretory immunity: levels of secretory IgA and natural antibodies in saliva. Pediatr Res. 1980 Oct;14(10):1111–1114. doi: 10.1203/00006450-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Burns C. A., Ebersole J. L., Allansmith M. R. Immunoglobulin A antibody levels in human tears, saliva, and serum. Infect Immun. 1982 Jun;36(3):1019–1022. doi: 10.1128/iai.36.3.1019-1022.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. F., Hsu S. D., Baum B. J., Bowen W. H., Sierra L. I., Aquirre M., Gillespie G. Specific and nonspecific immune factors in dental plaque fluid and saliva from young and old populations. Infect Immun. 1981 Mar;31(3):998–1002. doi: 10.1128/iai.31.3.998-1002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Glucose uptake by Streptococcus mutans, Streptococcus mitis, and Actinomyces viscosus in the presence of human saliva. Infect Immun. 1982 Dec;38(3):1060–1067. doi: 10.1128/iai.38.3.1060-1067.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Simple and rapid procedure for the selective removal of lysozyme from human saliva. Infect Immun. 1979 Dec;26(3):991–995. doi: 10.1128/iai.26.3.991-995.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., de Stoppelaar J. D., Harden L. Lysozyme insensitivity of bacteria indigenous to the oral cavity of man. J Dent Res. 1966 May-Jun;45(3):877–881. doi: 10.1177/00220345660450036201. [DOI] [PubMed] [Google Scholar]
- Korsrud F. R., Brandtzaeg P. Characterization of epithelial elements in human major salivary glands by functional markers: localization of amylase, lactoferrin, lysozyme, secretory component, and secretory immunoglobulins by paired immunofluorescence staining. J Histochem Cytochem. 1982 Jul;30(7):657–666. doi: 10.1177/30.7.6179983. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laible N. J., Germaine G. R. Adsorption of lysozyme from human whole saliva by Streptococcus sanguis 903 and other oral microorganisms. Infect Immun. 1982 Apr;36(1):148–159. doi: 10.1128/iai.36.1.148-159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;(8):25–47. [PubMed] [Google Scholar]
- Oberg S. G., Izutsu K. T., Truelove E. L. Human parotid saliva protein composition: dependence on physiological factors. Am J Physiol. 1982 Mar;242(3):G231–G236. doi: 10.1152/ajpgi.1982.242.3.G231. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N. M., Wolf R. O., Tylenda A. Free and total lysozyme determined in parotid saliva. Clin Chem. 1983 Aug;29(8):1551–1552. [PubMed] [Google Scholar]
- Peeters T. L., Vantrappen G. R. Factors influencing lysozyme determinations by the lysoplate method. Clin Chim Acta. 1977 Feb 1;74(3):217–225. doi: 10.1016/0009-8981(77)90288-1. [DOI] [PubMed] [Google Scholar]
- Samson R. R., Mirtle C., McClelland D. B. Secretory IgA does not enhance the bacteriostatic effects of iron-binding or vitamin B12-binding proteins in human colostrum. Immunology. 1979 Oct;38(2):367–373. [PMC free article] [PubMed] [Google Scholar]
- Shindler J. S., Childs R. E., Bardsley W. G. Peroxidase from human cervical mucus. The isolation and characterisation. Eur J Biochem. 1976 Jun 1;65(2):325–331. doi: 10.1111/j.1432-1033.1976.tb10345.x. [DOI] [PubMed] [Google Scholar]
- Skurk A., Krebs S., Rehberg J. Flow rate, protein, amylase, lysozyme and kallikrein of human parotid saliva in health and disease. Arch Oral Biol. 1979;24(10-11):739–743. doi: 10.1016/0003-9969(79)90033-5. [DOI] [PubMed] [Google Scholar]
- Spik G., Cheron A., Montreuil J., Dolby J. M. Bacteriostasis of a milk-sensitive strain of Escherichia coli by immunoglobulins and iron-binding proteins in association. Immunology. 1978 Oct;35(4):663–671. [PMC free article] [PubMed] [Google Scholar]
- Tabak L., Mandel I. D., Karlan D., Baurmash H. Alterations in lactoferrin in salivary gland disease. J Dent Res. 1978 Jan;57(1):43–47. doi: 10.1177/00220345780570011801. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Moldoveanu Z., Mestecky J., Pruitt K. M., Rahemtulla B. M. Interaction of specific and innate factors of immunity: IgA enhances the antimicrobial effect of the lactoperoxidase system against Streptococcus mutans. J Immunol. 1982 Feb;128(2):726–731. [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A., Smith K. W., Chwang A. K. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun. 1983 Feb;39(2):767–778. doi: 10.1128/iai.39.2.767-778.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina S., Barka T. Localization of peroxidase activity in the developing submandibular gland of normal and isoproterenol-treated rats. J Histochem Cytochem. 1972 Nov;20(11):855–872. doi: 10.1177/20.11.855. [DOI] [PubMed] [Google Scholar]


