Abstract
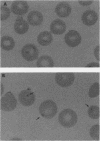
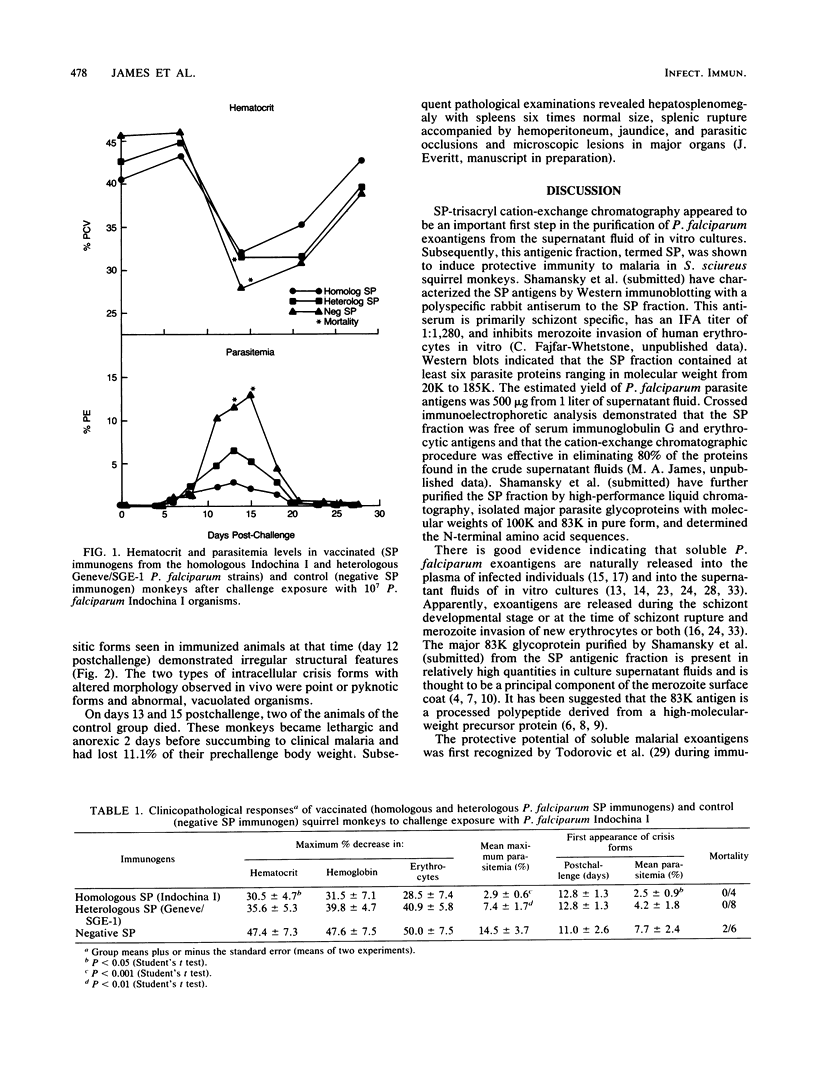
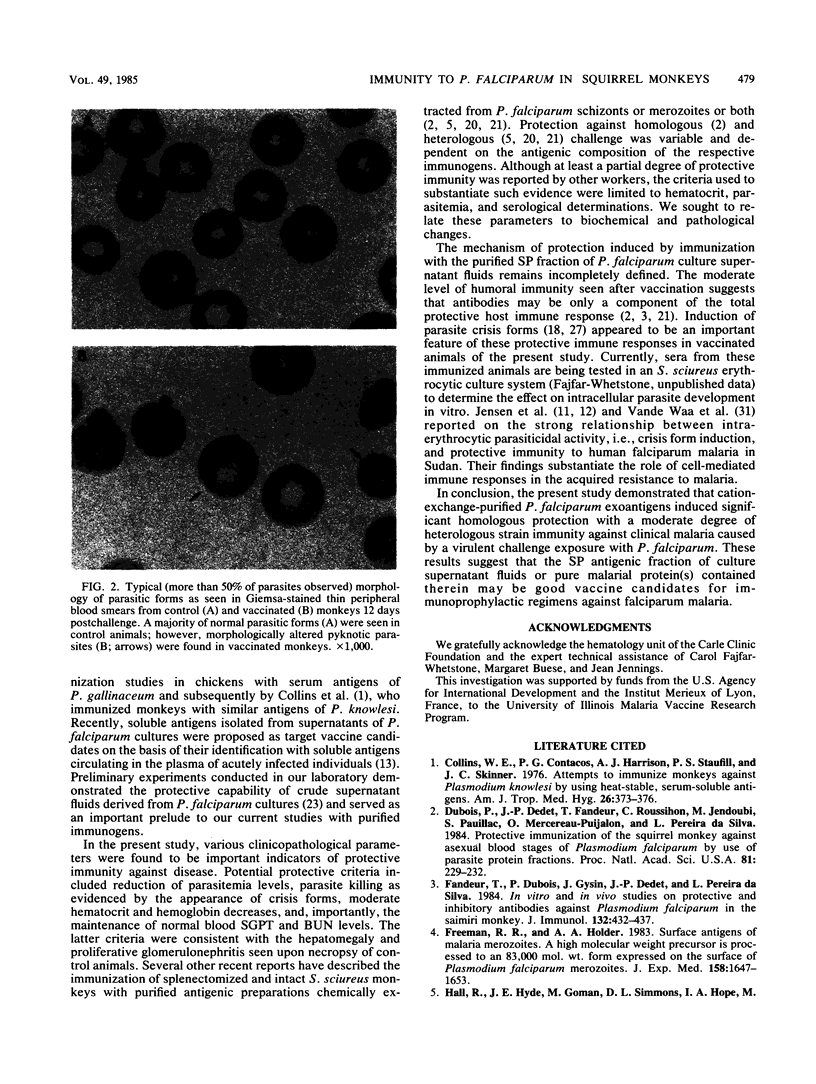
Soluble Plasmodium falciparum exoantigens in crude culture supernatant fluids induced protective immunity against experimental falciparum malaria in Bolivian Saimiri sciureus monkeys. Susceptible squirrel monkeys were vaccinated with an aluminum hydroxide-fortified fraction purified from culture supernatants of P. falciparum Indochina I and Geneve/SGE-1 by cation-exchange (sulfopropyl-trisacryl) chromatography. Animals immunized with sulfopropyl-purified and corresponding control immunogens were challenged with whole blood containing monkey-adapted virulent organisms of the Indochina I strain. Hematological, serological, and parasitological profiles, including the appearance of crisis forms, served as potential indicators of protection. This immunogen conferred significant clinical protection of squirrel monkeys against needle challenge with the homologous Indochina I strain and a moderate degree of heterologous strain immunity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins W. E., Contacos P. G., Harrison A. J., Stanfill P. S., Skinner J. C. Attempts to immunize monkeys against Plasmodium knowlesi by using heat-stable, serum-soluble antigens. Am J Trop Med Hyg. 1977 May;26(3):373–376. doi: 10.4269/ajtmh.1977.26.373. [DOI] [PubMed] [Google Scholar]
- Dubois P., Dedet J. P., Fandeur T., Roussilhon C., Jendoubi M., Pauillac S., Mercereau-Puijalon O., Pereira Da Silva L. Protective immunization of the squirrel monkey against asexual blood stages of Plasmodium falciparum by use of parasite protein fractions. Proc Natl Acad Sci U S A. 1984 Jan;81(1):229–232. doi: 10.1073/pnas.81.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandeur T., Dubois P., Gysin J., Dedet J. P., da Silva L. P. In vitro and in vivo studies on protective and inhibitory antibodies against Plasmodium falciparum in the Saimiri monkey. J Immunol. 1984 Jan;132(1):432–437. [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Strych W., Mrema J. E. Identification of surface and internal antigens from spontaneously released Plasmodium falciparum merozoites by radio-iodination and metabolic labelling. Z Parasitenkd. 1983;69(6):715–725. doi: 10.1007/BF00927421. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. F., Reese R. T. Synthesis of merozoite proteins and glycoproteins during the schizogony of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Mar;10(3):319–334. doi: 10.1016/0166-6851(84)90030-6. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Allan J. S., Carlin J. M., Vande Waa J. A., Divo A. A., Akood M. A. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun. 1983 Sep;41(3):1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B., Hoffman S. L., Boland M. T., Akood M. A., Laughlin L. W., Kurniawan L., Marwoto H. A. Comparison of immunity to malaria in Sudan and Indonesia: crisis-form versus merozoite-invasion inhibition. Proc Natl Acad Sci U S A. 1984 Feb;81(3):922–925. doi: 10.1073/pnas.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen S., Andersen B. J. Immunoadsorbent isolation of antigens from the culture medium of in vitro cultivated Plasmodium falciparum. Acta Pathol Microbiol Scand C. 1981 Apr;89(2):99–103. doi: 10.1111/j.1699-0463.1981.tb02671.x. [DOI] [PubMed] [Google Scholar]
- Jepsen S., Axelsen N. H. Antigens and antibodies in Plasmodium falciparum malaria studied by immunoelectrophoretic methods. Acta Pathol Microbiol Scand C. 1980 Oct;88(5):263–270. doi: 10.1111/j.1699-0463.1980.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Jepsen S. Inhibition of in vitro growth of Plasmodium falciparum by purified antimalarial human IgG antibodies. Isolation of target antigens from culture supernatants. Scand J Immunol. 1983 Dec;18(6):567–571. doi: 10.1111/j.1365-3083.1983.tb00893.x. [DOI] [PubMed] [Google Scholar]
- McColm A. A., Trigg P. I. Release of radio-isotope labelled antigens from Plasmodium knowlesi during merozoite re-invasion in vitro. Parasitology. 1980 Aug;81(1):199–-209. doi: 10.1017/s0031182000055153. [DOI] [PubMed] [Google Scholar]
- McGregor I. A., Turner M. W., Williams K., Hall P. Soluble antigens in the blood of African patients with severe plasmodium falciparum malaria. Lancet. 1968 Apr 27;1(7548):881–884. doi: 10.1016/s0140-6736(68)90237-7. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Loche M., Dedet J. P., Roussilhon C., Fandeur T. Immunization against Plasmodium falciparum asexual blood stages using soluble antigens. Clin Exp Immunol. 1984 Apr;56(1):67–72. [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez da Silva L., Loche M., Dayal R., Perrin L. H. Plasmodium falciparum polypeptides released during in vitro cultivation. Bull World Health Organ. 1983;61(1):105–112. [PMC free article] [PubMed] [Google Scholar]
- Smith R. D., James M. A., Ristic M., Aikawa M., Vega y Murguia C. A. Bovine babesiosis: protection of cattle with culture-derived soluble Babesia bovis antigen. Science. 1981 Apr 17;212(4492):335–338. doi: 10.1126/science.7209532. [DOI] [PubMed] [Google Scholar]
- Sulzer A. J., Wilson M. The fluorescent antibody test for malaria. CRC Crit Rev Clin Lab Sci. 1971;2(4):601–619. doi: 10.3109/10408367109151318. [DOI] [PubMed] [Google Scholar]
- Thelu J., Ambroise-Thomas P., Contat M., Kupka P. Antigènes excrétés-sécrétés par Plasmodium falciparum en cultures in vitro. Etude comparée avec les antigènes somatiques et les antigènes figurés. Bull World Health Organ. 1982;60(5):761–766. [PMC free article] [PubMed] [Google Scholar]
- Todorovic R., Ferris D., Ristic M. Immunogenic properties of serum antigens from chickens acutely infected with Plasmodium gallinaceum. Ann Trop Med Parasitol. 1967 Jun;61(2):117–124. doi: 10.1080/00034983.1967.11686467. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vande Waa J. A., Jensen J. B., Akood M. A., Bayoumi R. Longitudinal study on the in vitro immune response to Plasmodium falciparum in Sudan. Infect Immun. 1984 Aug;45(2):505–510. doi: 10.1128/iai.45.2.505-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]
- Wilson R. J., Bartholomew R. K. The release of antigens by Plasmodium falciparum. Parasitology. 1975 Oct;71(2):183–192. doi: 10.1017/s0031182000046631. [DOI] [PubMed] [Google Scholar]