Abstract
Reticular dysgenesis (RD) is an autosomal recessive form of human Severe Combined Immunodeficiency, characterized by an early differentiation arrest in the myeloid lineage and impaired lymphoid maturation. In addition, affected newborns have bilateral sensorineural deafness. We have identified biallelic mutations in the adenylate kinase 2 (AK2) gene in seven patients affected with RD. These mutations resulted in the absence or a strong decrease in protein expression. We then demonstrated that restoration of AK2 expression in the bone marrow cells of RD patients overcomes the neutrophil differentiation arrest underlining its specific requirement in the development of a restricted set of haematopoietic lineages. Lastly, we established that AK2 is specifically expressed in the stria vascularis region of the inner ear, which provides an explanation to the sensorineural deafness. These results suggest a novel mechanism regulating haematopoetic cell differentiation, and involved in one of the most severe human immunodeficiency syndromes.
The term “reticular dysgenesis” (RD), was coined in 1959 by de Vall and Seyneheve1 and relates to the histological findings in primary and secondary lymphohaematopoietic organs, where the scarcity of cells highlights the prominent reticular tissue framework. The lack of polymorphonuclear neutrophils (PMNs) in affected patients is responsible for the occurrence of severe infections earlier than is usually observed in other forms of SCID2,3. RD-associated neutropenia is characterized by lack of responsiveness to granulocyte-colony stimulating factor (G-CSF)4. The only available treatment for RD is allogeneic haematopoietic stem cell transplantation (HSCT), which formally demonstrates that the inherited defect is cellular and not micro-environmental5.
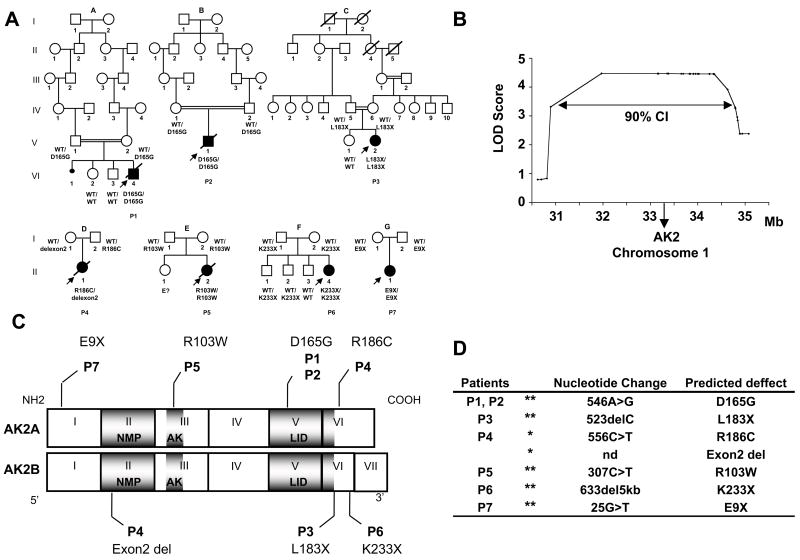
In order to determine the molecular genetic basis of RD, we took advantage of three separate consanguineous kindreds (family A, B & C, Fig. 1a). A genome-wide linkage scan generated a significant, multipoint LOD-score of 4.47 in a single region located on the short arm of chromosome 1 (1p31–p34). The map was then refined by genotyping 17 additional microsatellite markers in this region. The 90% confidence interval (CI) of the region of interest covered ~ 4 Mbs, stretching from 30.89 to 34.79 Mb (Fig. 1b). Interestingly, two patients originating from the same geographical area (P1 and P2) were found to be homozygous for the same haplotype in the region between 32.8 and 34.79 Mb (including 18 SNPs and 8 microsatellite markers), which strongly suggested a founder effect in these two families and reduced the prime region of interest to ~ 2 Mbs. The prime region of interest was then fully sequenced (Supplementary Table 1). Patients from the three consanguineous families (P1, P2 and P3) were found to be homozygous for mutations in the adenylate kinase 2 (AK2)-encoding gene. Similarly, we found AK2 mutations in four additional available RD patients born to non consanguineous parents (Fig. 1a).
Figure 1. AK2 gene mutations in seven patients with reticular dysgenesis.
(a) Genealogical trees for 7 families with children suffering from RD (indicated by filled symbols). Diagonal bars indicate deceased individuals. Double horizontal bars indicate consanguineous marriages. AK2 mutations are indicated for each patient and parent.
WT= wild type allele of the AK2 gene. E?= no material available.
(b) Genome-wide linkage analysis Multipoint LOD scores are plotted along the 1p30–p35 region of chromosome 1. CI= confidence interval
(c) Location of AK2 mutations in RD patients. Diagrammatic representation of the AK2A and AK2B encoding regions from exon 1 to 7. The AK2A isoform includes the 6 first exons, while AK2B has an additional exon (VII). The corresponding protein domains are shown in dark grey: NMP binding domain. (NMP), adenylate kinase domain (AK) and LID domain (LID).
(d) Description of the AK2 gene mutations in the 7 RD patients. In 6 patients the mutations were homozygous (**) and in 1 patient heterozygous mutations were found (*).
The AK2 gene encodes two types of mRNA (expressed via an alternative splicing mechanism): AK2A (6 exons) and AK2B which includes an additional seventh exon located in the 3′ part of the gene. AK2A and AK2B encodes, respectively, a 239-amino-acid (aa) and a 232-aa protein6. Homozygous mutations were prevalent among the defects found in the seven RD patients analyzed (Fig. 1c): 3 patients (P1, P2 and P5) showed homozygous missense mutations of highly conserved amino acid residues (Supplementary Fig. 1), two patients (P3 and P6) carried homozygous deletion (1 bp- and ~5 kb, respectively) that generate a premature stop codon and in one case (P7) a homozygous nonsense mutation was detected. P4 was found to be a compound heterozygote with a missense mutation and an exon 2 deletion (Fig. 1c and d). All mutations are located in different key domains of the AK2 protein (Fig. 1c) and are thus expected to severely hamper its stability and/or function. In all cases, the parents were found to be heterozygous for the mutated AK2 allele and the healthy siblings that we tested were heterozygous or homozygous for the wild-type allele (Fig. 1a).
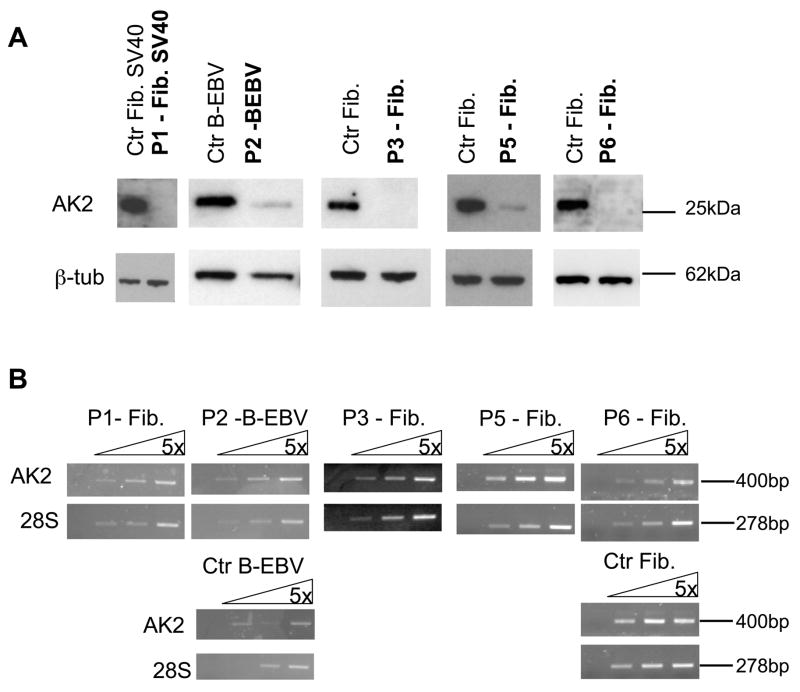
AK2 is expressed in the mitochondrial intermembrane space in a variety of tissues such as the liver, kidney, spleen and heart 6. We assessed the expression of the AK2 gene in fibroblasts (either primary cultures or SV40-transformed cells) obtained from patients P1, P3, P5 and P6 and in a B-EBV cell line from patient P2. Using Western blot analysis, no AK2 protein was detected in the fibroblasts from P1, P3 and P6 (Fig. 2a). The AK2 protein was detected in the B-EBV cell line and in fibroblasts derived from P2 and P5, respectively, but at markedly lower levels than in control cells (Fig. 2a). In contrast to the AK2 protein, AK2 mRNA was detected by RT-PCR at levels comparable to those found in control cell lines in all patient samples analyzed (Fig. 2b). These data strongly suggest that RD results from loss-of-function mutations in the AK2 gene.
Figure 2. Gene and protein expression analysis of AK2 in cell lines derived from RD patients.
(a) Western blot analysis of AK2 expression. Lysates from fibroblast (Fib) or B-EBV cell lines derived from RD patients were immunoblotted with an anti-AK2 antibody and with anti-β– tubulin as a loading control.
(b) RT-PCR analysis of AK2 expression. cDNA prepared from fibroblast or B-EBV cell lines derived from RD patients was analyzed by RT-PCR using 5-fold serial dilution. The cDNA input was normalized against 28S-rRNA gene expression.
Analysis of the immunological characteristics of RD patients showed that the profound neutropenia was invariably associated with an equally profound T- and natural killer (NK) cell lymphopenia, whilst the B cell lineage was variably affected (Table 1). The circulating monocyte count was normal or above normal, whereas red blood cell and platelet counts were normal or slightly diminished, as also observed by Levinsky RJ et al7. Morphological examination of the bone marrow revealed a profound block in granulopoiesis, with no detectable cells beyond the myelocyte stage, thus indicating that RD defect corresponds to a selective developmental arrest along T, NK lymphoid and granulocytic myeloid pathways. In semi-solid medium, CD34+ cells isolated from the BM of P2 did not generate granulocytic colonies and only a few myeloblasts and erythroblasts were observed before and after culture (Supplementary Fig. 2a). In patient CD34+ cells cultured in the presence of SCF, FLT-3L and GM-CSF or G-CSF, CD15+ CD11b+ neutrophil cell counts were two and four times lower, respectively, than in control cord blood (CB) cell cultures (Supplementary Fig. 2b) suggesting a potential role for AK2 in the G-CSF R mediated myeloid differentiation. This is in agreement with the G-CSF refractory neutropenia observed in RD patients.
Table 1.
Haematological and Immunological characteristics of the patients
| Patient* | P1 | P2 | P3 | P4 | P5 | P6 | P7 | Age matched- control values0–1month |
|---|---|---|---|---|---|---|---|---|
| WBCs(10−9 cells/l) | 0.5 | 1.63 | 0.39 | 1.8 | 1.4 | 0.3 | 0.85 | 7.20–18.00 |
| Lymphocytes(10−9 cells/l) | 0.30 | 0.67 | 0.26 | 0.9 | 0.8 | 0.07 | 0.35 | 3.40–7.60 |
| T-lymphocytes(10−9 cells/l) | 0.27 | 0.04 | 0.01 | 0.09 | 0.1 | 0.001 | 0.02 | 2.50–5.50 |
| T-CD4+(10−9 cells/l) | 0.10 | 0.001 | 0.004 | NA | 0.09 | 0 | 0.01 | 1.60–4.00 |
| T-CD8+(10−9 cells/l) | 0.03 | 0.04 | 0.004 | NA | 0 | 0 | 0.006 | 0.56–1.70 |
| B-lymphocytes(10−9 cells/l) | 0 | 0.556 | 0.165 | NA | 0.5 | 0 | 0.3 | 0.3–2.00 |
| NK cells(10−9 cells/l) | 0.017 | 0.053 | 0.008 | 0.09 | 0.056 | NA | 0.03 | 0.17–1.10 |
| PMNs(10−9 cells/l) | 0 | 0.03 | 0 | 0.15 | 0.15 | 0 | 0.10 | 1.50–8.50 |
| Monocytes(10−9 cells/l) | 0.2 | 0.555 | 0.15 | 0.55 | 0.4 | 0.11 | 0.4 | 0.20–1.00 |
| Platelets(10−9 cells/l) | 275 | 62 | 100 | 442 | 300 | 172 | 200 | 175–500 |
| Hemoglobin (g/dl) | 8 | 15 | 10 | 15.5 | 12 | 11.5 | 14.6 | 14.0–17.0 |
Abbreviations:
WBC = white blood cells
PMN = polymorphonuclear cells
NK = natural killer cells
NA = not available
All patients have been diagnosed within the first month of life.
As a first step towards elucidating the function of AK2 in haematopoiesis, we performed an RT-PCR AK2 gene expression profile analysis that revealed significant AK2 upregulation in the various haematopoietic subsets as compared to a fibroblast cell line (Supplementary Fig. 3). These results fit with a differential and specific role of AK2 in different cell types.
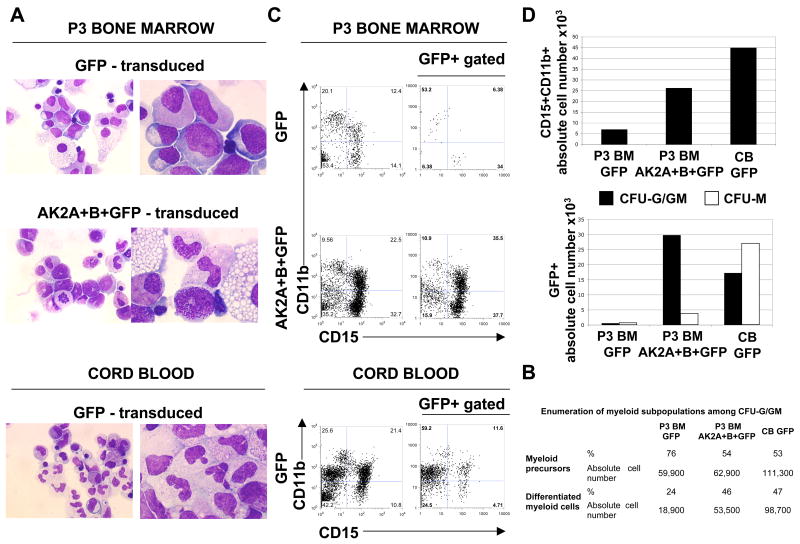
In order to prove unequivocally that AK2 mutations were responsible for the profound neutropenia, we set out to restore myeloid differentiation in vitro by transducing bone-marrow CD34+ cells from RD patients with an AK2 A+B cDNA-encoding lentivirus. Granulocyte/granulocyte-monocyte (G/GM) colonies derived from BM cells of P3 and transduced with a control GFP vector were characterized by the presence of immature myeloblasts, promyelocytes, myelocytes and very few mature polynucleated cells. In contrast, G/GM colonies derived from BM cells transduced with AK2A+B+GFP contained around 46% of mature myeloid cells, metamyelocytes and polymorphonuclear cells (Fig. 3a and b) exhibiting the unambiguous characteristics of mature myeloid cells. These mature granulocytes were very similar to those observed in colonies cultured from control healthy cord blood cells (Fig. 3a). We confirmed these morphological observations by flow cytometry analysis (Fig. 3c) and showed that the CD15+CD11b+ cell count was 3.7-fold higher in the AK2A+B+GFP condition than in the GFP condition (Fig. 3d). Furthermore, the overall count of GFP+AK2+ complemented cells in the G/GM colonies was 53-fold higher than that of GFP+ cells isolated from non-complemented BM cells (Fig. 3d). Restoration of neutrophil differentiation was confirmed in BM cell from P6 (Supplementary Fig. 4). These data demonstrate that AK2 complementation corrects the defective granulopoiesis in RD.
Figure 3. Complementation of the neutrophil differentiation defect by restoration of AK2 expression.
CD34+ cells isolated from the bone marrow (BM) of patient P3 were transduced with either two bicistronic lentivirus vectors (encoding AK2A and AK2B, respectively, together with a GFP reporter gene: AK2A+B+GFP) or a lentivirus encoding the GFP reporter only (GFP). As a positive control, cord-blood-derived CD34+ cells were transduced with GFP. After lentiviral transduction, 104 CD34+ cells from the BM of P3 were seeded in semi-solid medium for each condition, corresponding to 500 complemented cells (based on a transduction efficiency of 5%). As a control, 500 CD34+ cord blood cells were seeded in semi-solid medium (transduction efficiency of 35%).
(a) After 13 days, CFU-G/GM colonies were collected and cytospin preparations of each condition (P3 BM GFP; P3 BM AK2A+B+GFP; CB GFP) were analyzed by May-Grunwald/Giemsa staining. Representative pictures of various stages of neutrophil maturation are shown at magnifications of 50× (left panel) or 100× (right panel).
(b) Enumeration of myeloid subpopulations among CFU-G/GM colonies. Myeloid precursors (blasts, myeloblasts and promyelocytes) and differentiated myeloid cells (myelocytes, metamyelocytes and granulocytes) are represented in percentage and in absolute cell numbers.
(c) Representative flow cytometry analysis of CD15 and CD11b expression in CFU-G/GM colonies (left-hand dot plots) and on transduced GFP+ CFU-G/GM colonies (right-hand dot plots)
(d) Absolute number of double-positive CD15+CD11b+ labelled cells are indicated for each condition (left panel). The absolute number of GFP+ cells isolated from the CFU-G/GM colonies and from the CFU-M colonies are indicated (right panel).
In order to confirm the specific role of AK2 in neutrophil differentiation, we have developed a RNA interference strategy through lentiviral-mediated gene transfer of AK2 short hairpin RNAs (shAK2), using the previously published shAK2 target sequence 8. The downregulation of AK2 expression in human CD34+ cell induced a 17 to 27-fold reduction in myeloid cells and granulocyte precursors associated with a profound arrest in neutrophil differentiation as determined by counting of CD15+CD11b+ cells and by morphological examination (Supplementary Fig. 5). Conversely, no decrease in the proliferation rate wa observed in human primary fibroblasts transduced with the same AK2 shRNA lentivirus vector (data not shown).
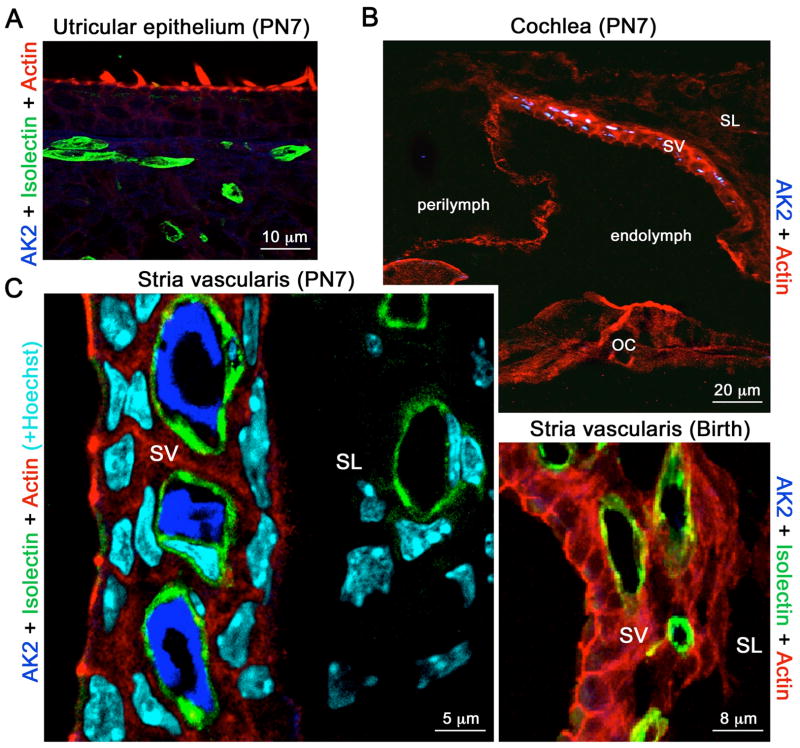
In order to investigate the pathogenic process underlying the hearing impairment observed in all 7 RD patients, we analyzed the distribution of AK2 in the mouse inner ear by immunohistolabelling. AK2 expression could not be detected in the vestibule, at any developmental stage (Fig. 4a). In contrast, the assesment of the cochlea revealed an intense AK2 staining observed uniquely in the stria vascularis (SV) at post-natal day 7 but not at birth (Fig 4b). Co-immunolabelling of AK2 and isolectin showed that AK2 is present within the lumen of the SV capillaries (Fig 4c), thus suggesting that it could be here functioning as an ecto-enzyme. Notably, AK2 could not be detected in the capillaries or vessels of the adjacent connective tissue (Fig 4c).
Figure 4. AK2 distribution in the mouse inner ear.
(a) AK2 is not detected in the vestibular epithelium. (b) Top panel. In the post-natal day 7 (PN7) cochlea, AK2 is only detected in the stria vascularis (SV). It is absent from the spiral ligament (SL) and organ of Corti (OC). Lower panel. AK2 is not detected in the SV at birth. (c) In the PN7 SV, AK2 labelling (dark blue) is restricted to the lumen of the capillaries and terminal blood vessels, which are stained green with isolectin. Blood vessels in the spiral ligament (SL) do not contain AK2. Cell nuclei are stained in light blue by Hoechst staining.
Our data provide evidence that recessive mutations of the human AK2 gene are responsible for RD- a very rare form of SCID characterized by a profound impairment of the lymphomyeloid compartment associated with deafness. AK2 plays a major role during development, as shown in Drosophila where AK2−/− larvae are not viable9. This enzyme regulates the homeostasis of cellular adenine nucleotides by converting ADP into ATP and AMP 9 and thus may be critical in specific key subcellular or extracellular compartments. Our observations in the inner ear suggest a new function for AK2 in the SV. Indeed, failure of the SV to produce the endocochlear potential or secrete K+ in the endolymph results in hearing impairment10,11. Moreover, serum ADP is thought to be one of the most damaging factors for endothelial integrity, in view of its pro-inflammatory and thrombotic effects12. In this respect, ecto-AK2 in the SV microvasculature might contribute to the control of local ADP levels via reverse transphosphorylation into ATP and AMP.
In many human and murine cells, AK2 is localized in the mitochondrial intermembrane space, suggesting a role for the protein in providing the energy required for the proliferation of haematopoietic precursors and/or in controlling cell apoptosis. With respect to the latter hypothesis, our findings are reminiscent of the results reported in another type of severe congenital neutropenia caused by HAX-1 deficiency13. HAX-1 is also located in the mitochondrial intermembrane space and is required to prevent apoptosis of mouse lymphocytes, granulocytes and neurons14,15. AK2 might also be involved in the regulation of apoptosis via an as yet unknown mechanism which may nevertheless involve its release from the mitochondria, together with cytochrome c16,17. More recently, AK2 has been implicated in a novel, intrinsic apoptosis pathway, where it forms a complex with FADD and caspase 108. In this latter report, truncated, mislocated AK2 products were found in the cytosol of human cells and were seen to induce apoptosis. However, our western blot analysis did not reveal the presence of any truncated AK2 protein. This in agreement with the observation of a strong decrease in cell proliferation/survival as well as a block in myeloid differentiation induced by the downregulation of AK2 expression in CD34+ cells. The discrepancy with the published data may be related to the usage of different cell subsets, i.e. HeLa in Lee’s work8 and CD34+ cells in ours. Indeed, no effect on fibroblast cell survival was induced by downregulation of AK2. It is nevertheless possible that formation of an AK2/FADD/caspase 10 complex is required for commitment to the T/NK lymphoid and neutrophil differentiation pathway. Indeed, the recent description of thrombocytopenia in patients carrying mutations in the cytochrome c gene18 emphasizes a possible lineage-specific control of caspase activation that may not be related to the classic cell death mechanism19,20. Further investigation of the potential involvement of AK2 in regulating apoptosis in certain cell lineages is required.
Taken as a whole, we identified mutations in human AK2 responsible for Reticular Dysgenesis, one of the most severe immunodeficiencies affecting both innate and adaptive immunity and associated with sensorineural deafness. These data enlight the key role of the AK2 protein in haematopoiesis and emphasize a novel cell-lineage restricted pathways controlling energy metabolism and/or cell growth and survival.
METHODS
Patients
Seven families (each with one affected child) were analyzed in the present study. Three were unrelated consanguineous families (A, B, & C), with two originating from Cape Verde (Western Africa) and the third from Turkey. The four other unrelated non-consanguineous families originated from France (D), Portugal (E), the Netherlands (F) and Central America (G), respectively. All the probands were admitted to the hospital very soon after birth (from day 1 up to 1 month) because of multiple, life-threatening infections. The blood cell count and the bone marrow (BM) status prompted a diagnosis of RD. All patients were later found to be deaf. All received an allogeneic hematopoietic stem cell transplant from one of the two parents or pheno-compatible cord blood (P6). This treatment resulted in a cure in 3 (P3, P6 & P7) of the 7 patients.
Genetic, sequencing and gene expression analysis
Linkage analysis of the two Cape Verde families (A, B) and the Turkish family (C), performed by homozygosity mapping21, was performed at the Centre National de Genotypage (CNG, Evry, France) and 17 additional microsatellite markers were genotyped in the region of interest by using standard methods. DNA samples from the RD patients and their relatives were purified from peripheral blood cells by using genomic DNA Maxiprep kits (Qiagen). For target gene sequencing, PCR primers were designed for the 2 Mb region and standard Sanger sequencing was performed at the NIH Intramural Sequencing Center (NISC). Coding regions of AK2 (exons 1–7) were completely covered by sequencing and had very high sequence trace quality scores. Exons 1 and 7 had double sequence coverage (4 traces per patient – 2 forward and 2 reverse traces). On average, each amplimer sequenced 645 targeted bases (median: 671; mode: 700). Traces were processed to detect single nucleotide and insertion/deletion (indel) variation using POLYPHRED software (version 6.11). We screened healthy individuals (100 chromosomes) for the presence of AK2 gene mutation and found none.
Cell samples and purification of human CD34+ cells
Control human cord blood (CB) samples were harvested on delivery of full-term, healthy pregnancies in the Antoine Béclère Hospital (Clamart, France) and the Pitié-Salpetrière Hospital (Paris, France). Human BM from RD patients was collected under general anaesthesia for the installation of the central line required for allogeneic BM transplantation. Our study was performed with informed consent obtained from each patient or the patient’s family and was approved by the Hôpital Necker independent ethics committee and the NHGRI IRB. Mononuclear cells (MNCs) and CD34+ cells were purified as previously described22.
For patients P1, P3, P5 and P6, primary fibroblast cell lines were obtained from skin biopsies and fibroblasts were immortalized with SV40, as previously described23. B-EBV cell lines (for EBV transformed B-cell lines) were generated from BM samples, as described elsewhere24.
In vitro myeloid differentiation assays
CD34+ cells differentiation was evaluated by clonal assay in methylcellulose (methoCultR H4435), as previously described22. CD34+ cells were also cultured in X-Vivo 20 medium (BioWhittaker) supplemented with 10 ng/ml of stem cell factor (SCF, Amgen), and 100 ng/ml of granulocyte macrophage colony stimulating factor (GM-CSF, Amgen) or 100ng/ml granulocyte colony stimulating factor (G-CSF, Amgen). After 2 weeks, cells were harvested and analyzed by May-Grunwald/Giemsa staining. Cells were also analyzed by flow cytometry using a combination of fluorescein isothiocyanate (FITC)-conjugated anti-CD15 (HI98), phycoerythrin (PE)-conjugated anti-CD11b (ICRF44) and allophycocyanin (APC)-conjugated anti-CD14 (M5E2) antibodies (all provided by Becton Dickinson, BD Biosciences). Analysis was performed on a FACSCalibur (BD Biosciences) using the FlowJo software.
Gene expression analysis
Total RNA was reverse transcribed using random hexamers with MultiScribe™ MuLV reverse transcriptase (Applied Biosystems). RT-PCR was performed on each patient sample using primers specific for AK2 and 28S (Supplementary Table 2).
Comparative real-time RT-PCR assays25,26 were performed for each sample in triplicate in a final reaction volume of 50 μl. The endogenous gene (GAPDH) or the gene of interest (AK2A or AK2B) were amplified using around 20 ng of cDNA, and AK2A (Hs00761823), AK2B (Hs00797700) or GAPDH (Hs00266705) FAM-labelled probes (Applied Biosystems). All reactions were carried out using an ABI Prism 7900 sequence detection system (Applied Biosystems). The data were analyzed using the comparative cycle threshold method and presented as the fold change in gene expression, normalized against the calibrator sample, corresponding to a fibroblast cell line.
Western blots
Control or RD fibroblast cell lines (either primary or SV40-transformed) were lysed in a buffer (containing 20mM Tris, pH 7.9; 300mM NaCl; 1% NP40) supplemented with protease and phosphatase inhibitors. Cell extracts were separated by SDS-PAGE, blotted and stained with a polyclonal antibody against AK2 (H65, Santa Cruz) or with a monoclonal antibody against β tubulin (TUB2.1, Sigma). After staining with an HRP-conjugated secondary antibody, the immunoblot was developed using an ECL+ kit (Amersham).
Construction and production of the lentiviral vectors
The backbone of the replication-defective, self-inactivating pWPI lentiviral vector was provided by Addgene. The constructs were generated using PmeI (Ozyme) ligation of the coding sequence of human AK2A (pWPI-AK2A) or AK2B (pWPI-AK2B). For construction of AK2 short hairpin RNA (shAK2) and the negative control Scramble shRNA (shScramble), the forward and reverse primers (Supplementary Table 2) containing target sequences from AK2 corresponding to the previously published shRNA#18 and from a control Scramble were cloned into the MluI and ClaI sites of the pLVTHM lentiviral vector, provided by Addgene. The latter vectors enable coexpression of GFP under the control of an EF1α promoter. High-titer lentivirus supernatants were produced by Vectalys. Using this shAK2 lentivector, AK2 expression was effectively knocked-down in cord blood derived CD34+ cells as observed by quantitative RT-PCR (data not shown).
Transduction of human CD34+ cells
Bone-marrow mononuclear cells or purified CD34+ cells were cultured overnight in X-vivo 20 medium supplemented with SCF (300 ng/ml), Flt3-ligand (Flt3-L, 300 ng/ml), interleukin 3 (60 ng/ml IL-3) and thrombopoietin (100 ng/ml TPO) (R&D Systems). Cells were then transferred onto culture plates coated with 50 μg/ml of retronectin-CH296 (Takara Bio) and transduced (at 2×106/ml) or not with the lentiviral supernatant (multiplicity of infection = 100) in X-vivo 20 medium supplemented with the cytokines described above and 4 μg/ml of protamine sulfate (Choay). After an overnight culture, cells were harvested and seeded for the myeloid differentiation assay.
Immunofluorescence staining on inner ear sections
Experiments were performed using 10-μm-thick, frozen cochlear sections. We prepared cochlear sections by dissecting the temporal bones and fixing them by immersion in 4% paraformaldehyde. The samples were then decalcified in 0.5 M EDTA, immersed in 20% sucrose and frozen in OCT compound embedding medium. After blocking in 20% goat serum, the sections were incubated with the primary antibody at 4°C for 12 h, followed by incubation with the secondary antibody (and with phalloidin and/or Hoechst dyes) for 1 h at room temperature and, lastly, embedded in Fluorsave (Calbiochem) for confocal microscopy analysis (with the LSM510 META from Zeiss). We used the following antibodies: polyclonal anti-AK2 (Abcam), Cy5 goat anti-rabbit Ig (H+L), Cy3 goat anti-mouse Ig (H+L) (Amersham), and Alexa Fluor 488-conjugated isolectin GS-IB4 (Invitrogen).
Supplementary Material
Acknowledgments
We wish to thank the Antoine Béclère Hospital (Clamart, France) and the Pitié-Salpetrière Hospital (Paris, France) for providing cord blood samples, the French National Genotyping Centre (Evry, France) for contributing to the genome-wide linkage scan and Dr. Praveen Cherukuri and the NISC Comparative Sequencing Program (NISC, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA) for their help in sequencing the region of interest. We are grateful to Dr. I. André-Schmutz, Dr. S. Blanche, Dr. J.L. Casanova, Dr. J. Chinen, Dr. C. Chomienne, Dr. L. Coulombel, Dr. L. dal Cortivo, Dr. F. Le Deist, Dr. G. de Saint-Basile and Dr. J.P. de Villartay for fruitful discussions and assistance, and to all staff nurses who cared for the patients. We also thank the patients’ families for their participation. We acknowledge C. Hue, C. Martinache, J. Rouiller, C. Soudais and M.C. Stolzenberg for their expert technical assistance, as well as David Fraser for his language editing review.
The study was funded by the French National Institute for Health and Medical Research (INSERM), the French Institute for Rare Diseases (GIS-Institut des maladies rares), the French National Research Agency (ANR), and the Intramural Research Program of the National Human Genome Research Institute (NIH).
References
- 1.De Vall O, Seyneheve V. Reticular dysgenesis. Lancet. 1959;2:1123–1125. doi: 10.1016/s0140-6736(59)90105-9. [DOI] [PubMed] [Google Scholar]
- 2.Heltzer ML, Paessler M, Raffini L, Bunin N, Perez EE. Successful haploidentical bone marrow transplantation in a patient with reticular dysgenesis: three-year follow-up. J Allergy Clin Immunol. 2007;120:950–2. doi: 10.1016/j.jaci.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Reubsaet LL, Boelens JJ, Rademaker C, Smal J, Wulffraat NM. Successful cord blood transplantation in a premature and dysmature neonate of 1700 g with reticular dysgenesis. Bone Marrow Transplant. 2007;39:307–8. doi: 10.1038/sj.bmt.1705577. [DOI] [PubMed] [Google Scholar]
- 4.Bujan W, Ferster A, Sariban E, Friedrich W. Effect of recombinant human granulocyte colony-stimulating factor in reticular dysgenesis. Blood. 1993;82:1684. [PubMed] [Google Scholar]
- 5.Antoine C, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361:553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 6.Noma T, Song S, Yoon YS, Tanaka S, Nakazawa A. cDNA cloning and tissue-specific expression of the gene encoding human adenylate kinase isozyme 2. Biochim Biophys Acta. 1998;1395:34–9. doi: 10.1016/s0167-4781(97)00193-0. [DOI] [PubMed] [Google Scholar]
- 7.Levinsky RJ, Tiedeman K. Successful bone-marrow transplantation for reticular dysgenesis. Lancet. 1983;1:671–2. doi: 10.1016/s0140-6736(83)91968-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, et al. AK2 activates a novel apoptotic pathway through formation of a complex with FADD and caspase-10. Nat Cell Biol. 2007;9:1303–10. doi: 10.1038/ncb1650. [DOI] [PubMed] [Google Scholar]
- 9.Noma T. Dynamics of nucleotide metabolism as a supporter of life phenomena. J Med Invest. 2005;52:127–36. doi: 10.2152/jmi.52.127. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Salmon M, et al. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A. 2007;104:6229–34. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heine P, Braun N, Heilbronn A, Zimmermann H. Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur J Biochem. 1999;262:102–7. doi: 10.1046/j.1432-1327.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 13.Klein C, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease) Nat Genet. 2007;39:86–92. doi: 10.1038/ng1940. [DOI] [PubMed] [Google Scholar]
- 14.Chao JR, et al. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452:98–102. doi: 10.1038/nature06604. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson G, Fasth A. Infantile genetic agranulocytosis, morbus Kostmann: presentation of six cases from the original “Kostmann family” and a review. Acta Paediatr. 2001;90:757–64. [PubMed] [Google Scholar]
- 16.Kohler C, et al. Release of adenylate kinase 2 from the mitochondrial intermembrane space during apoptosis. FEBS Lett. 1999;447:10–2. doi: 10.1016/s0014-5793(99)00251-3. [DOI] [PubMed] [Google Scholar]
- 17.Munoz-Pinedo C, et al. Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration. Proc Natl Acad Sci U S A. 2006;103:11573–8. doi: 10.1073/pnas.0603007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morison IM, et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat Genet. 2008;40:387–9. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
- 19.Hao Z, et al. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–91. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Solary E, Giordanetto F, Kroemer G. Re-examining the role of cytochrome c in cell death. Nat Genet. 2008;40:379–80. doi: 10.1038/ng0408-379. [DOI] [PubMed] [Google Scholar]
- 21.Lander ES, Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987;236:1567–70. doi: 10.1126/science.2884728. [DOI] [PubMed] [Google Scholar]
- 22.Six EM, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204:3085–93. doi: 10.1084/jem.20071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicolas N, et al. A human severe combined immunodeficiency (SCID) condition with increased sensitivity to ionizing radiations and impaired V(D)J rearrangements defines a new DNA Recombination/Repair deficiency. jem. 1998;188:627–634. doi: 10.1084/jem.188.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosato G, Cohen JI. Generation of Epstein-Barr Virus (EBV)-immortalized B cell lines. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im0722s76. Chapter 7, Unit 7 22. [DOI] [PubMed] [Google Scholar]
- 25.Peters UR, et al. Distinct expression patterns of the p53-homologue p73 in malignant and normal hematopoiesis assessed by a novel real-time reverse transcription-polymerase chain reaction assay and protein analysis. Cancer Res. 1999;59:4233–6. [PubMed] [Google Scholar]
- 26.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.