Abstract
Bone marrow-derived mesenchymal stem cells (MSC) are being extensively studied as potential therapeutic agents for various diseases and have demonstrated tremendous promise to date. To reduce immunological and inflammatory reaction upon delivery of MSC in situ, the cells are often suspended in protein-free and nutrient-poor buffered saline solution at high titers and kept on ice (0 °C) until completion of the transplantation procedure. This study investigated the effects of suspending MSC (5 × 106 cells/mL) in phosphate buffered saline (PBS) with and without calcium, over a time course of 90 and 180 min, at temperatures of 0 and 37 °C. The results at 0 °C showed a small but significant decrease in cell viability within calcium-free PBS after 180 min, whereas no significant changes in cell viability were observed with PBS containing calcium. Additionally, it was observed that significant aggregation of MSC into cellular clumps occurred when incubated in PBS at 0 °C, with a higher degree of aggregation occurring under calcium-free conditions. By contrast at 37 °C, there was a more pronounced decrease in cell viability after 90 and 180 min, but lesser aggregation of MSC both in the presence and absence of calcium. The aggregation of MSC into cellular clumps could pose an embolic hazard if delivered into the arterial vasculature in cardiac applications, can clog-up injection or infusion catheters utilized for cell delivery during surgery, and can also possibly reduce the overall efficacy of transplantation therapy.
Keywords: Aggregation, Mesenchymal, Saline, Stem cells, Transplantation
Introduction
Bone marrow-derived mesenchymal stem cells (MSC) hold tremendous promise in treating cardiac diseases particularly the post-infarcted myocardium (Schäfer and Northoff 2008; Grauss et al. 2007) as well as in chronic heart failure (Abdel-Latif et al. 2007), bone defects (Siddappa et al. 2007), cartilage lesions (Jorgensen et al. 2008), neurodegenerative diseases (Dezawa 2008), traumatic injury to the central nervous system (Nandoe Tewarie et al. 2006); Graft versus Host disease (Le Blanc et al. 2008), Crohn’s disease (Lanzoni et al. 2008) and diabetes (Urbán et al. 2008). Because it is possible to extract only relatively small numbers of MSC from patients, these cells are often subjected to extensive ex vivo proliferation within prolonged durations of in vitro culture, so as to yield sufficient numbers for transplantation therapy. There are several excellent reviews on the various technical aspects of MSC isolation and ex vivo culture (Sensebé 2008; Pountos et al. 2007).
The MSC to be transplanted are usually suspended in protein-free and nutrient-poor buffered saline solution, because the presence of foreign proteins and serum may provoke an inflammatory reaction, which in turn could reduce the efficacy of transplantation therapy. Very often, the practice is to keep high titers (typically several millions per mL) of MSC suspended in saline on ice (0 °C) for varying durations of time until completion of the cell delivery procedure. It is therefore imperative to assess any detrimental effect on MSC upon prolonged durations of exposure to saline at low temperature, since the length of time that elapses between laboratory preparation of the cells (i.e. thawing and re-suspension) and actual delivery in situ would be expected to vary according to the exact nature of procedures being performed on patients.
This study investigated the effects of suspending MSC in phosphate buffered saline (PBS) over a time duration up to 180 min, at temperatures of 0 and 37 °C. Although the primary interest of this study was on the low temperature of 0 °C, the effects of protein and nutrient deprivation in PBS at the physiological temperature of 37 °C for comparison was also investigated. Besides quantifying cell viability with the trypan blue exclusion assay through an automated cell counter, the possible aggregation of MSC into cellular clumps was also observed. Gravitational settling of a high titer cell suspension within a tube over time is very common. This often leads to cellular aggregation or clumping, which in turn could pose an embolic hazard upon transplantation into the arterial vasculature of the brain or in the left ventricle during intramyocardial injections for cardiac indications. Invariably, cellular clumps may also contribute towards clogging of needles or infusion catheters and other delivery systems utilized for cell delivery leading to possible procedural complications and loss of valuable cells. Because calcium is known to influence cell adhesion (Hirano et al. 1987) as well as various other aspects of cell physiology (Huang and Miller 2007; Hidalgo and Donoso 2008), this study also investigated whether the presence or absence of calcium influenced MSC aggregation and viability over a prolonged duration of incubation in PBS.
Materials and methods
Cells, culture media and reagents
Bone marrow-derived Human mesenchymal stem cells (Cat No: PT-2501, Batch no: 6F4382, cryopreserved at the 2nd passage) were purchased from Lonza Inc. (Walkersville, MD, USA). Unless otherwise stated, all reagents and chemicals were purchased from Sigma–Aldrich Inc. (St Louis, MO, USA), all culture media and serum were purchased from Lonza Inc. (Walkersville, MD, USA), and all labware consumables were purchased from Becton-Dickinson Inc. (Franklin Lakes, NJ, USA).
Prolonged exposure of mesenchymal stem cells to phosphate buffered saline, in the presence and absence of calcium
Cryopreserved MSC were thawed and cultured up to five passages upon purchase from Lonza Inc. (Walkersville, MD, USA), prior to being utilized for this study. After trypsinization, MSC were reconstituted to a titer of 5.0 × 106 cells/mL in Dulbecco’s phosphate buffered saline (PBS) with and without calcium (Invitrogen Inc., Carlsbad, CA, USA; Cat No. 14040-133 and Cat No. 14190-144, respectively), placed within a polycarbonate microcentrifuge tube and subsequently incubated at either 0 °C (ice) or 37 °C (incubator). At time points of 0, 90 and 180 min, 100 μL aliquots of the MSC suspension in PBS (with and without calcium) were then analyzed for cell viability with the trypan blue exclusion assay, by utilizing an automated cell counter (Vi-Cell® XR analyzer, Cat No. 383556; Beckman Coulter Inc., Fullerton, CA, USA). Concurrently at each of the three time-points, 20 μL aliquots of the MSC suspensions were placed under a bright-line hemocytometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and observed under phase-contrast microscopy, to detect the presence of cellular aggregation and formation of cell clumps. Altogether, there were 12 experimental groups for MSC incubated at either 0 or 37 °C in PBS with and without calcium, at three time-points of 0, 90 and 180 min.
Assessment of cell viability and aggregation with an automated cell counter (Vi-Cell® XR analyzer)
Aliquots of MSC suspension from all 12 experimental groups were subjected to cell viability assessment (trypan-blue exclusion) with an automated cell counter (Vi-CELL® XR analyzer, Cat No. 383556; Beckman Coulter Inc., Fullerton, CA, USA), utilizing the Vi-CELL® XR Quad Pak Reagent Kit (Cat. No. 383198, Beckman-Coulter Inc., Fullerton, CA, USA) and accessory sample vials (Cat No. 383721, Beckman-Coulter Inc., Fullerton, CA, USA). The cell suspension was diluted 20 times in PBS for cell viability analysis (30 μL of sample in 570 μL of PBS) within the sample vial. The automated cell counter then mixed the cell suspension with an equal volume of 0.4% (w/v) trypan blue solution (600 μL), prior to drawing in 50 × 20 μL aliquots of the mixture into its counting chamber. Each reading by the automated cell counter is therefore obtained by averaging the results from 50 separate images. For each experimental group, there were three replicate readings. Besides percentage viability, the Vi-CELL® XR analyzer also gave counts of total cell titers and viable cell titers, from which the degree of cellular aggregation and clumping can also be assessed. There is a filter system with the Vi-CELL® XR analyzer that would exclude any cell clump above 70 μm in size. Cellular aggregates lesser than 70 μm in size are able to enter the counting chamber within the instrument, but the Vi-CELL® XR analyzer is able to discern the total number of cells within the smaller clumps through its imaging software. Hence the drop in cell titer over the time duration of 180 min would yield a quantitative measurement of the degree of cellular aggregation and clumping that take place in PBS in the presence and absence of calcium.
Statistical analysis of data
There were three replicates for each experimental group, and the results from each data set were expressed as mean ± standard deviations. Differences between data sets were assessed by the students t-test, with a value of p < 0.05 being considered significantly different.
Results
Effects of prolonged exposure of mesenchymal stem cells to phosphate buffered saline at 0 °C
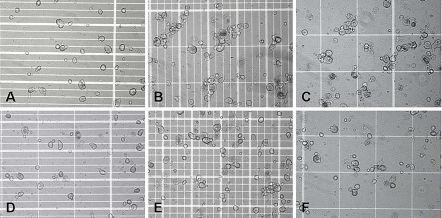
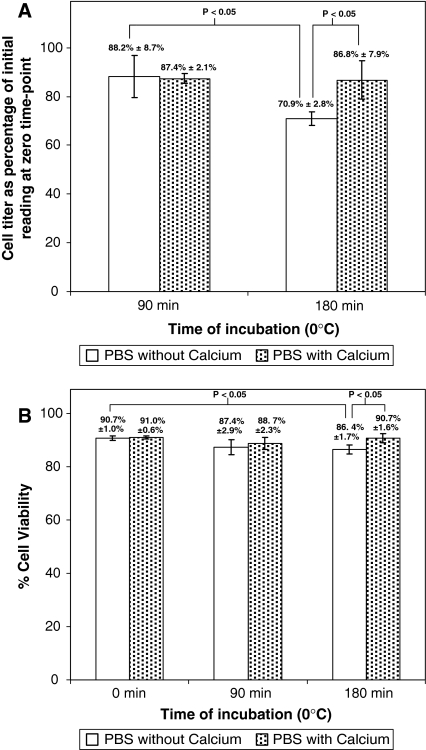
Substantial aggregation was observed in the MSC suspension that was kept on ice at 0 °C (Fig. 1), with a higher degree of aggregation occurring in calcium-free PBS (Fig. 1a–c) as compared to PBS with calcium (Fig. 1d–f). This was confirmed by cell titer measurements with the Vi-CELL® XR analyzer (Fig. 2a), which showed a significantly larger percentage drop in cell titer within calcium-free PBS, as compared to PBS with calcium, after 180 min of incubation on ice at 0 °C (70.9 ± 2.8% vs. 86.8 ± 7.9%, respectively, p < 0.05). At the 90 min time-point, there were statistically significant decreases (p < 0.05) in cell titer compared to the zero time-point, but the results with calcium-free PBS were not significantly different from PBS with calcium (88.2 ± 8.7% vs. 87.4 ± 2.1%, respectively, p > 0.05). Subsequently, cell viability assessment with the Vi-CELL® XR analyzer (Fig. 2b) showed that in the presence of calcium, there were no statistically significant changes (p > 0.05) in cell viability after 90 min (88.7 ± 2.3%) and 180 min (90.7 ± 1.6%) of incubation at 0 °C, as compared to the zero time-point (91.0 ± 0.6%). However, in the absence of calcium, the cell viability at the 180 time point (86.4 ± 1.7%) was significantly lower (p < 0.05) than at the zero time-point (90.7 ± 1.0%).
Fig. 1.
MSC incubated at 0 °C in a PBS without calcium after 0 min, b PBS without calcium after 90 min, c PBS without calcium after 180 min, d PBS with calcium after 0 min, e PBS with calcium after 90 min, f PBS with calcium after 180 min
Fig. 2.
MSC incubated at 0 °C were analyzed for cell viability and titer with the Vi-CELL® XR analyzer at the 0, 90 and 180 min time points. a cell titer after incubation for 90 and 180 min, expressed as a percentage of the initial reading at zero time point, b cell viability at the 0, 90 and 180 min time points
Effects of prolonged exposure of mesenchymal stem cells to phosphate buffered saline at 37 °C
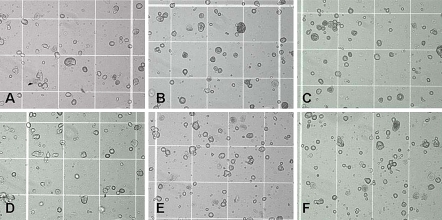
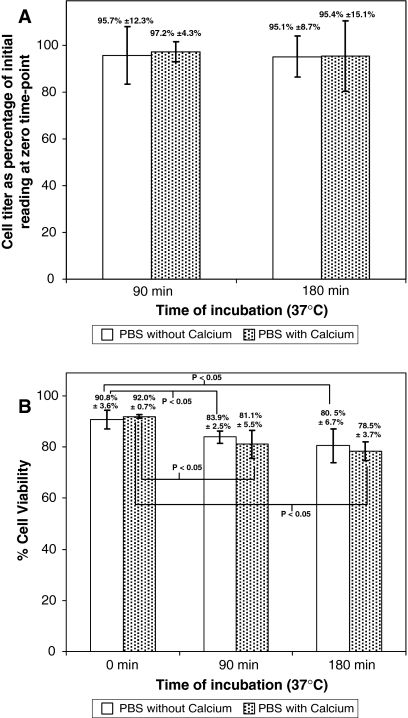
When MSC suspensions were incubated at physiological temperature of 37 °C, lesser degree of aggregation into cellular clumps (Fig. 3) was observed, as compared to that at 0 °C (Fig. 1). This was subsequently confirmed by cell titer measurements with the Vi-CELL® XR analyzer (Fig. 4a), which showed no significant changes in cell titer at the 0, 90 and 180 min time-points, in either the presence or absence of calcium. However, subsequent cell viability assessment (Fig. 4b) showed significant decreases (p < 0.05) in cell viability at the 90 and 180 min time-point compared to the zero time-point, in both the presence and absence of calcium.
Fig. 3.
MSC incubated at 37 °C in a PBS without calcium after 0 min, b PBS without calcium after 90 min, c PBS without calcium after 180 min, d PBS with calcium after 0 min, e PBS with calcium after 90 min, f PBS with calcium after 180 min
Fig. 4.
MSC incubated at 37 °C were analyzed for cell viability and titer with the Vi-CELL® XR analyzer at the 0, 90 and 180 min time points. a cell titer after incubation for 90 and 180 min, expressed as a percentage of the initial reading at zero time point, b cell viability at the 0, 90 and 180 min time points
Discussion
In the overwhelming majority of MSC transplantation studies on animal models and human clinical trials, there is always a significant time delay between cell preparation and actual cell delivery in situ. This time duration varies according to the actual clinical procedures being performed, any non-cell related procedural complications and the familiarity and skill-set of the clinician or clinical team performing the procedure. If this duration is prolonged, there could possibly be an adverse effect on the MSC, which are suspended in protein-free and nutrient-poor buffered saline solution at high titers. To mitigate against nutrient starvation and serum deprivation in saline, the usual practice is to slow down cellular metabolism by exposure to low temperature i.e. keeping on ice at 0 °C. In a previous study by Muraki et al. (2006), it was demonstrated that after 24 h incubation in PBS at 4 °C, 81% of MSC remained viable, as compared to 70% and 62% viability upon incubation at 24 and 37 °C, respectively. Subsequently, Pal et al. (2008) also reported that MSC retained higher viability at 4 °C compared to physiological and room temperature (37 and 22 °C, respectively). Nevertheless, what has not yet been investigated is the effect of temperature on cellular aggregation and its potential relation to viability.
As expected, the results do indeed show that MSC viability in protein-free and nutrient-poor saline is best maintained at low temperatures, most probably due to a slower cellular metabolism at 0 °C compared to 37 °C. This is consistent with the previous findings of Muraki et al. (2006) and Pal et al. (2008). Nevertheless, this study analyzed cell viability only with the Trypan blue exclusion assay because other techniques such as the MTT (Mosmann 1983) and WST-1 assay (Hamasaki et al. 1996) are deemed to be unsuitable. MTT assay requires dye extraction from stained adherent cells by dimethyl sulfoxide (Mosmann 1983), and is therefore clearly unsuited for characterizing MSC in free suspension. The application of the WST-1 assay would be hindered by two major experimental artifacts encountered in this study. Firstly, the presence of cellular aggregation and clumping would mean that not all viable cells would be uniformly exposed to the dye, nor would there be uniform secretion of the blue formazan product by all viable cells. In particular, viable cells located within the interior of clumps and aggregates would be less exposed to the dye, and consequently secrete less of the blue formazan product. Secondly, for cells exposed to 0 °C for either 90 or 180 min, and then suddenly incubated at 37 °C for the WST-1 assay, there is probably a ‘lag period’ whereby the cellular metabolism needs to switch from its suspended animation at 0 °C to ‘full gear’ at 37 °C.
The results showed a much higher degree of cellular aggregation and clumping at 0 °C compared to 37 °C. This is surprising as cellular adhesion function is normally expected to be active only at physiological temperature. Previously, Moscona (1961) showed that aggregation of chick embryonic cells could only occur at temperatures above 15 °C. In another study by Owen et al. (1978) on trypsin-dissociated HeLa cells, it was shown that reduction of temperature from 37 to 25 °C significantly reduced cellular aggregation. Nevertheless these studies were conducted in culture media and did not examine cellular aggregation at the much lower temperature of 0 °C in nutrient-deficient saline.
Additionally, it was observed that a higher degree of MSC aggregation occurred under calcium-free conditions at 0 °C, although there were no significant differences in cellular aggregation within calcium-free and calcium-containing PBS at 37 °C. Again, these results are unexpected, because calcium ions in solution are known to facilitate cellular adhesion. In fact, enzyme-free dissociation of cell monolayers works on the principle of removing free calcium ions in solution through chelating agents like EDTA (1999).
It is difficult to hypothesize on the mechanism by which MSC display a higher degree of cellular aggregation at low temperature under calcium-free conditions. Nevertheless, it is speculated this could either be due to changes in plasma membrane fluidity at low temperatures (Dynlacht and Fox 1992), or changes in the electrostatic and conformational properties of surface proteins under calcium-free conditions. It is well-known that the physiological function of various proteins and enzymes are altered upon association with free calcium ions, which induces changes in their electrostatic properties and conformational structure (Bo and Pawliszyn 2006). How exactly this relates to cellular aggregation of MSC observed in this study, certainly warrants further investigation.
The aggregation of MSC observed in this study could in turn have adverse consequences in transplantation therapy. The presence of cellular clumps could pose an embolic hazard, and may also clog-up injection or infusion catheters utilized for cell delivery during cell delivery procedures. Moreover, one could speculate that the delivery of cell clumps in situ as opposed to a single cell suspension, might adversely affect the efficacy of transplantation therapy. Additionally, it may also be worthwhile to investigate whether the supplementation of surfactants could inhibit cellular aggregation in saline, but addition of any unknown agent to the suspension media will need to be stringently evaluated in terms of its effect on the cells themselves and also on the recipient.
References
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B (2007) Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 167(10):989–997. doi:10.1001/archinte.167.10.989 [DOI] [PubMed]
- Bo T, Pawliszyn J (2006) Role of calcium binding in protein structural changes and phospholipid–protein interactions studied by capillary isoelectric focusing with whole column imaging detection. Anal Chim Acta 559:1–8. doi:10.1016/j.aca.2005.11.047 [DOI] [PubMed]
- Dezawa M (2008) Systematic neuronal and muscle induction systems in bone marrow stromal cells: the potential for tissue reconstruction in neurodegenerative and muscle degenerative diseases. Med Mol Morphol 41(1):14–19. doi:10.1007/s00795-007-0389-0 [DOI] [PubMed]
- Dynlacht JR, Fox MH (1992) Heat-induced changes in the membrane fluidity of Chinese hamster ovary cells measured by flow cytometry. Radiat Res 130(1):48–54. doi:10.2307/3578478 [DOI] [PubMed]
- Grauss RW, Winter EM, van Tuyn J, Pijnappels DA, Steijn RV, Hogers B, van der Geest RJ, de Vries AA, Steendijk P, van der Laarse A, Gittenberger-de Groot AC, Schalij MJ, Atsma DE (2007) Mesenchymal stem cells from ischemic heart disease patients improve left ventricular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol 293(4):H2438–H2447. doi:10.1152/ajpheart.00365.2007 [DOI] [PubMed]
- Hamasaki K, Kogure K, Ohwada K (1996) A biological method for the quantitative measurement of tetrodotoxin (TTX): tissue culture bioassay in combination with a water-soluble tetrazolium salt. Toxicon 34(4):490–495. doi:10.1016/0041-0101(95)00151-4 [DOI] [PubMed]
- Hidalgo C, Donoso P (2008) Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal 10(7):1275–1312. doi:10.1089/ars.2007.1886 [DOI] [PubMed]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M (1987) Calcium-dependent cell–cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol 105:2501–2510. doi:10.1083/jcb.105.6.2501 [DOI] [PMC free article] [PubMed]
- Huang C, Miller RT (2007) The calcium-sensing receptor and its interacting proteins. J Cell Mol Med 11(5):923–934. doi:10.1111/j.1582-4934.2007.00114.x [DOI] [PMC free article] [PubMed]
- Jorgensen C, Djouad F, Bouffi C, Mrugala D, Noël D (2008) Multipotent mesenchymal stromal cells in articular diseases. Best Pract Res Clin Rheumatol 22(2):269–284. doi:10.1016/j.berh.2008.01.005 [DOI] [PubMed]
- Lanzoni G, Roda G, Belluzzi A, Roda E, Bagnara GP (2008) Inflammatory bowel disease: moving toward a stem cell-based therapy. World J Gastroenterol 14(29):4616–4626. doi:10.3748/wjg.14.4616 [DOI] [PMC free article] [PubMed]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O, Developmental Committee of the European Group for Blood, Marrow Transplantation (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371(9624):1579–1586. doi:10.1016/S0140-6736(08)60690-X [DOI] [PubMed]
- Moscona A (1961) Effect of temperature on adhesion to glass and histogenetic cohesion of dissociated cells. Nature 190:408–409. doi:10.1038/190408a0 [DOI] [PubMed]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4 [DOI] [PubMed]
- Muraki K, Hirose M, Kotobuki N, Kato Y, Machida H, Takakura Y, Ohgushi H (2006) Assessment of viability and osteogenic ability of human mesenchymal stem cells after being stored in suspension for clinical transplantation. Tissue Eng 12(6):1711–1719. doi:10.1089/ten.2006.12.1711 [DOI] [PubMed]
- Nandoe Tewarie RD, Hurtado A, Levi AD, Grotenhuis JA, Oudega M (2006) Bone marrow stromal cells for repair of the spinal cord: towards clinical application. Cell Transplant 15(7):563–577. doi:10.3727/000000006783981602 [DOI] [PubMed]
- Owen E, Clifford J, Marson A (1978) The effects of surfactants on cell aggregation. J Cell Sci 32:363–376 [DOI] [PubMed]
- Pal R, Hanwate M, Totey SM (2008) Effect of holding time, temperature and different parenteral solutions on viability and functionality of adult bone marrow-derived mesenchymal stem cells before transplantation. J Tissue Eng Regen Med 2(7):436–444 [DOI] [PubMed]
- Pountos I, Corscadden D, Emery P, Giannoudis PV (2007) Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury 38 (suppl 4):S23–S33. doi:10.1016/S0020-1383(08)70006-8 [DOI] [PubMed]
- Salzmann J (1999) Cultured fibroblast or epithelial cell dissociation method using sulfated polysaccharide and chelator. US Patent 5906939, 25 May 1999. http://www.freepatentsonline.com/5906939.html. Accessed 30 Oct 2008
- Schäfer R, Northoff H (2008) Cardioprotection and cardiac regeneration by mesenchymal stem cells. Panminerva Med 50(1):31–39 [PubMed]
- Sensebé L (2008) Clinical grade production of mesenchymal stem cells. Biomed Mater Eng 18 (suppl 1):S3–S10 [PubMed]
- Siddappa R, Fernandes H, Liu J, van Blitterswijk C, de Boer J (2007) The response of human mesenchymal stem cells to osteogenic signals and its impact on bone tissue engineering. Curr Stem Cell Res Ther 2(3):209–220. doi:10.2174/157488807781696267 [DOI] [PubMed]
- Urbán VS, Kiss J, Kovács J, Gócza E, Vas V, Monostori E, Uher F (2008) Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells 26(1):244–253. doi:10.1634/stemcells.2007-0267 [DOI] [PubMed]