Abstract
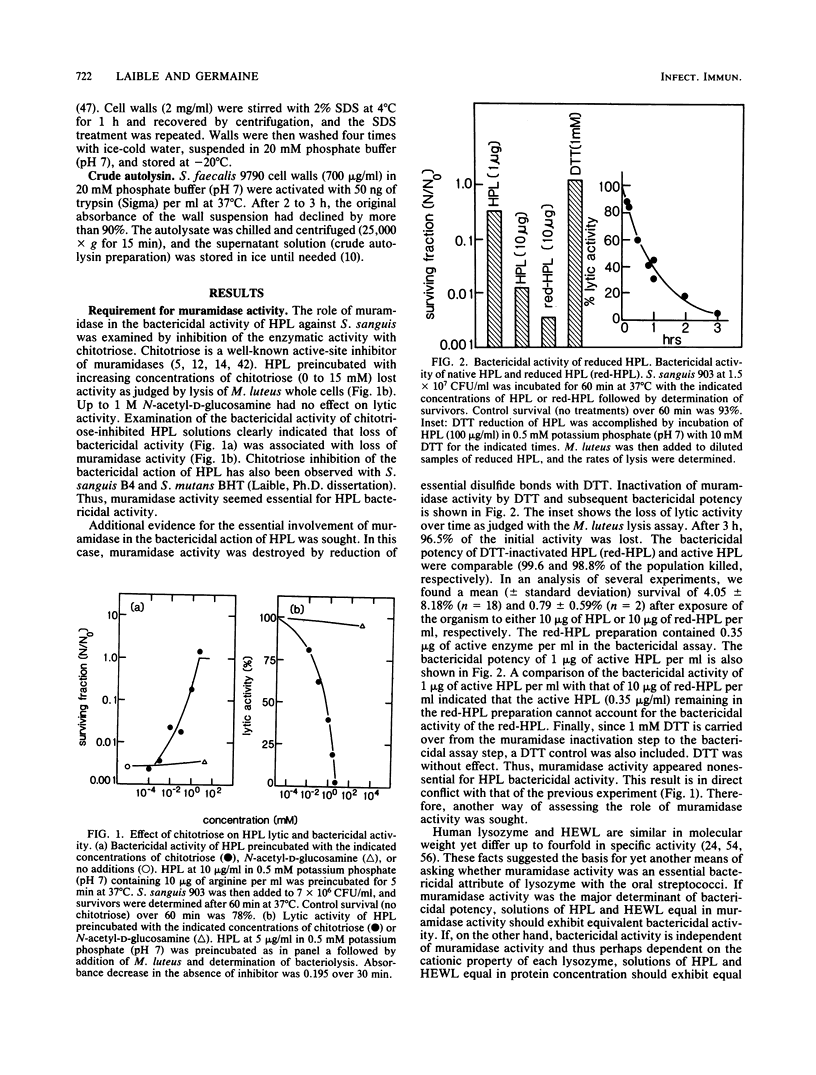
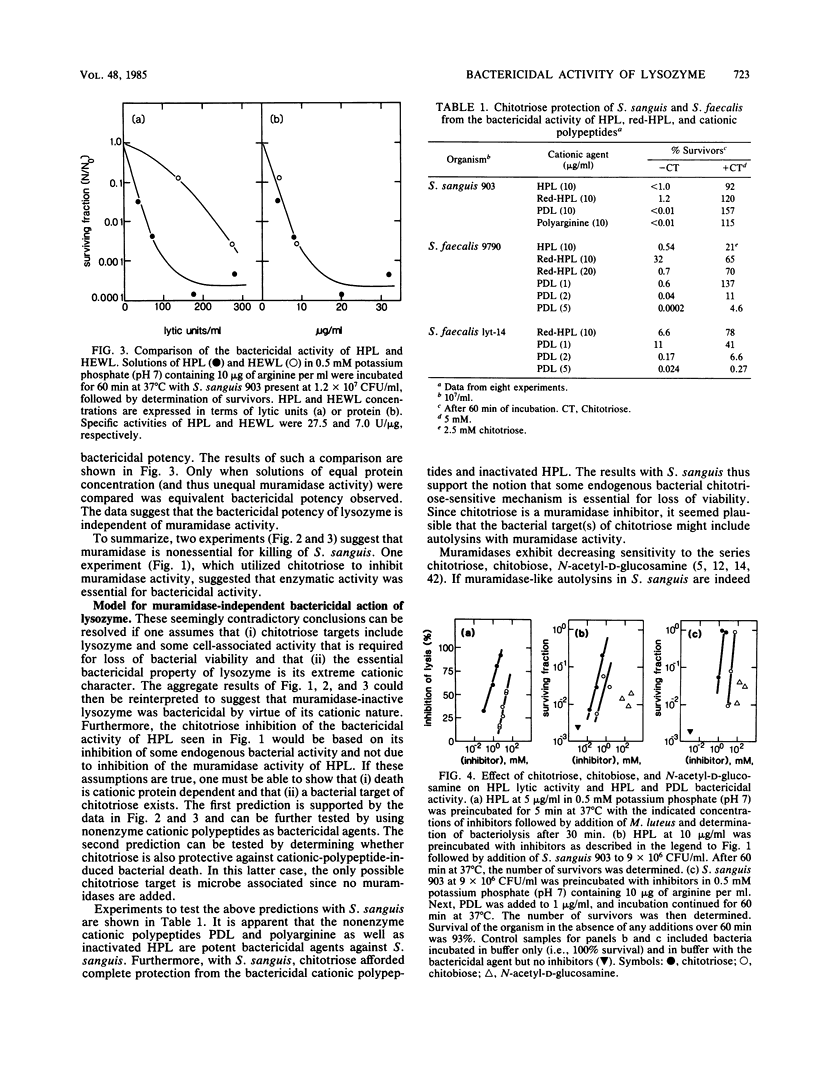
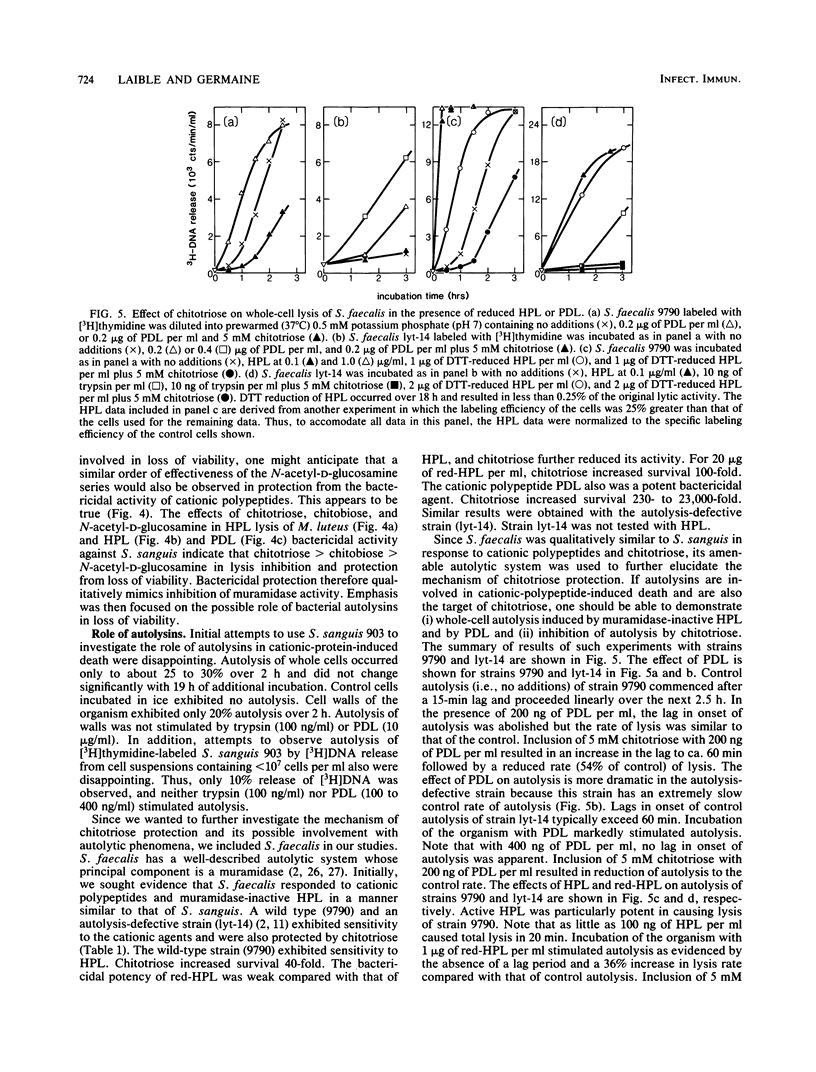
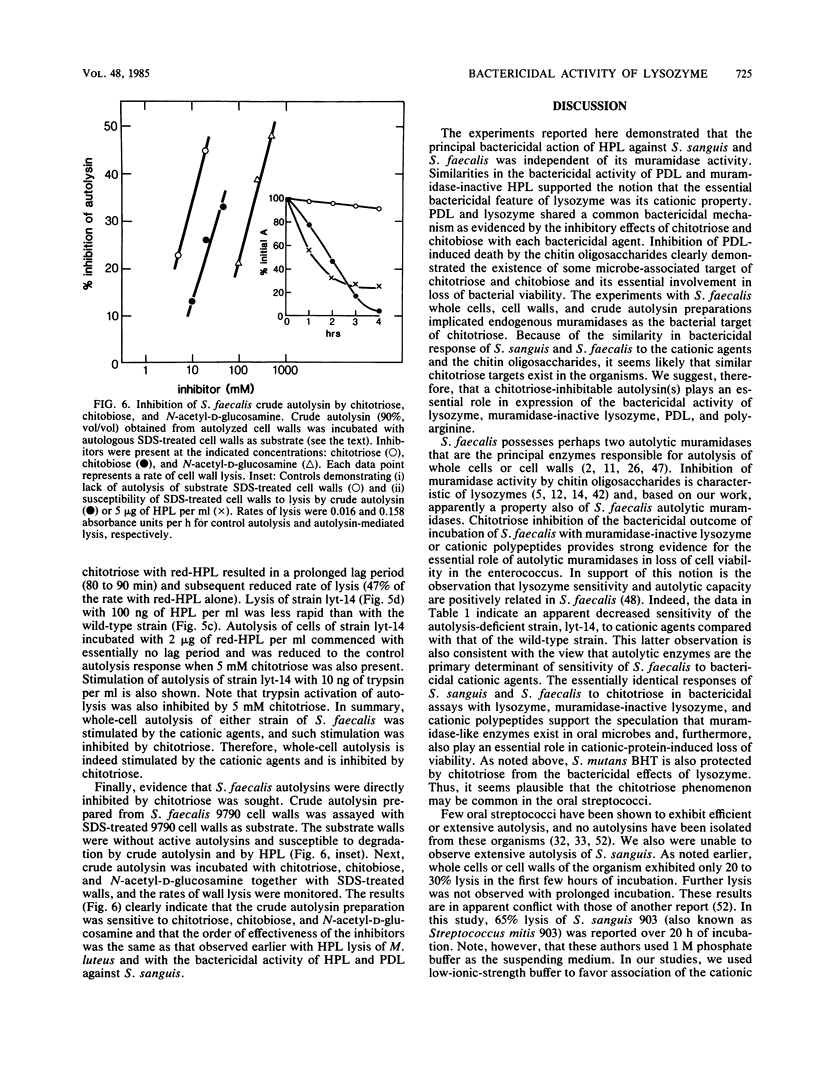
The basis of the bactericidal activity of human lysozyme against Streptococcus sanguis was studied. Experiments were designed to evaluate the role of lysozyme muramidase activity in its bactericidal potency. Inactivation of the muramidase activity of lysozyme was achieved by reduction of essential disulfides with dithiothreitol (DTT) or by incubation with the chitin oligosaccharides chitotriose and chitobiose. Muramidase-inactive lysozyme, prepared by reduction with DTT, was equal in bactericidal potency to native lysozyme. Solutions of native chicken egg white lysozyme and human lysozyme exhibited equal bactericidal potency yet differed ca. fourfold with respect to lytic (muramidase) activity. The above results suggested that the bactericidal activity of lysozyme is not dependent upon muramidase activity. Chitotriose and chitobiose were found to inhibit both lytic and bactericidal activities of lysozyme. The bactericidal activity of muramidase-inactive lysozyme (reduction with DTT) was also inhibited by chitotriose and chitobiose. Further investigations demonstrated that chitotriose and chitobiose were also potent inhibitors of the bactericidal activity of the cationic homopolypeptides poly-L-arginine and poly-D-lysine. These latter results suggested that the essential bactericidal property of lysozyme was its extreme cationic nature and that some bacterial endogenous activities, inhibitable by chitotriose and chitobiose, were essential for expression of the bactericidal activity of either native or muramidase-inactive lysozyme or of the cationic homopolypeptides. Experiments with Streptococcus faecalis whole cells, cell walls, and crude autolysin preparations implicated endogenous autolytic muramidases as the bacterial targets of chitotriose and chitobiose. The essentially identical responses of S. sanguis and S. faecalis to chitotriose in bactericidal assays with muramidase-inactive lysozyme and polylysine suggested that muramidase-like enzymes exist in S. sanguis and, furthermore, play an essential role in cationic protein-induced loss of viability of the oral microbe.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. O-acetyl groups in the cell wall of Streptococcus faecalis. J Biol Chem. 1958 Feb;230(2):949–959. [PubMed] [Google Scholar]
- Barrett J. F., Schramm V. L., Shockman G. D. Hydrolysis of soluble, linear, un-cross-linked peptidoglycans by endogenous bacterial N-acetylmuramoylhydrolases. J Bacteriol. 1984 Aug;159(2):520–526. doi: 10.1128/jb.159.2.520-526.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen W. H. Defense mechanisms in the mouth and their possible role in the prevention of dental caries: a review. J Oral Pathol. 1974;3(6):266–278. doi: 10.1111/j.1600-0714.1974.tb01721.x. [DOI] [PubMed] [Google Scholar]
- Chipman D. M., Grisaro V., Sharon N. The binding of oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid to lysozyme. The specificity of binding subsites. J Biol Chem. 1967 Oct 10;242(19):4388–4394. [PubMed] [Google Scholar]
- Cho M. I., Holt S. C., Iacono V. J., Pollock J. J. Effects of lysozyme and inorganic anions on the morphology of Streptococcus mutans BHT: electron microscopic examination. J Bacteriol. 1982 Sep;151(3):1498–1507. doi: 10.1128/jb.151.3.1498-1507.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. F., Hsu S. D., Baum B. J., Bowen W. H., Sierra L. I., Aquirre M., Gillespie G. Specific and nonspecific immune factors in dental plaque fluid and saliva from young and old populations. Infect Immun. 1981 Mar;31(3):998–1002. doi: 10.1128/iai.31.3.998-1002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover M. J., Thompson J. S., Shockman G. D. Autolytic enzyme of Streptococcus faecalis: release of soluble enzyme from cell walls. Biochem Biophys Res Commun. 1966 Jun 13;23(5):713–719. doi: 10.1016/0006-291x(66)90459-1. [DOI] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist F. W., Jao L., Raftery M. On the binding of chitin oligosaccharides to lysozyme. Proc Natl Acad Sci U S A. 1966 Jul;56(1):26–30. doi: 10.1073/pnas.56.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamizo T., Torikata T., Kuhara S., Hayashi K. Human lysozyme-catalyzed reaction of chitooligosaccharides. J Biochem. 1982 Sep;92(3):709–716. doi: 10.1093/oxfordjournals.jbchem.a133982. [DOI] [PubMed] [Google Scholar]
- Gennaro R., Dolzani L., Romeo D. Potency of bactericidal proteins purified from the large granules of bovine neutrophils. Infect Immun. 1983 May;40(2):684–690. doi: 10.1128/iai.40.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli S., Tomasz A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J Bacteriol. 1984 Jun;158(3):1188–1190. doi: 10.1128/jb.158.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Bactericidal action of histone. J Exp Med. 1958 Dec 1;108(6):925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L. V., Gooder H. Specificity of the autolysin of Streptococcus (Diplococcus) pneumoniae. J Bacteriol. 1974 Feb;117(2):796–804. doi: 10.1128/jb.117.2.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Iacono V. J., Boldt P. R., MacKay B. J., Cho M. I., Pollock J. J. Lytic sensitivity of Actinobacillus actinomycetemcomitans Y4 to lysozyme. Infect Immun. 1983 May;40(2):773–784. doi: 10.1128/iai.40.2.773-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., DiRienzo S., Pollock J. J. Selective antibacterial properties of lysozyme for oral microorganisms. Infect Immun. 1980 Aug;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., Grossbard B. L., DiRienzo S., Pollock J. J. Lysozyme binding by a polyglycerol phosphate polymer of the oral bacterium Streptococcus mutans BHT. Arch Oral Biol. 1982;27(4):347–354. doi: 10.1016/0003-9969(82)90165-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Nakamura R., Watanabe T., Tsunemitsu A. Purification and amino acid analysis of human parotid saliva lysozyme. J Dent Res. 1970 Sep-Oct;49(5):1104–1110. doi: 10.1177/00220345700490051801. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Watanabe T., Tsunemitsu A., Fukui Z., Moriyama T. Lysis of streptococci by lysozyme from human parotid saliva and sodium lauryl sulfate. J Dent Res. 1971 Nov-Dec;50(6):1688–1688. doi: 10.1177/00220345710500066201. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- Lahav M., Ginsburg I. Effect of leukocyte hydrolases on bacteria. X. The role played by leukocyte factors, cationic polyelectrolytes, and by membrane-damaging agents in the lysis of Staphylococcus aureus: relation to chronic inflammatory processes. Inflammation. 1977 Jun;2(2):165–177. doi: 10.1007/BF00918678. [DOI] [PubMed] [Google Scholar]
- Laible N. J., Germaine G. R. Adsorption of lysozyme from human whole saliva by Streptococcus sanguis 903 and other oral microorganisms. Infect Immun. 1982 Apr;36(1):148–159. doi: 10.1128/iai.36.1.148-159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Germaine G. R. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect Immun. 1981 Mar;31(3):935–941. doi: 10.1128/iai.31.3.935-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder L., Andersson C., Sund M. L., Shockman G. D. Protoplast formation and localization of enzymes in Streptococcus mitis. Infect Immun. 1983 Jun;40(3):1146–1154. doi: 10.1128/iai.40.3.1146-1154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder L. Extraction of cell-bound hyaluronidase and aminopeptidase from Streptococcus mitis, ATCC 903. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):593–601. doi: 10.1111/j.1699-0463.1974.tb00225.x. [DOI] [PubMed] [Google Scholar]
- MacFarlane T. W., Mason D. K. Changes in the oral flora in Sjögren's syndrome. J Clin Pathol. 1974 May;27(5):416–419. doi: 10.1136/jcp.27.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay B. J., Pollock J. J., Iacono V. J., Baum B. J. Isolation of milligram quantities of a group of histidine-rich polypeptides from human parotid saliva. Infect Immun. 1984 Jun;44(3):688–694. doi: 10.1128/iai.44.3.688-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik D. Sorption of Streptococci to glass: Effects of macromolecular solutes. Acta Pathol Microbiol Scand B. 1977 Feb;85B(1):47–53. [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupley J. A., Butler L., Gerring M., Hartdegen F. J., Pecoraro R. Studies on the enzymic activity of lysozyme, 3. The binding of saccharides. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1088–1095. doi: 10.1073/pnas.57.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957 Dec;21(4):273–294. doi: 10.1128/br.21.4.273-294.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPITZNAGEL J. K. Antibacterial effects associated with changes in bacterial cytology produced by cationic polypeptides. J Exp Med. 1961 Dec 1;114:1079–1091. doi: 10.1084/jem.114.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M. N., Lahav M., Ginsburg I. Effect of leukocyte hydrolases on bacteria. IX. The release of lipoteichoic acid from group A streptococci and from Strep. mutans by leukocyte extracts and by lysozyme: relation to tissue damage in inflammatory sites. Inflammation. 1977 Jun;2(2):151–164. doi: 10.1007/BF00918677. [DOI] [PubMed] [Google Scholar]
- Sela M. N., Ofek I., Lahav M., Ginsburg I. The effect of leukocyte hydrolases on bacteria. XI. Lysis by leukocyte extracts and by myeloperoxidase of a Staphylococcus aureus mutant which is deficient in teichoic acid, and the inhibition of bacteriolysis by lipoteichoic acid. Proc Soc Exp Biol Med. 1978 Oct;159(1):126–130. doi: 10.3181/00379727-159-40297. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Autolysis in strains of viridans streptococci. J Gen Microbiol. 1976 Sep;96(1):87–94. doi: 10.1099/00221287-96-1-87. [DOI] [PubMed] [Google Scholar]
- Tortosa M., Cho M. I., Wilkens T. J., Iacono V. J., Pollock J. J. Bacteriolysis of Veillonella alcalescens by lysozyme and inorganic anions present in saliva. Infect Immun. 1981 Jun;32(3):1261–1273. doi: 10.1128/iai.32.3.1261-1273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasstrand E. N., Jensen H. B. Affinity chromatography of human saliva lysozyme and effect of pH and ionic strength on lytic activity. Scand J Dent Res. 1980 Jun;88(3):219–228. doi: 10.1111/j.1600-0722.1980.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Wecke J., Lahav M., Ginsburg I., Giesbrecht P. Cell wall degradation of Staphylococcus aureus by lysozyme. Arch Microbiol. 1982 Mar;131(2):116–123. doi: 10.1007/BF01053992. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Tobiishi M., Tsuru D. Affinity chromatographic purification of human lysozyme, with special reference to human leukemia lysozyme. J Biochem. 1976 Oct;80(4):703–709. doi: 10.1093/oxfordjournals.jbchem.a131329. [DOI] [PubMed] [Google Scholar]


