Abstract
The ATM kinase has previously been shown to respond to the DNA damage induced by reoxygenation following hypoxia by initiating a Chk 2-dependent cell cycle arrest in the G2 phase. Here we show that ATM is both phosphorylated and active during exposure to hypoxia in the absence of DNA damage, detectable by either comet assay or 53BP1 focus formation. Hypoxia-induced activation of ATM correlates with oxygen concentrations low enough to cause a replication arrest and is entirely independent of hypoxia-inducible factor 1 status. In contrast to damage-activated ATM, hypoxia-activated ATM does not form nuclear foci but is instead diffuse throughout the nucleus. The hypoxia-induced activity of both ATM and the related kinase ATR is independent of NBS1 and MRE11, indicating that the MRN complex does not mediate the DNA damage response to hypoxia. However, the mediator MDC1 is required for efficient activation of Kap1 by hypoxia-induced ATM, indicating that similarly to the DNA damage response, there is a requirement for MDC1 to amplify the ATM response to hypoxia. However, under hypoxic conditions, MDC1 does not recruit BRCA1/53BP1 or RNF8 activity. Our findings clearly demonstrate that there are alternate mechanisms for activating ATM that are both stress-specific and independent of the presence of DNA breaks.
Sensing and responding to DNA damage is crucial for maintaining cellular homeostasis and preventing the development of cancer. The cellular response to DNA damage can be divided into three parts: sensing the type of damage, activating DNA damage signaling pathways, and repairing the damage. The proteins involved in these three processes act as sensors, transducers, and effectors of the DNA damage response (41, 54). The ATM kinase is one of the key transducers of the DNA double-stranded break response. ATM has been identified as the product mutated or inactivated in ataxia telangiectasia (AT) patients and belongs to the phosphatidyl inositol-3-kinase-like kinase (PIKK) family, together with its family members ATR, DNA-PK, and mTOR. Elegant studies have demonstrated that ATM is present as an inactive dimer that undergoes rapid autophosphorylation on serine 1981 after DNA damage (4) and is recruited to sites of DNA strand breaks (2). Various proteins, such as the MRE11-Rad50-NBS1 (MRN) complex (49) and MDC1 (20, 43), have been described to be essential for the efficient response of ATM to DNA damage. Activated ATM phosphorylates downstream targets, such as p53, Chk2, and BRCA 1 and 2, that are involved in DNA repair, cell cycle control, and apoptosis.
Low-oxygen tension or hypoxia is a common feature in all solid tumors (15). It is strongly associated with tumor development, malignant progression, metastatic outgrowth, and resistance to therapy and is considered an independent prognostic indicator for poor patient prognosis in various tumor types. Tumor hypoxia results from an imbalance between the cellular oxygen consumption rate of cells and the delivery of oxygen to cells (50). Interestingly, the level of tumor hypoxia varies between tumors of the same histology. Hypoxia can result from the consumption of oxygen by successive layers of tumor cells distal to the lumen of the blood vessel (46). Due to the nature of tumor vasculature, hypoxic regions can also result from temporary vessel closure. Hypoxia itself does not induce detectable DNA damage, but significant levels of damage have been observed in response to reoxygenation that occurs when vessels reopen (25). This reoxygenation-induced damage is thought to result from the production of reactive oxygen species, as free radical scavengers prevent the activation of the DNA damage response under these conditions (26). Although hypoxia does not induce a DNA damage response, Chk2 is phosphorylated and activated in an ATM-dependent manner (16, 19). As with the S-phase arrest induced by DNA damage, hypoxia alone induces a rapid S-phase arrest, although the underlying mechanism of this arrest is poorly defined. However, it is clear that oxygen levels of 0.5% or below are needed to induce this arrest, whereas oxygen concentrations above 0.5% have little effect on proliferation (18, 22, 25). Clinical data indicate that oxygen levels within a tumor reach levels low enough to induce an S-phase arrest, contributing to an increase in resistance to chemotherapeutic agents which target rapidly proliferating cells (51).
Recently it was proposed that the DNA damage response acted as a barrier to tumorigenesis and that hypoxia may also contribute to this barrier (5, 21). We and others have demonstrated that DNA damage signaling pathways are initiated in response to hypoxia and hypoxia-reoxygenation (16, 19, 47). For example, the ATR kinase is active under hypoxic conditions and phosphorylates numerous targets, including p53 and Chk 1 (25). Previous studies also indicated that ATM might play a more significant role during the reoxygenation phase of hypoxia-reoxygenation due to the induction of DNA damage by reactive oxygen species. In addition, ATM is required to maintain phosphorylation of p53 and initiates a cell cycle arrest in the G2 phase after reoxygenation (16, 26). In this study we investigate if ATM is both phosphorylated and active during hypoxia in the absence of reoxygenation and investigate how the mechanism of hypoxia-induced ATM activation differs from DNA damage-inducing stress.
MATERIALS AND METHODS
Cell lines.
GM0536, GM1526, and Seckel lymphoblastoid cell line (LCL) cells were grown in RPMI medium supplemented with 15% fetal calf serum (FCS). Both GM0536 (ATM+/+) and GM1526 (ATM−/−) are Epstein-Barr virus-immortalized LCLs (11). The MO59J, MO59K, and MJ-L24 cells lines were received from Ben Chen and were grown in Dulbecco modified Eagle medium (DMEM) with 10% FCS. HCT116 cells (wild type and ATR−/flox), mouse embryo fibroblasts (MEFs) (MDC1+/+ and MDC1−/−), U20S cells, and RKO cells were maintained in DMEM with 10% FCS. pEBS (ATM−/−) and YZ3 (ATM+/+) fibroblasts were grown in DMEM with 10% FCS supplemented with 100 μM hygromycin (Sigma-Aldrich) (55). F02/98 hTERT (Seckel) and 1BR hTERT were a generous gift from Penny Jeggo (University of Sussex). A549 and A549 [rho0] cells were a gift from Darren Magda (PCYC, Inc.) All cell lines were mycoplasma tested and found to be negative. The inhibitors used were, for ATM, 2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one (Calbiochem number 118500) and for DNA-PK, 2-(morpholin-4-yl)-benzo[h]chromen-4-one (Calbiochem number 260961).
Hypoxia treatment.
All hypoxia treatments were carried out in a Bactron II anaerobic chamber (Shell Labs) at an oxygen concentration of <0.02%, unless indicated otherwise. Unless specified otherwise, cells were plated on glass dishes. Where no period of reoxygenation is indicated, cells were harvested inside the chamber with equilibrated solutions.
Immunoblotting.
Cells were lysed in UTB (9 M urea, 75 mM Tris-HCl, pH 7.5, and 0.15 M β-mercaptoethanol) and sonicated briefly. The antibodies used were anti-ATM MAT3 (Sigma), anti-ATM protein kinase S1981 (Rockland), anti-ATM, phospho-S1981 (Upstate), ATR (Santa Cruz), DNA-PKcs phospho T-2609 (Abcam), hypoxia-inducible factor 1α (HIF-1α) (BD Transduction Laboratories), Kap-1 and Phospho Kap-1 (S824) (Bethyl Laboratories), Phospho-p53 (S15) (Cell Signal), GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Fitzgerald), MRE11 (Novus Biologicals), p53BP1 (Bethyl), histone 4 (Cell Signaling), H4me2K20 (Upstate/Millipore), and α-tubulin (Research Diagnostics, Inc). Proteins were detected by enhanced chemiluminescence using horse radish peroxidase secondary antibody with the ECL Plus substrate (Promega) or Alexa Fluor 680 secondary antibodies (Invitrogen) and Odyssey infrared imaging technology.
Immunofluorescence.
Cells were grown on coverslips at a density of 5 × 104 per slide. After treatment, cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min. Irradiated cells were exposed to 6 Gy infrared and fixed after 4 h of incubation under normal conditions. The chamber slides were washed three times with PBS and permeabilized in 0.25% Triton-X in PBS for 10 min. After being washed twice with PBS, slides were incubated in primary antibody 1:500 in 1% bovine serum albumin (BSA) in PBS for 1 h in a humidified chamber at 37°C. Cells were rinsed three times with PBS, and samples were incubated in secondary antibody Alexa Fluor 488 green and/or Alexa Fluor 594 red (Invitrogen) in 1% BSA in PBS for 1 h in a humidified chamber at 37°C. Antibodies used for immunofluorescence were ATM-S1981 (Upstate), ATM (Sigma-Aldrich), CENP-F (Abcam), γ-H2AX-S139 (Upstate), FK2 (Santa Cruz), and BRCA1 (Santa Cruz). MDC1 and 53BP1 antibodies were a kind gift from Grant Stewart, and replication protein A 32 (RPA32) was from Kai Rothkamm. Cells were mounted using Vectashield (VectorLabs) HardSet mounting media with DAPI (4′,6-diamidino-2-phenylindole). The microscopes used were a Radiance Bio-Rad confocal microscope and a Nikon 90i epifluorescence microscope.
Biochemical fractionation.
Biochemical fractionation was carried out as described in reference 2. In brief, hypoxia-, neocarzinostatin (NCS)-, or mock-treated cells were washed with PBS. Cells were fractionated in three consecutive steps with two buffers (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA) containing 0.2% Nonidet P-40 (for fractions I and II) or 0.5% Nonidet P-40 (for fraction III), supplemented with protease inhibitors (complete EDTA free; Roche) and phosphatase inhibitors (Phospho-Stop; Roche). The pellets were lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer (fraction IV). Equal aliquots of each fraction were separated on 6% sodium dodecyl sulfategels, and Western blotting was carried out.
RESULTS
ATM is phosphorylated and active during hypoxia/reoxygenation.
GM0536 (ATM+/+) cells were exposed to various oxygen concentrations, the HIF-inducing agent CoCl2, and the DNA strand-breaking agent NCS. Cells were then harvested without reoxygenation in a hypoxia chamber, and Western blotting was carried out for the proteins or phospho-proteins indicated. Phosphorylation of ATM on serine 1981 was observed only at 0.02% oxygen or in response to NCS treatment (Fig. 1A). Phosphorylation of p53 on serine 15 by hypoxia and NCS paralleled the phosphorylation pattern of ATM (25). This oxygen dependency of the phosphorylation of ATM indicates that there is no requirement for HIF-1 as part of this response, since HIF-1α is induced by all hypoxic levels and by CoCl2, but only low levels of oxygen were able to induce ATM phosphorylation. However, to fully investigate the possibility that ATM phosphorylation is a consequence of hypoxia-induced HIF-1 signaling, we have examined ATM phosphorylation in HIF-1α knockout cells in comparison with control cells (13) (Fig. 1B). HCT116 and HCT116HIF-1α−/− were exposed to hypoxia or NCS, and Western blotting was carried out for total ATM, ATM-S1981, and HIF-1α. There was no significant difference in the levels of ATM-S1981 in response to hypoxia in the two cell lines, indicating that HIF-1 signaling does not play a role in the ATM-mediated response to hypoxia. It has previously been shown that Chk2 is phosphorylated by ATM during both hypoxia and reoxygenation (16, 19). We show here that ATM remains phosphorylated after hypoxia and during subsequent reoxygenation for at least 60 min and attribute this to the significant level of DNA damage induced upon reoxygenation (Fig. 1C). We have further investigated the hypoxia-induced activity of ATM by considering other downstream targets. We found that both Kap 1 and DNA-PKcs are phosphorylated in response to hypoxia and that this occurs in an ATM-dependent manner. Figure 1D shows that when a chemical inhibitor of ATM is added to cells exposed to hypoxia, the levels of Kap 1-S824 are significantly diminished, indicating that this phosphorylation event is ATM dependent. Cells lacking ATM also show reduced phosphorylation of DNA-PKcs-T2609 (Fig. 1E). Interestingly, we found that DNA-PKcs was not phosphorylated at residue S2056 during exposure to hypoxia (data not shown). This phosphorylation event is associated with the autophosphorylation activity of DNA-PKcs (14, 37). It should be noted that using either the chemical inhibition or genetic loss of ATM, there is some residual phosphorylation of ATM target proteins. This can be attributed to either the failure of the chemical to inhibit ATM completely or the activity of another kinase, such as ATR. Most importantly, these findings indicate that hypoxia-induced phosphorylation of ATM correlates with activity.
FIG. 1.
ATM is phosphorylated and active during both hypoxia and reoxygenation. GM0536 cells were exposed to the levels of hypoxia shown, to hypoxia-mimetic CoCl2 for 6 h, or, as a positive control, to the DNA-damaging agent NCS (5 ng/ml) for 10 min. Shown is a Western blot for the proteins or protein modifications indicated. (A) GAPDH is shown as a loading control. (B) HCT116wt and HCT116HIF-1α−/− cells were exposed to hypoxia (Hyp) for 16 h or NCS for 2 h. Western blotting was carried out for the proteins indicated. (C) The GM0536 cell line was exposed to hypoxia as shown and then reoxygenated for the time periods indicated. The levels of phosphorylated and total ATM are shown. (D) LCLs from either a healthy control (GM0536) or an AT patient (GM1526) were exposed to hypoxia for the times indicated, and Western blotting was carried out for Kap1-S824, DNA-PKcs-T2609, and GAPDH as a loading control. (E) GM0536 cells were exposed to hypoxia for 8 h in the presence of the ATM inhibitor (ATMi) (10 μM). Cells were also treated with NCS for 10 min as a positive control. The levels of phosphorylated Kap1 (residue serine 824) are shown. wt, wild type; N, normoxic.
ATM undergoes autophosphorylation under hypoxic conditions.
Previous reports have described how, in response to the presence of DNA breaks, hypertonic salt, and HDAC inhibitors, ATM undergoes autophosphorylation and separates, from an inactive dimer, into active monomers (4). To address the role of PIKK family members in ATM activation under hypoxia, we investigated the requirement of DNA-PK, ATR, and ATM to phosphorylate ATM in response to hypoxia. To asses any involvement of DNA-PK in the hypoxia-induced phosphorylation of ATM, we initially used the MO59 cell lines, which have been described and classified extensively within the literature (17). However, these cells lines proved to be unsuitable to study ATM autophosphorylation because the loss of DNA-PK also correlated with decreased ATM levels. The levels of ATM protein increased after the reintroduction of DNA-PKcs, as seen in the MO59J derivative MJ-L24, indicating that DNA-PK plays a role in maintaining basal levels of ATM (Fig. 2A). As an alternative, we used a pharmacological inhibitor of DNA-PK to rapidly inactivate DNA-PK (Fig. 2B) (52). Addition of the DNA-PK inhibitor had no effect on the total levels of ATM or the hypoxia-induced induction of ATM phosphorylation, indicating that DNA-PK activity is not required. We then investigated the contribution of ATR to hypoxia-induced ATM phosphorylation. As a result of a truncating mutation in one ATR allele, Seckel patients have very low levels of the ATR protein and have an impaired DNA damage response (39). We compared the phosphorylation of ATM during hypoxia in cells isolated from patients with either AT or Seckel cells to that from a healthy individual (Fig. 2C). Decreased levels of ATR had no effect on ATM phosphorylation under hypoxic conditions. The HCT116ATR/flox cell line has a significantly reduced level of ATR expression compared to the parental cell line, but as in the previous experiment, we did not find any impairment in the ATM response to hypoxia (Fig. 2D). These findings are in contrast to those showing that in response to UV exposure, ATR phosphorylates ATM (45). It should be noted, however, that both the Seckel and HCT116ATR/flox cells express low levels of functional ATR, which could be responsible for phosphorylating ATM in response to hypoxia. We are unable to reduce ATR levels further in these systems, either by using small interfering RNA or expressing the Cre recombinase in the HCT116ATR/flox cell line, as we have demonstrated that this leads to the accumulation of DNA damage, which will in turn lead to the activation of ATM (27). We asked if hypoxia-induced phosphorylation of ATM was the result of an autophosphorylation event, as has been described, in response to DNA damage. GM0536 (ATM+/+) cells were treated with hypoxia or NCS in the presence or absence of a pharmacological inhibitor of ATM. Immunoblotting shows the levels of phosphorylated ATM and GAPDH as a loading control (Fig. 2E). We found that the inhibitor had a significant effect on both hypoxia and the NCS-induced levels of ATM phosphorylation. Data from three independent experiments are also shown and indicate that the inhibitor reduces hypoxia-induced ATM phosphorylation by approximately 40%, while ATM levels are reduced by 60% during NCS treatment. These data indicate that phosphorylation of serine 1981 under hypoxic conditions is primarily the result of ATM autophosphorylation.
FIG. 2.
Hypoxia-induced ATM-S1981 is a result of an autophosphorylation event. (A) MO59K (DNA-PKcs+/+), MO59J (DNA-PKcs−/−, and MJ-L24 (complemented DNA-PKcs+/+) cell lines were exposed to hypoxia, and Western blotting was carried out for ATM-S1981 and ATM. (B) GM0536 (ATM+/+) cells were exposed to hypoxia (Hyp) for 7 h in the presence or absence of DNA-PK inhibitor II (10 μM) as indicated. The levels of total ATM and phosphorylated ATM are shown. (C) GM0536 (ATM+/+), GM1526 (ATM−/−), and DKOO64 (ATR/Seckel) were exposed to hypoxia for 6 h, and then Western blotting was carried out for total and phosphorylated ATM. (D) HCT116 or HCT116ATR−/+ cells were exposed to 0.02% O2 for 16 h. Western blotting was carried out for total and phosphorylated ATM. (E) GM0536 (ATM+/+) cells were exposed to 0.02% O2 for 6 h, plus and minus the ATM inhibitor (ATMi) (10 μM). The results of three identical experiments are also shown graphically. Con, control.
Mitochondrial signaling is not required for hypoxia-induced ATM phosphorylation.
Recently ATM has been described as being partially cytoplasmic in localization and having specific roles to play in the mitochondria (1). We have further investigated both the cellular localization of ATM and the role of the mitochondria in hypoxia-induced ATM signaling. In order to determine whether mitochondrial signaling may be involved in the ATM-activating signal during hypoxic exposure, we took two complementary approaches; firstly we exposed HCT116 cells to either 2% or 0.02% oxygen in the presence of mitochondrial inhibitors. The inhibitors used were myxothiazol, which inhibits the coenzyme Q-cytochrome c reductase complex (complex II) and antimycin A, which inhibits the oxidation of ubiquinol in complex III in the cytochrome b subunit of the electron transport chain. The addition of mitochondrial inhibitors had no effect on hypoxia-induced phosphorylation of ATM, indicating that this signaling is not required for ATM activation in hypoxic conditions. In contrast, the levels of HIF-1α induced at 2% oxygen were decreased in the presence of mitochondrial inhibitors (Fig. 3A), consistent with previous reports (6, 12). Exposure of cells to 2% oxygen induced a small increase in the phosphorylation of ATM. Our second approach was to compare hypoxia-induced ATM activities in normal cells (A549) and those lacking mitochondria DNA (A549 [rho0]). Cells were exposed to either hypoxia or NCS (Fig. 3B). Despite the complete lack of mitochondrial DNA, hypoxia-induced phosphorylation of Kap1 is unchanged, indicating that mitochondrion-associated signaling is not required for ATM activation under these conditions.
FIG. 3.

Mitochondrial signaling is not required for hypoxia-induced activation of ATM. (A) GM0536 (ATM+/+) cells were exposed to 0.02% or 2% O2 for 8 h in the presence of mitochondrial inhibitors (1 μM myxothiazol [Myx] or 10 μM antimycin A[AntA]). Western blotting was then carried out for ATM-S1981, HIF-1α, and p53-S15. Con, control. (B) A549 and A549 [rho0] cells were exposed to hypoxia (Hyp) for 16 h or NCS for 4 h. The levels of Kap1-S824 were then determined as a marker of ATM activity. wt, wild type. N, normoxic.
The MRN complex does not mediate the ATM response to hypoxia.
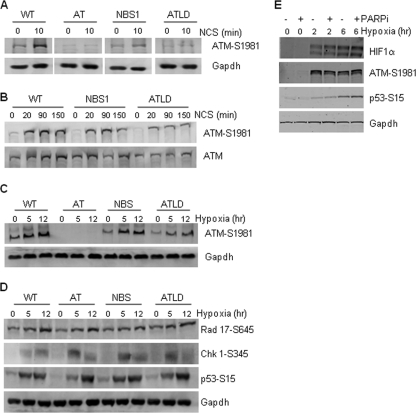
The complex of MRE11, Rad50, and NBS1 (MRN complex) has been described as mediating the ATM response (49). Studies have shown that ATM activation is influenced by the presence of MRN and that MRN is required for the initial and rapid ATM response to DNA damage. Wild-type, NBS1, and ATLD cells were exposed to NCS for 10 min before harvesting and immunoblotting for phospho-ATM (Fig. 4A). Under these conditions, there is a strong dependency on the presence of both MRE11 and NBS1 for serine 1981 phosphorylation, as cells lacking either MRE11 or NBS1 show almost no induction of phosphorylated ATM compared to wild-type cells. However, when this experiment was repeated with longer exposure times to NCS, the contribution of MRE11 or NBS1 becomes less apparent, indicating that the MRN complex affects the early kinetics of ATM phosphorylation (Fig. 4B). We investigated whether either MRE11 or NBS1 is required for hypoxia-induced phosphorylation of ATM using the same cell lines. We carried out these experiments over a range of time points in order to determine if, like with the response to NCS, there was a dependency at some time points but not others. In contrast to what occurred after treatment with NCS, at no point was the hypoxia-induced phosphorylation of ATM affected by the loss of either MRE11 or NBS1. Time points for 5 and 12 h of exposure to hypoxia are shown in Fig. 4C. Our experiments ranged from 2 to 24 h, and we did not see phosphorylation of ATM until 3 to 4 h for these cell lines (data not shown).
FIG. 4.
The MRN complex is not required for the ATM response to hypoxia. LCLs of the genotypes shown were used for these experiments. (A) Cells were treated with NCS (5 ng/ml) for 10 min before harvesting. (B) A longer time course was then carried out with NCS (5 ng/ml). (C and D) Cells were then exposed to 0.02% O2 for the times indicated, and Western blotting was carried out for the proteins/modifications shown. (E) RKO cells were exposed to hypoxia for the times indicated in the presence/absence of PARP inhibitor (PARPi), 4-amino-1,8-naphthalimide (10 μM). Western blotting was carried out for the proteins indicated. WT, wild type.
A recent report describes a novel p53 gain-of-function mutation in inducing genetic instability by inactivating ATM (42). Mutations in the DNA binding region (R248W and R273H) were proposed to interact with MRE11 and suppress the binding of the MRN complex to DNA double-stranded breaks and therefore abrogate ATM activation after exposure to ionizing radiation or UV treatment. We investigated this using both human tumor cells carrying known p53 mutations and a p53-inducible system. In support of our finding that the MRN complex is not required for hypoxia-induced activation of ATM, we saw no difference between the levels of ATM-S1981 induced in the presence of either the wild type or these p53 DNA binding domain mutations (data not shown). Recently it was shown that the MRN complex is also involved in mediating the ATR response to UV (44). We have previously shown that the ATR kinase is active under hypoxic conditions, and so we investigated whether the MRN complex was involved in this signaling (Fig. 4D) (25). As with our findings on the independence of ATM on MRN, we found that ATR signaling to any of the targets tested, Rad 17, p53, or Chk 1, was unaffected by the loss of either MRE11 or NBS1. These findings indicate that the MRN complex has little effect on ATM and ATR under hypoxic conditions. More recent findings have demonstrated that poly(ADP-ribose) polymerase (PARP) also has a role in the initial sensing of double-stranded breaks and the subsequent activation of ATM signaling (24). We have investigated the role of PARP in hypoxia-induced ATM activation (Fig. 4E). Cells were exposed to short periods of hypoxia in the presence or absence of a specific PARP inhibitor, and Western blotting was carried out. There was no observable decrease in the levels of hypoxia-induced ATM-S1981 in the presence of the PARP inhibitor, indicating that functional PARP is not required for this response. The levels of phosphorylated p53 were also unaltered, which suggests that PARP inhibition also had no effect on hypoxia-induced ATR signaling.
Nuclear localization of hypoxia-induced ATM.
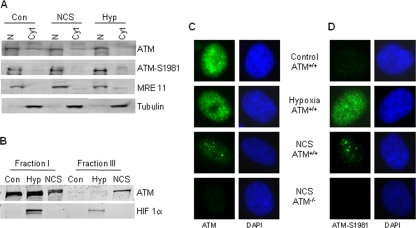
In order to determine the subcellular localization of hypoxia-induced ATM, we prepared nuclear and cytoplasmic extracts of HCT116 cells treated with either NCS or hypoxia and probed for both total ATM and phospho-ATM. The efficiency of the fractionation was determined by blotting for MRE11, which is a nuclear protein, and α-tubulin, which is a cytoplasmic protein (Fig. 5A). In untreated or NCS-treated cells, ATM was found to be predominantly in the nucleus, although a small fraction (<10%) of the total ATM appeared cytoplasmic. Under hypoxic conditions, ATM appeared entirely nuclear. Western blotting of the fractions for phosphorylated ATM showed that this was entirely nuclear under all conditions. It has previously been shown that in response to DNA damage, ATM is retained at the sites of DNA damage and as a result becomes more resistant to biochemical extraction (2). We have used this approach to determine if, in response to hypoxia, ATM also becomes more associated with chromatin. Our data indicate that in contrast to treatment with NCS, hypoxia exposure does not lead to a stronger interaction between ATM and chromatin (Fig. 5B). This finding prompted us to examine the cellular localization of ATM immunohistochemically. In response to agents which induce double-stranded breaks, ATM has been found to relocalize to the sites of breaks and in doing so form large nuclear foci. We have investigated the nuclear distribution of ATM during hypoxia treatment which, as previously mentioned, does not induce detectable double-stranded breaks. Wild-type and ATM-deficient cells were exposed to either hypoxia or NCS and then stained for total ATM (Fig. 5C) or phosphorylated ATM (Fig. 5D). In response to NCS, both total ATM and ATM-S1981 can be seen as discrete, large nuclear foci, as would be expected in response to DNA damage. In contrast, this did not occur in the cells exposed to hypoxia. Instead, during hypoxia, total ATM remained diffuse throughout the nucleus and, although the phospho-ATM appears somewhat punctuate, it is clearly distinct from the pattern seen in response to NCS. Previous studies have reported that in response to non-DNA damaging agents, such as HDAC inhibitors, high salt, or chloroquine, ATM is activated but does not form foci (4, 30). Our immunofluorescence staining of ATM in hypoxic cells indicates that hypoxia represents a unique stress and that the activation of ATM in response to this stress may be through an alternate mechanism.
FIG. 5.
Hypoxia-activated ATM is nuclear but does not form foci. (A) GM0536 cells were treated with NCS (5 ng/ml) for 20 min or hypoxia (Hyp) for 8 h. Cell fractionation was then carried out to isolate cytoplasmic (Cyt) or nuclear (N) preparations. MRE11 was used as a nuclear marker, and tubulin was used for the cytoplasm. (B) Cell fractionation was carried out as described previously (2). HeLa cells were treated for 1 h with 5 ng/ml NCS or hypoxia (18 h). The levels of ATM and HIF-1α are shown for fractions I and III. (C and D) ATM does not form large nuclear foci in response to hypoxia. YZ3 (ATM+/+) and pEBS (ATM−/−) cells were exposed to either hypoxia (18 h) or NCS (2 h, 5 ng/ml). Cells were then stained for total ATM protein (C) or ATM-S1981 (D).
The pattern of hypoxia-induced ATM was surprising in part because we have previously described the formation of γ-H2AX foci in response to hypoxia, despite the absence of detectable double-stranded breaks (26). We have characterized the response of H2AX to hypoxia further here. Firstly, we show that both phosphorylation and focus formation of H2AX in response to hypoxia is independent of ATM status. pEBSATM−/− and YZ3ATM+/+ cells were exposed to hypoxia for the time periods indicated, and Western blotting was carried out for γ-H2AX (Fig. 6A). In parallel, cells were also immunostained for γ-H2AX (Fig. 6B). These data demonstrate that there is no role for ATM in hypoxia-induced H2AX phosphorylation and focus formation. In contrast, cells with reduced ATR activity showed a decrease in hypoxia-induced H2AX phosphorylation (Fig. 6C). In support of this finding we also show here that hypoxia-induced phosphorylation of H2AX is oxygen dependent and that this correlates with the conditions in which we have previously observed replication arrest and ATR activity (25). RKO cells were exposed to a range of oxygen concentrations for 8 h, and Western blotting was carried out for γ-H2AX (Fig. 6D). There was little or no phosphorylation detected until a level of 0.1% O2 was reached. The most significant increase, however, was observed at 0.02% O2, approximately 10-fold the levels seem in normoxic conditions. We noted previously and here that not all cells exposed to hypoxia accumulate γ-H2AX. In order to investigate this further, we carried out fluorescence-activated cell sorter analysis for γ-H2AX under hypoxic conditions (Fig. 6E). We have compared the percentages of γ-H2AX-positive cells in U2OS cells exposed to either 0.2 or 0.02% O2 and found that at the lower of these concentrations almost 50% of cells are positively stained. We found this to be similar in other cell lines and that this percentage did not increase with extended times under hypoxia. This suggests that only cells in certain phases of the cell cycle form γ-H2AX foci in response to hypoxia. To test this hypothesis, we exposed cells to hypoxia and then immunostained for both γ-H2AX and the G2/M marker CENP-F (Fig. 6F). The levels of CENP-F have been shown to increase throughout the G2 phase and reach maximum levels in mitosis. Our data indicate that there was no overlap between CENP-F staining and phosphorylation of H2AX, indicating that this does not occur in G2/M. Previously, we proposed that in response to hypoxia, replication arrest occurs due to stalling of replication forks, and we demonstrated this through the visualization of BrdU in single-stranded regions of DNA (27). Here, we have used the single-stranded DNA binding protein RPA to confirm that replication fork stalling occurs during exposure to hypoxia and that cells with RPA foci also accumulate γ-H2AX (Fig. 6G). RPA32 foci were clearly visible in approximately 50% of cells during exposure to hypoxia, indicating the presence of single-stranded DNA. The same cells that exhibited RPA32 foci also had γ-H2AX staining; we found no cells that stained positively for one and not the other. Interestingly, when we merged the RPA32 and γ-H2AX images, we found that although there was a significant colocalization, there was not a total overlap. This raises the possibility that as with DNA double-stranded breaks, the γ-H2AX signal may extend away from the stalled replication fork. In the majority of cells, hypoxia-induced γ-H2AX is found in foci, whereas hypoxia-induced ATM is diffuse throughout the nucleus. While some cells had clear γ-H2AX foci which were indistinguishable from those induced by NCS, other cells had a more diffuse staining pattern, like that observed for ATM (data not shown).
FIG. 6.
Hypoxia-induced γ-H2AX is dependent on ATR in S-phase cells. (A) pEBS (ATM−/−) and YZ3 (ATM+/+) cells were exposed to hypoxia for the time periods indicated. The levels of γ-H2AX are shown. (B) In an experiment parallel to the one shown in panel A, cells were also immunostained for γ-H2AX. (C) FO2/98/hTERT (Seckel) cells and a matched control, IBR hTERT, were treated as shown, and the induction of γ-H2AX was determined. (D) RKO cells were exposed to the oxygen tensions shown for 8 h. Western blotting was carried out for γ-H2AX and β actin. (E) U2OS cells were exposed to either 0.2% or 0.02% O2 for 16 h. γ-H2AX-positive cells were then quantified with a fluorescence-activated cell sorter. (F) U2OS cells were exposed to hypoxia for 6 h or NCS for 6 h. Cells were then costained for γ-H2AX and CENP-F. (G) U2OS cells were exposed to hypoxia for 6 h and then stained for RPA32 and γ-H2AX. DAPI was used as a nuclear stain.
The mediator protein 53BP1 has been described as having a role in sensing alterations in chromatin structure and consequently contributing to the ATM response, and it is also often used as an alternate to γ-H2AX as a marker of DNA breaks (3). We investigated the pattern of 53BP1 staining in response to hypoxia. U2OS cells were exposed to NCS or hypoxia, as indicated, and then stained for 53BP1 and γ-H2AX. In response to NCS, we saw clear, large nuclear 53BP1 foci which completely colocalized with γ-H2AX foci, as would be expected. In contrast, in the hypoxia-treated cells, we saw no 53BP1 foci, despite the presence of γ-H2AX foci (Fig. 7A). We investigated the nuclear localization of 53BP1 over a range of exposure times to hypoxia (30 min to 18 h) and detected no foci at any point (data not shown). These findings support the concept that hypoxic induction of ATM is not the consequence of DNA double-stranded breaks but are in direct contrast to the results of staining for γ-H2AX and suggest that in response to hypoxia, ATM is activated through a unique mechanism. We then examined the levels of both total 53BP1 and phosphorylated 53BP1 (p53BP1) during hypoxia. Despite the lack of hypoxia-induced 53BP1 foci, we saw a robust phosphorylation in response to hypoxia. This was very apparent by 4 h but decreased at later time points (8 and 16 h). This phosphorylation was entirely abrogated in the pEBS cell line, which lacks ATM, indicating that it is dependent on ATM (Fig. 7B). Intrigued by the decrease in p53BP1 observed at longer hypoxia exposure (8 h), we asked if this was a cell line-specific observation. We investigated the kinetics of 53BP1 phosphorylation in both the RKO and U2OS cell lines (Fig. 7C and data not shown) and found that in both cases, 53BP1 was rapidly phosphorylated, but equally rapid levels dropped back to control levels. Interestingly, in both the RKO and U2OS cell lines, the total levels of 53BP1 also appeared to decrease during hypoxia exposure, although this was not observed in the YZ3 and pEBS cells. We and others have previously shown that components of the DNA damage response, for example BRCA1 and Rad51, can be repressed at the protein level by exposure to hypoxia (7, 9). Decreased levels of 53BP1 could, in part, explain why we do not observe hypoxia-induced 53BP1 foci after longer exposures (16 h) but do not explain why the p53BP1 seen after 4-h exposure does not form foci. In response to DNA damage, the tudor domain of 53BP1 has been shown to interact with dimethylated lysine 20 (K20) on histone 4, which is responsible for the relocalization of 53BP1 to sites of damage (10). Exposure to hypoxia has been demonstrated to induce a novel signature of chromatin modifications, although to our knowledge, histone H4 (me)2K20 has not been studied (31). We hypothesized that if this histone modification was affected by hypoxia exposure, this might explain the failure of 53BP1 to form foci. RKO cells were exposed to hypoxia, and Western blotting was carried out for both H4 (me)2K20 and total H4 (Fig. 7C). We saw no change in the levels of H4 or the modified H4, suggesting that this is not the cause of the lack of hypoxia-induced 53BP1 foci. Previous studies have shown that while hypoxia does not lead to an accumulation of double-stranded breaks, reoxygenation is a potent inducer of damage (26). We hypothesized that reoxygenation-induced damage would lead to the formation of 53BP1 foci. In order to investigate this, U2OS cells were exposed to hypoxia and then reoxygenated for varying time periods. We found that 53BP1 did indeed form foci after reoxygenation and that this occurred as early as 1 h and persisted for at least 16 h (Fig. 7D and data not shown). Reoxygenation-induced 53BP1 focus formation was found to be dependent on ATM activity, as cells treated with an ATM inhibitor prior to reoxygenation did not accumulate foci (data not shown).
FIG. 7.
53BP1 and BRCA1 do not form nuclear foci in response to hypoxia. (A) U2OS cells were treated with hypoxia (6 h) or NCS (5 ng/ml 6 h) and stained for 53BP1 and γ-H2AX. (B) YZ3(ATM+/+) and pEBS(ATM−/−) cells were exposed to hypoxia for the times indicated, and Western blotting was carried out for p53BP1, 53BP1, and GAPDH. (C) RKO cells were exposed to hypoxia (Hyp) as shown, and Western blotting was carried out for the proteins and modifications indicated. (D) U2OS cells were exposed to hypoxia for 16 h followed by periods of reoxygenation; shown is 1 h of reoxygenation (Reox). Cells were then stained for 53BP1 and DAPI. U2OS cells were treated as described for panel A and stained for BRCA1 (E) and mono- and polyubquitinated substrates using the FK2 antibody (F). Con, control.
In response to DNA damage, chromatin-bound substrates, such as H2AX, H2A, and others, are ubquitylated and, as a result, recruit factors, including 53BP1 and BRCA1 (29, 33, 36, 53). In the case of BRCA1, this is via the intermediate Rap80 (32). We show here that in contrast to how it responds to NCS, BRCA1 does not form nuclear foci in response to hypoxia (Fig. 7E). The E3 ubiquitin ligase RNF8 has been shown to ubiquitinate H2AX and to be required for focus formation by both 53BP1 and BRCA1 (29). The FK2 antibody, which recognizes mono- and polyubiquitinated proteins, has been widely used as a means of visualizing DNA damage-associated ubiquitin conjugates (29, 40). We hypothesized that 53BP1 and BRCA1 may not form foci at hypoxia-induced replication forks due to a lack of the required chromatin remodeling. Using the FK2 antibody, we stained cells which had been treated with either NCS or hypoxia (Fig. 7F). As expected, we saw an increase in nuclear foci with FK2 in NCS-treated cells but, in support of our hypothesis, did not observe any foci in the hypoxia-treated cells, indicating that the lack of ubiquitination could explain the lack of 53BP1 and BRCA1 foci.
MDC1 amplifies the hypoxia-induced ATM response.
Recently it was shown that MDC1 is required for both 53BP1 phosphorylation and focus formation in response to DNA damage, suggesting that MDC1 is an upstream regulator of 53BP1 (38). Data from several complementary studies suggest that this is through the recruitment of RNF8 by MDC1 to the sites of DNA damage. We proposed that the lack of ubiquitinated substrates detectable with the FK2 antibody in hypoxia-treated cells may result from a failure of MDC1 to relocalize to hypoxia-induced stalled replication forks. We have therefore investigated the cellular distribution of MDC1 in hypoxia-treated cells. We found that, in contrast to ATM and 53BP1, MDC1 did form nuclear foci in hypoxia-treated cells which also had RPA foci (Fig. 8A). From this we concluded that MDC1 relocalized in hypoxic S-phase cells, although as we saw with γ-H2AX, the overlap with RPA32 did not always colocalize exactly, indicating that MDC1 may not be present exactly at the stalled fork but in a region surrounding it. We determined that MDC1 focus formation was independent of ATM status, as indistinguishable foci formed in both YZ3ATM+/+ and pEBSATM−/− cells in response to hypoxia (Fig. 8B). However, analysis of the MDC1 foci formed in hypoxic conditions showed a complete overlap with γ-H2AX foci (Fig. 8C). In contrast to the MRN complex, the MDC1 protein, although also described as a mediator of the DNA damage checkpoint, has also been shown to amplify the ATM response without being required for the initial activation or phosphorylation of ATM (35, 43). We have investigated the requirement of MDC1 for both ATM phosphorylation and activity in response to hypoxia. MEFs, MDC1+/+, and MDC1−/− were exposed to hypoxia or NCS for the times indicated and then to Western blotting for ATM and Kap1 (Fig. 8D). Our data show that ATM is phosphorylated at normal levels in response to hypoxia, despite the loss of MDC1, whereas Kap1 shows significantly diminished phosphorylation in the absence of MDC1. Phosphorylation of Kap1 remained low in MDC1−/− cells for 16 to 24 h compared to that in MDC1+/+ cells (data not shown). These data indicate that, as reported for DNA damage, MDC1 is required to amplify the ATM signal induced by hypoxia. In conclusion, we have described here the activation of ATM by a physiologically relevant non-DNA-damaging stress and have compared this activation to the response to DNA damage-induced ATM. It seems that while the pathways downstream of ATM may be similar in both cases, the initial signals and mediator requirements are significantly different.
FIG. 8.
MDC1 is required to amplify the ATM response to hypoxia. (A) U20S cells were exposed to hypoxia for 6 h and then stained for MDC1 and RPA32. (B) YZ3 (ATM+/+) and pEBS (ATM−/−) cells were exposed to hypoxia (Hyp) for 16 h and then stained for MDC1, γ-H2AX, and DAPI. Con, control. (C) Cells were exposed to either 6 h of hypoxia or 6 Gy. Irradiated cells were fixed 4 h after treatment. Cells were stained for MDC1 and γ-H2AX, and nuclei were visualized using DAPI. (D) MDC1−/− and MDC1+/+ MEFs were exposed to hypoxia, and Western blotting was carried out for the proteins indicated.
DISCUSSION
The DNA damage response is initiated by both classical DNA-damaging agents, such as NCS, and nondamaging agents, such as hypoxia, high salt concentrations, histone deacetylase inhibition, and chloroquine (4, 16). Of these nondamaging agents, hypoxia is the most relevant to tumorigenesis, as regions of hypoxia occur early and in most, if not all, solid tumors. Most models on the mechanism of ATM activation rely on the induction of DNA damage and do not include situations where DNA damage is not detected, such as hypoxia. In this study we have shown that in response to hypoxia, ATM is not only phosphorylated but also active. In contrast to its response to DNA-damaging agents, the ATM response to hypoxia is independent of the MRN complex. This finding is supportive of a model whereby the MRN complex acts upstream of ATM in the rapid detection of DNA breaks and then recruits ATM. However, the MRN complex is not needed in the absence of DNA damage for the activation of ATM. It should be noted that NBS1 is phosphorylated during hypoxia, most likely by both ATR and ATM, and so, although it may not have a role in the initial activation of the DNA damage response, it is activated as part of the overall response (28). We have also shown that PARP activity is not required for hypoxia-induced activation of ATM. This is also supportive of a model whereby hypoxia induces ATM activity in the absence of DNA breaks and hence is not reliant on molecules which act as sensors of breaks (24). We predict that other molecules demonstrated to function in this way, for example, Aven and FoxO3a, may also be of less importance to the hypoxia response, although this remains to be formally tested (23, 48). Once activated, hypoxia-induced ATM seems to behave in a similar manner to damage-activated ATM, in that it phosphorylates downstream targets, and this is, in part, dependent on MDC1. However, another critical difference between hypoxic and DNA damage-induced activation of ATM is the cellular localization of ATM. In response to hypoxia, ATM remains diffuse through the nucleus, as does phosphorylated ATM. The staining pattern we observe is reminiscent of that seen in response to high salt and chloroquine, indicating that there may be a similar mechanism of activation for non-DNA-damaging stresses (4). The mechanism(s) by which stresses such as chloroquine exposure or high salt induce ATM phosphorylation has not been described but has been broadly attributed to chromatin modifications. Given the replication arrest induced by the levels of hypoxia used in this study, it is conceivable that chromatin modifications may also be the activating signal induced by hypoxia. As a part of this study, we have investigated the involvement of other DNA damage response proteins under hypoxic conditions. In contrast to ATM, both γ-H2AX and MDC1 formed clear nuclear foci in response to hypoxia which partially overlapped RPA foci. The implications of this are twofold; firstly this confirms the presence of single-stranded DNA in hypoxic cells, indicating the presence of stalled replication forks. Secondly, this demonstrates that foci involving some of the DNA damage response proteins do form in hypoxic S-phase cells. From these data we can start to build a picture of the unique DNA damage response signaling that occurs in the absence of an initiating double-stranded break. We propose that during hypoxia exposure, replication stalling leads to the accumulation of regions of single-stranded DNA; these in turn become RPA bound and signal to the ATR-ATRIP complex and potentially also ATM. The ATR kinase then phosphorylates H2AX in the region including and surrounding the stalled fork. Once phosphorylated, γ-H2AX recruits MDC1 to the stalled replication fork in hypoxic S-phase cells. In the response to DNA damage, phosphorylated MDC1 recruits the RNF8/Ubc13 ligases, which then ubiquitinate γ-H2AX. Our data suggest that while MDC1 and H2AX are present at stalled forks in a similar manner to that seen for DNA double-stranded breaks and while MDC1 contributes to hypoxia-induced ATM activity, the downstream signaling pathways dependent on ATM diverge at this point. We saw no foci with the FK2 antibody, indicating a failure to ubiquitinate γ-H2AX, most probably due to a failure of MDC1 to recruit RNF8. We hypothesize that RNF8 would also fail to form foci in hypoxic cells. The consequence of this is that neither BRCA1 nor 53BP1 is recruited to chromatin, and thus, they fail to form foci. This is compounded by the falling levels of these proteins, which we observed under hypoxic conditions. It was recently shown that 53BP1 phosphorylation and colocalization are required for correct phosphorylation of Chk2 (38). We show here and showed previously that 53BP1 is phosphorylated after short exposures to hypoxia before levels begin to decrease but that Chk2 phosphorylation is robust and constant throughout hypoxia and reoxygenation (16).
There have been several studies recently describing the downregulation of DNA repair pathways in response to hypoxia, most notably homologous recombination but also mismatch repair (7, 8, 34). It is unclear what the evolutionary benefit is to a hypoxic cell to repress repair, although the finding that this occurs under conditions of extreme levels of hypoxia where DNA damage has not been detected may be the answer. When a cell reaches a level of hypoxia that leads to replication stalling, it also enters a state where it is difficult to maintain protein levels, and energy needs to be reserved; global transcription and translation are both repressed at these oxygen tensions. The undesirable consequence of this is that if a tumor cell becomes reoxygenated as, for example, a result of improved blood flow, the cell sustains reoxygenation-induced damage but lacks the machinery to repair it. Our previous work, however, showed that one of the roles of hypoxia/reoxygenation-induced ATM is to induce a cell cycle arrest in the G2 phase, which allows time for the reexpression of DNA proteins to promote repair and replication restart. The recruitment of MDC1 to hypoxia-induced γ-H2AX foci but not 53BP1 is intriguing, in particular, and indicates that in response to hypoxia, ATR and ATM induce pathways associated more with cell cycle checkpoints than with DNA repair.
Acknowledgments
We are grateful to Grant Stewart, University of Birmingham, for technical assistance. A.J.G. is supported by NIH grant CA008480 awarded to A.J.G. E.M.H., M.R.K., Z.B., and I.M.P. are funded by CRUK grant C6515/A9321, awarded to E.M.H.
Footnotes
Published ahead of print on 3 November 2008.
REFERENCES
- 1.Ambrose, M., J. V. Goldstine, and R. A. Gatti. 2007. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum. Mol. Genet. 162154-2164. [DOI] [PubMed] [Google Scholar]
- 2.Andegeko, Y., L. Moyal, L. Mittelman, I. Tsarfaty, Y. Shiloh, and G. Rotman. 2001. Nuclear retention of ATM at sites of DNA double strand breaks. J. Biol. Chem. 27638224-38230. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, L., C. Henderson, and Y. Adachi. 2001. Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol. Cell. Biol. 211719-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421499-506. [DOI] [PubMed] [Google Scholar]
- 5.Bartkova, J., Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J. M. Nesland, C. Lukas, T. Orntoft, J. Lukas, and J. Bartek. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434864-870. [DOI] [PubMed] [Google Scholar]
- 6.Bell, E. L., T. A. Klimova, J. Eisenbart, P. T. Schumacker, and N. S. Chandel. 2007. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol. Cell. Biol. 275737-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bindra, R. S., S. L. Gibson, A. Meng, U. Westermark, M. Jasin, A. J. Pierce, R. G. Bristow, M. K. Classon, and P. M. Glazer. 2005. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 6511597-11604. [DOI] [PubMed] [Google Scholar]
- 8.Bindra, R. S., and P. M. Glazer. 2005. Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat. Res. 56975-85. [DOI] [PubMed] [Google Scholar]
- 9.Bindra, R. S., P. J. Schaffer, A. Meng, J. Woo, K. Maseide, M. E. Roth, P. Lizardi, D. W. Hedley, R. G. Bristow, and P. M. Glazer. 2004. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol. Cell. Biol. 248504-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botuyan, M. V., J. Lee, I. M. Ward, J. E. Kim, J. R. Thompson, J. Chen, and G. Mer. 2006. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 1271361-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 2811677-1679. [DOI] [PubMed] [Google Scholar]
- 12.Chandel, N. S., D. S. McClintock, C. E. Feliciano, T. M. Wood, J. A. Melendez, A. M. Rodriguez, and P. T. Schumacker. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 27525130-25138. [DOI] [PubMed] [Google Scholar]
- 13.Dang, D. T., F. Chen, L. B. Gardner, J. M. Cummins, C. Rago, F. Bunz, S. V. Kantsevoy, and L. H. Dang. 2006. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res. 661684-1936. [DOI] [PubMed] [Google Scholar]
- 14.Douglas, P., X. Cui, W. D. Block, Y. Yu, S. Gupta, Q. Ding, R. Ye, N. Morrice, S. P. Lees-Miller, and K. Meek. 2007. The DNA-dependent protein kinase catalytic subunit is phosphorylated in vivo on threonine 3950, a highly conserved amino acid in the protein kinase domain. Mol. Cell. Biol. 271581-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endrich, B., H. S. Reinhold, J. F. Gross, and M. Intaglietta. 1979. Tissue perfusion inhomogeneity during early tumor growth in rats. J. Natl. Cancer Inst. 62:387-395. [PubMed] [Google Scholar]
- 16.Freiberg, R. A., E. M. Hammond, M. J. Dorie, S. M. Welford, and A. J. Giaccia. 2006. DNA damage during reoxygenation elicits a Chk2-dependent checkpoint response. Mol. Cell. Biol. 261598-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried, L. M., C. Koumenis, S. R. Peterson, S. L. Green, P. van Zijl, J. Allalunis-Turner, D. J. Chen, R. Fishel, A. J. Giaccia, J. M. Brown, and C. U. Kirchgessner. 1996. The DNA damage response in DNA-dependent protein kinase-deficient SCID mouse cells: replication protein A hyperphosphorylation and p53 induction. Proc. Natl. Acad. Sci. USA 9313825-13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner, L. B., F. Li, X. Yang, and C. V. Dang. 2003. Anoxic fibroblasts activate a replication checkpoint that is bypassed by E1a. Mol. Cell. Biol. 239032-9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson, S. L., R. S. Bindra, and P. M. Glazer. 2005. Hypoxia-induced phosphorylation of Chk2 in an ataxia telangiectasia mutated-dependent manner. Cancer Res. 6510734-10741. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg, M., M. Stucki, J. Falck, D. D'Amours, D. Rahman, D. Pappin, J. Bartek, and S. P. Jackson. 2003. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature 421952-956. [DOI] [PubMed] [Google Scholar]
- 21.Gorgoulis, V. G., L. V. Vassiliou, P. Karakaidos, P. Zacharatos, A. Kotsinas, T. Liloglou, M. Venere, R. A. Ditullio, Jr., N. G. Kastrinakis, B. Levy, D. Kletsas, A. Yoneta, M. Herlyn, C. Kittas, and T. D. Halazonetis. 2005. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434907-913. [DOI] [PubMed] [Google Scholar]
- 22.Green, S. L., R. A. Freiberg, and A. J. Giaccia. 2001. p21Cip1 and p27Kip1 regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol. Cell. Biol. 211196-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo, J. Y., A. Yamada, T. Kajino, J. Q. Wu, W. Tang, C. D. Freel, J. Feng, B. N. Chau, M. Z. Wang, S. S. Margolis, H. Y. Yoo, X. F. Wang, W. G. Dunphy, P. M. Irusta, J. M. Hardwick, and S. Kornbluth. 2008. Aven-dependent activation of ATM following DNA damage. Curr. Biol. 18933-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haince, J. F., S. Kozlov, V. L. Dawson, T. M. Dawson, M. J. Hendzel, M. F. Lavin, and G. G. Poirier. 2007. Ataxia telangiectasia mutated (ATM) signaling network is modulated by a novel poly(ADP-ribose)-dependent pathway in the early response to DNA-damaging agents. J. Biol. Chem. 28216441-16453. [DOI] [PubMed] [Google Scholar]
- 25.Hammond, E. M., N. C. Denko, M. J. Dorie, R. T. Abraham, and A. J. Giaccia. 2002. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 221834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond, E. M., M. J. Dorie, and A. J. Giaccia. 2003. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J. Biol. Chem. 27812207-12213. [DOI] [PubMed] [Google Scholar]
- 27.Hammond, E. M., M. J. Dorie, and A. J. Giaccia. 2004. Inhibition of ATR leads to increased sensitivity to hypoxia/reoxygenation. Cancer Res. 646556-6562. [DOI] [PubMed] [Google Scholar]
- 28.Hammond, E. M., S. L. Green, and A. J. Giaccia. 2003. Comparison of hypoxia-induced replication arrest with hydroxyurea and aphidicolin-induced arrest. Mutat. Res. 532205-213. [DOI] [PubMed] [Google Scholar]
- 29.Huen, M. S., R. Grant, I. Manke, K. Minn, X. Yu, M. B. Yaffe, and J. Chen. 2007. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt, C. R., R. K. Pandita, A. Laszlo, R. Higashikubo, M. Agarwal, T. Kitamura, A. Gupta, N. Rief, N. Horikoshi, R. Baskaran, J. H. Lee, M. Lobrich, T. T. Paull, J. L. Roti Roti, and T. K. Pandita. 2007. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 673010-3017. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, A. B., N. Denko, and M. C. Barton. 2008. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat. Res. 640174-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, H., J. Chen, and X. Yu. 2007. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 3161202-1205. [DOI] [PubMed] [Google Scholar]
- 33.Kolas, N. K., J. R. Chapman, S. Nakada, J. Ylanko, R. Chahwan, F. D. Sweeney, S. Panier, M. Mendez, J. Wildenhain, T. M. Thomson, L. Pelletier, S. P. Jackson, and D. Durocher. 2007. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 3181637-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koshiji, M., K. K. To, S. Hammer, K. Kumamoto, A. L. Harris, P. Modrich, and L. E. Huang. 2005. HIF-1α induces genetic instability by transcriptionally downregulating MutSα expression. Mol. Cell 17793-803. [DOI] [PubMed] [Google Scholar]
- 35.Lou, Z., K. Minter-Dykhouse, S. Franco, M. Gostissa, M. A. Rivera, A. Celeste, J. P. Manis, J. van Deursen, A. Nussenzweig, T. T. Paull, F. W. Alt, and J. Chen. 2006. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell 21187-200. [DOI] [PubMed] [Google Scholar]
- 36.Mailand, N., S. Bekker-Jensen, H. Faustrup, F. Melander, J. Bartek, C. Lukas, and J. Lukas. 2007. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131887-900. [DOI] [PubMed] [Google Scholar]
- 37.Meek, K., P. Douglas, X. Cui, Q. Ding, and S. P. Lees-Miller. 2007. trans autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining. Mol. Cell. Biol. 273881-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minter-Dykhouse, K., I. Ward, M. S. Huen, J. Chen, and Z. Lou. 2008. Distinct versus overlapping functions of MDC1 and 53BP1 in DNA damage response and tumorigenesis. J. Cell Biol. 181727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Driscoll, M., V. L. Ruiz-Perez, C. G. Woods, P. A. Jeggo, and J. A. Goodship. 2003. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 33497-501. [DOI] [PubMed] [Google Scholar]
- 40.Polanowska, J., J. S. Martin, T. Garcia-Muse, M. I. Petalcorin, and S. J. Boulton. 2006. A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. EMBO J. 252178-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3155-168. [DOI] [PubMed] [Google Scholar]
- 42.Song, H., M. Hollstein, and Y. Xu. 2007. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat. Cell Biol. 9573-580. [DOI] [PubMed] [Google Scholar]
- 43.Stewart, G. S., B. Wang, C. R. Bignell, A. M. Taylor, and S. J. Elledge. 2003. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 421961-966. [DOI] [PubMed] [Google Scholar]
- 44.Stiff, T., C. Reis, G. K. Alderton, L. Woodbine, M. O'Driscoll, and P. A. Jeggo. 2005. Nbs1 is required for ATR-dependent phosphorylation events. EMBO J. 24:199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stiff, T., S. A. Walker, K. Cerosaletti, A. A. Goodarzi, E. Petermann, P. Concannon, M. O'Driscoll, and P. A. Jeggo. 2006. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 25:5775-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomlinson, R. H., and L. H. Gray. 1955. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 9539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.To, K. K., O. A. Sedelnikova, M. Samons, W. M. Bonner, and L. E. Huang. 2006. The phosphorylation status of PAS-B distinguishes HIF-1alpha from HIF-2alpha in NBS1 repression. EMBO J. 254784-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai, W. B., Y. M. Chung, Y. Takahashi, Z. Xu, and M. C. Hu. 2008. Functional interaction between FOXO3a and ATM regulates DNA damage response. Nat. Cell Biol. 10460-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uziel, T., Y. Lerenthal, L. Moyal, Y. Andegeko, L. Mittelman, and Y. Shiloh. 2003. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 225612-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaupel, P., S. Briest, and M. Hockel. 2002. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien. Med. Wochenschr. 152334-342. [DOI] [PubMed] [Google Scholar]
- 51.Vaupel, P., K. Schlenger, C. Knoop, and M. Hockel. 1991. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 513316-3322. [PubMed] [Google Scholar]
- 52.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 2695241-5248. [PubMed] [Google Scholar]
- 53.Wang, B., and S. J. Elledge. 2007. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc. Natl. Acad. Sci. USA 10420759-20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408433-439. [DOI] [PubMed] [Google Scholar]
- 55.Ziv, Y., A. Bar-Shira, I. Pecker, P. Russell, T. J. Jorgensen, I. Tsarfati, and Y. Shiloh. 1997. Recombinant ATM protein complements the cellular A-T phenotype. Oncogene 15159-167. [DOI] [PubMed] [Google Scholar]