Abstract
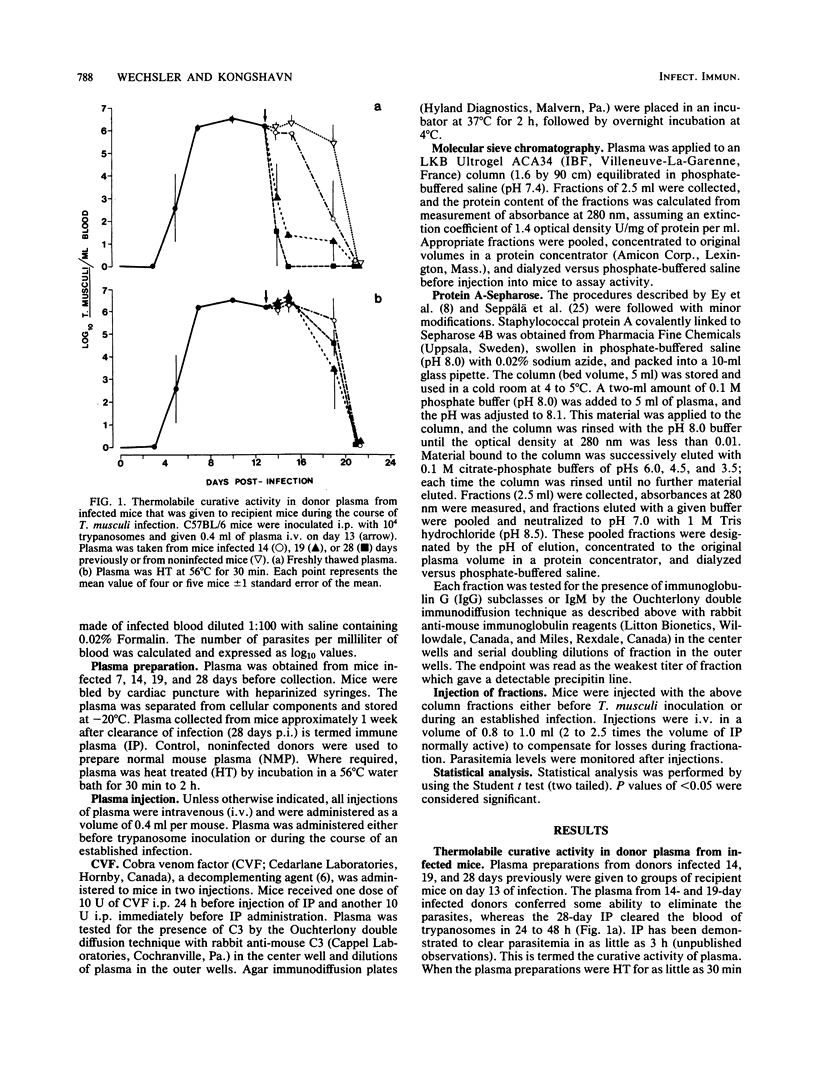
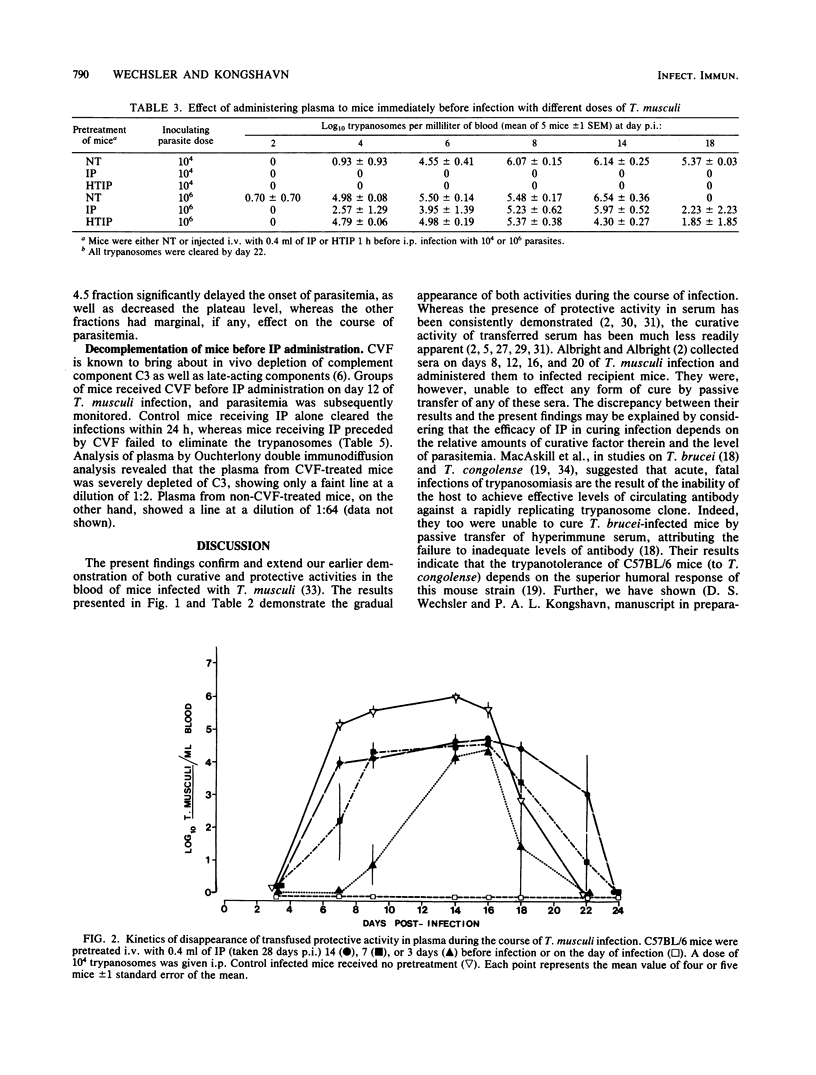
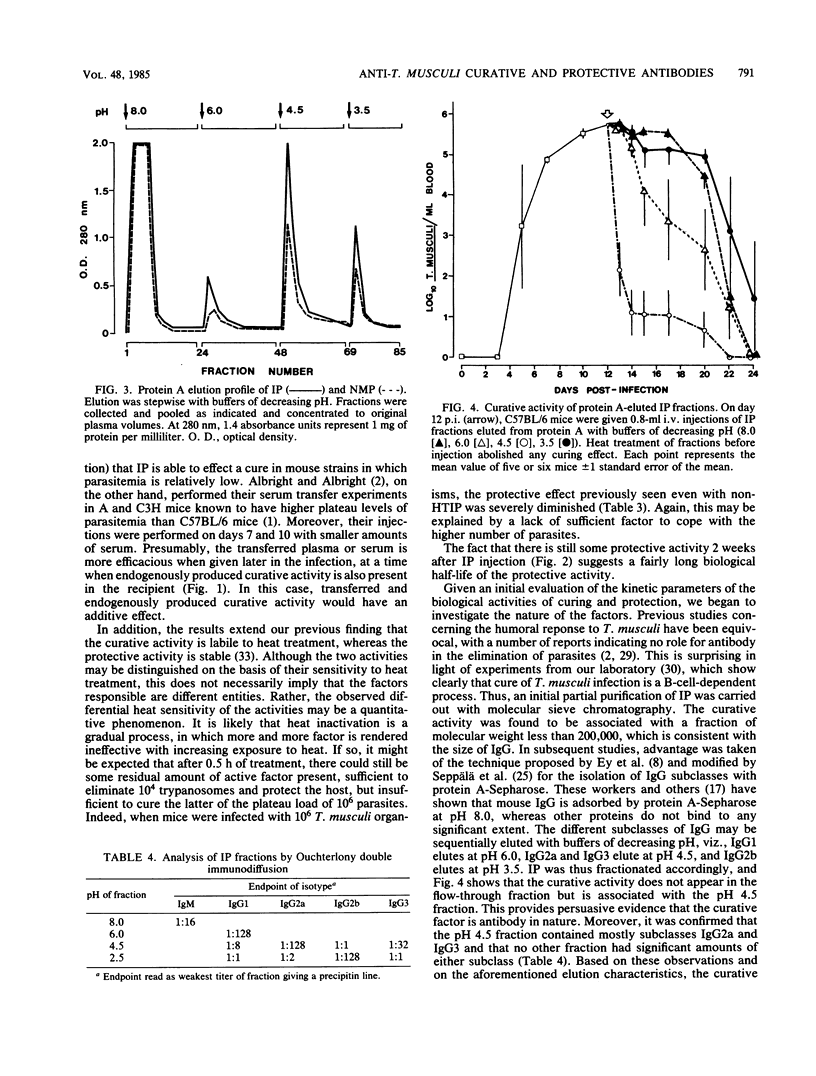
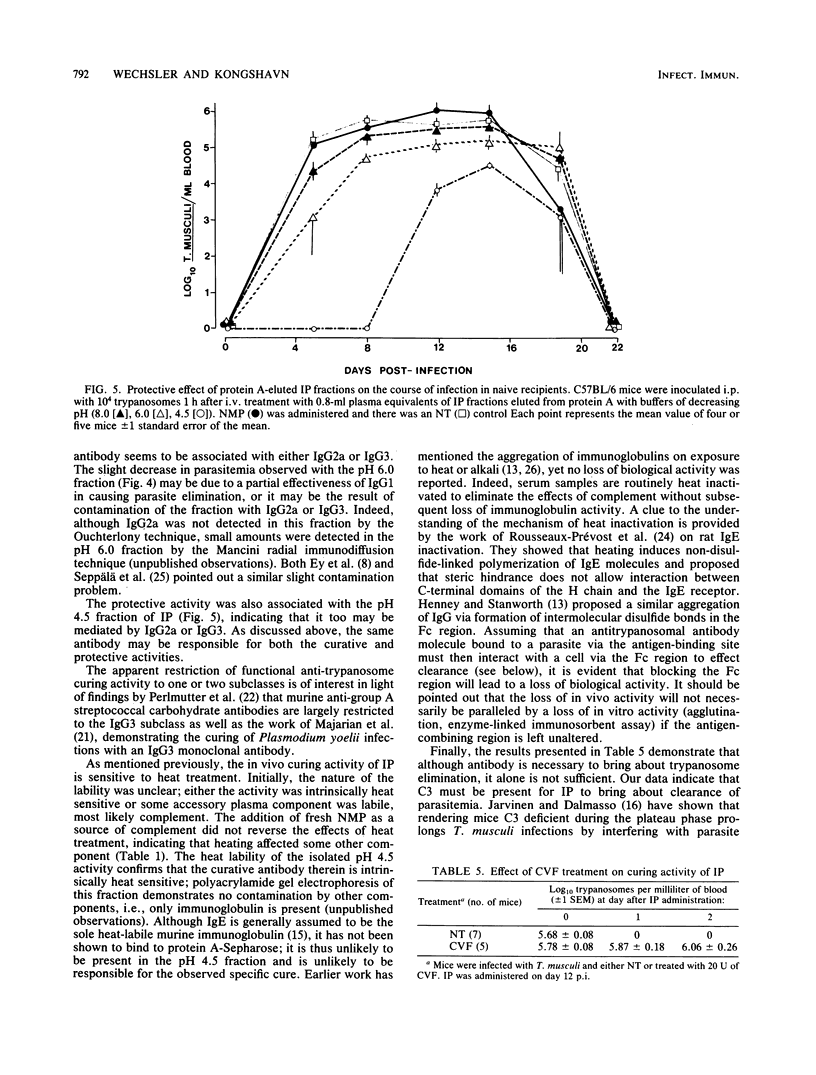
Plasma samples were collected from mice infected with Trypanosoma musculi at different times postinfection and administered to naive recipient mice either before or during T. musculi infection. The protective and curative activities of these plasma samples were shown to increase as the time of collection postinfection increased; plasma collected at 14 days postinfection was partially protective and partially curative, whereas that collected at 28 days postinfection was completely protective and curative. The curative activity was labile to heat treatment (30 min at 56 degrees C), whereas the protective activity was heat stable. Additional kinetic parameters relating to the efficacy of protection were investigated. Evidence is presented that both activities are immunoglobulin in nature. Protein A-Sepharose chromatography indicated that the activities are associated with the immunoglobulin G2a or immunoglobulin G3 subclasses of immunoglobulin G. The curative antibody appears to be intrinsically heat labile, since heat treatment of a purified immunoglobulin preparation abolished the ability to cure. Studies on the mechanism of parasite elimination from blood suggest that the process not only requires antibody but is also complement dependent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Albright J. F. Differences in resistance to Trypanosoma musculi infection among strains of inbred mice. Infect Immun. 1981 Aug;33(2):364–371. doi: 10.1128/iai.33.2.364-371.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. The decline of immunological resistance of aging mice to Trypanosoma musculi. Mech Ageing Dev. 1982 Dec;20(4):315–330. doi: 10.1016/0047-6374(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Brenière S., Viens P. Trypanosoma musculi: transfer of immunity from mother mice to litter. Can J Microbiol. 1980 Sep;26(9):1090–1095. doi: 10.1139/m80-181. [DOI] [PubMed] [Google Scholar]
- Brooks B. O., Reed N. D. Thymus dependency of Trypanosoma musculi elimination from mice. J Reticuloendothel Soc. 1977 Dec;22(6):605–608. [PubMed] [Google Scholar]
- Büngener W. Verlauf con Trypanosoma musculi-Infektionen in NMIR-Mäusen. Tropenmed Parasitol. 1975 Sep;26(3):281–284. [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Dusanic D. G. Trypanosoma musculi infections in complement-deficient mice. Exp Parasitol. 1975 Apr;37(2):205–210. doi: 10.1016/0014-4894(75)90071-5. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Jenkin C. R. Evidence implicating the mononuclear phagocytic system of the rat in immunity to infections with Trypanosoma lewisi. Aust J Exp Biol Med Sci. 1978 Apr;56(2):201–209. doi: 10.1038/icb.1978.22. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Jenkin C. R. The role of the macrophage in immunity to Trypanosoma lewisi infections in the rat. Cell Immunol. 1979 Feb;42(2):327–335. doi: 10.1016/0008-8749(79)90198-9. [DOI] [PubMed] [Google Scholar]
- Greenblatt H. C., Diggs C. L., Aikawa M. Antibody-dependent phagocytosis of Trypanosoma rhodesiense by murine macrophages. Am J Trop Med Hyg. 1983 Jan;32(1):34–45. doi: 10.4269/ajtmh.1983.32.34. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Stanworth D. R. The reactivity of rheumatoid factor with human gamma G globulin. Immunology. 1965 Aug;9(2):139–150. [PMC free article] [PubMed] [Google Scholar]
- Holmes P. H., MacAskill J. A., Whitelaw D. D., Jennings F. W., Urquhart G. M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. I. Aspects of the radiolabelling technique. Immunology. 1979 Mar;36(3):415–420. [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Menzel A. E. Physicochemical properties of reaginic antibody. VI. Effect of heat on gamma-E-, gamma-G- and gamma-A-antibodies in the sera of ragweed sensitive patients. J Immunol. 1967 Sep;99(3):610–618. [PubMed] [Google Scholar]
- Jarvinen J. A., Dalmasso A. P. Trypanosoma musculi infections in normocomplementemic, C5-deficient, and C3-depleted mice. Infect Immun. 1977 May;16(2):557–563. doi: 10.1128/iai.16.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark R., Thorén-Tolling K., Sjöquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Methods. 1983 Aug 12;62(1):1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- MacAskill J. A., Holmes P. H., Whitelaw D. D., Jennings F. W., Urquhart G. M. Immune mechanisms in C57B1 mice genetically resistant to Trypanosoma congolense infection. II. Aspects of the humoral response. Parasite Immunol. 1983 Nov;5(6):577–586. doi: 10.1111/j.1365-3024.1983.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Macaskill J. A., Holmes P. H., Jennings F. W., Urquhart G. M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. III. Studies in animals with acute infections. Immunology. 1981 Aug;43(4):691–698. [PMC free article] [PubMed] [Google Scholar]
- Macaskill J. A., Holmes P. H., Whitelaw D. D., McConnell I., Jennings F. W., Urquhart G. M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. II. Mechanisms in immune animals. Immunology. 1980 Aug;40(4):629–635. [PMC free article] [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Rank R. G., Roberts D. W., Weidanz W. P. Chronic infection with Trypanosoma musculi in congenitally athymic nude mice. Infect Immun. 1977 May;16(2):715–716. doi: 10.1128/iai.16.2.715-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux-Prévost R., Rousseaux J., Bazin H., Biserte G. Formation of biologically inactive polymers is responsible for the thermal inactivation of rat IgE. Int Arch Allergy Appl Immunol. 1983 Mar;70(3):268–276. doi: 10.1159/000233334. [DOI] [PubMed] [Google Scholar]
- Seppälä I., Sarvas H., Péterfy F., Mäkelä O. The four subclasses of IgG can be isolated from mouse serum by using Protein A-Sepharose. Scand J Immunol. 1981 Oct;14(4):335–342. doi: 10.1111/j.1365-3083.1981.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Soltis R. D., Hasz D., Morris M. J., Wilson I. D. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology. 1979 Jan;36(1):37–45. [PMC free article] [PubMed] [Google Scholar]
- Targett G. A., Viens P. The immunological response of CBA mice to Trypanosoma musculi: elimination of the parasite from the blood. Int J Parasitol. 1975 Apr;5(2):231–234. doi: 10.1016/0020-7519(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Vargas F. D., Viens P., Kongshavn P. A. Trypanosoma musculi infection in B-cell-deficient mice. Infect Immun. 1984 Apr;44(1):162–167. doi: 10.1128/iai.44.1.162-167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P., Caristan A., Pautrizel R. Macrophage function during Trypanosoma musculi infection in mice. Infect Immun. 1981 Nov;34(2):378–381. doi: 10.1128/iai.34.2.378-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Cure of Trypanosoma musculi infection by heat-labile activity in immune plasma. Infect Immun. 1984 Jun;44(3):756–759. doi: 10.1128/iai.44.3.756-759.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw D. D., MacAskill J. A., Holmes P. H., Jennings F. W., Urquhart G. M. Immune mechanisms in C57Bl mice genetically resistant to Trypanosoma congolense infection. I. Effects of immune modulation. Parasite Immunol. 1983 Jan;5(1):85–94. doi: 10.1111/j.1365-3024.1983.tb00726.x. [DOI] [PubMed] [Google Scholar]


