Abstract
Background and Aims
The isolation and culture of primary enteric neurons is a difficult process and yields a small number of neurons. We developed fetal (IM-FEN) and postnatal (IM-PEN) enteric neuronal cell lines using the H-2Kb-tsA58 transgenic mice that have a temperature sensitive mutation of the SV-40 large tumor antigen gene under the control of an interferon γ-inducible H-2Kb promoter element.
Methods
Enteric neuronal precursors were isolated from the intestines of E13-mouse fetuses and second day post natal mice using magnetic immunoselection with a p75NTR antibody. The cells were maintained at the permissive temperature, 33°C and IFN-γ for 24 or 48 h and then transferred to 39°C in the presence of GDNF for 7 days for further differentiation. Neuronal markers were assessed by RT-PCR, western blot and immunocytochemistry. Neuronal function was assessed by transplanting these cells into the colons of Piebald or nNOS−/− mice.
Results
Expression analysis of cells showed the presence of neuronal markers peripherin, PGP9.5, HuD, Tau, synaptic marker Synaptophysin, characteristic receptors of enteric neurons, Ret and 5-HT receptor subtypes at 33°C and 39°C. Nestin, S-100β and α-SMA were minimally expressed at 39°C. GDNF resulted in increased phosphorylation of Akt in these cells, similar to primary enteric neurons. Transplantation of cells into the piebald or nNOS−/− mice colon improved colonic motility.
Conclusions
We have developed novel enteric neuronal cell lines that have neuronal characteristics similar to primary enteric neurons. These cells can help us in understanding newer therapeutic options for Hirschsprung’s disease.
Keywords: Immorto, Enteric neurons, GDNF, proliferation, differentiation, Piebald, nNOS−/−
The enteric nervous system (ENS) is a complex network of neurons and glia derived from vagal, sacral and upper thoracic neural crest cells1. The neural crest cells migrate into the gut where they proliferate and then differentiate into a variety of distinct cell types that controls the motility and secretion of the gastrointestinal tract. Several growth factors like glial cell line-derived neurotrophic factor (GDNF) and neurturin2 influence the proliferation and survival of the ENS. GDNF binds to its receptor Ret, a transmembrane tyrosine kinase receptor, which requires one of the four glycosylphosphatidylinositol (GPI)-linked co-receptors (GFRα1–4) for its activation. GDNF/GFRα1/Ret signaling is essential for the migration, proliferation, maturation and survival of ENS precursors during embryonic development3. GDNF-deficient mice, GFRα1-deficient mice or Ret −/− mice have renal agenesis, total intestinal aganglionosis and defects in the sympathetic, parasympathetic and sensory nervous system4–6.
Currently the only method to study enteric neurons in culture is by primary isolation of fetal enteric neurons or adult myenteric neurons. This is a tedious process and yields only few cells. Therefore the generation of an enteric neuronal cell line would be a great resource to study the enteric nervous system and the diseases that affect it. Using the well established primary culture method for enteric neurons7 we generated immorto fetal enteric neuronal (IM-FEN) and immorto post-natal enteric neuronal (IM-PEN) cell lines. The immortomouse was used to derive conditionally immortalized cell lines, because it has already been shown to be very successful in establishing cell lines from various tissues that have proved difficult to culture in vitro8.
The origins and characteristics of H-2Kb-tsA58 transgenic mice have been described in detail8. In brief, these transgenic mice have stably integrated the SV40 mutant strain tsA58 thermolabile TAg gene, a conditional immortalizing gene, under the control of an interferon-γ (IFN-γ) inducible H-2Kb promoter element in every cell of the body. The tsA58 TAg gene product is functional at the permissive temperature of 33°C but is rapidly degraded at the nonpermissive temperature of 39°C. The H-2Kb promoter is active in a wide variety of tissues at various levels, and expression can be increased above basal levels in most cells by exposure to IFN-γ.
To our knowledge, this is the first description of the establishment of both fetal and postnatal enteric neuronal cell lines. We have characterized the neuronal marker expression, the receptor expression, growth curve and differentiation properties in these cell lines. The functional properties of these neurons have been established in vivo using intestinal transplantation. The Ret activation stimulated PI-3-kinase activity9 that has been demonstrated in primary enteric neurons10 was also seen in our enteric neuronal cell lines.
Materials and Methods
Reagents
Collagenase and dispase (Worthington Biochemical), Neurobasal-A medium, B-27 serum free-supplement (Invitrogen), Recombinant Ms IFN-γ (Chemicon), RNeasy mini kit (Qiagen), iScript cDNA Synthesis kit, SYBR Green I (Bio-Rad), PCR primers (IDT, Coralville, IA), GoTaq DNA polymerase (Promega, Madison, WI), 6 or 24-well plates coated with poly-D-lysine and laminin (BD Biosciences), prefilter and columns for magnetic separation (Miltenyi Biotec), pEGFP DNA vector (BD Biosciences), Caspase-1 inhibitor II (Calbiochem), Lipofectamine 2000 (Invitrogen), Guanethidine sulfate (TCI America). All other chemicals were purchased from Sigma. GDNF was produced as previously described11.
Antibodies to p75NTR, PGP9.5, Peripherin, HuD, Nestin, α-smooth muscle actin, GFP, Anti Goat IgG HRP-linked (Chemicon International); GFAP, phospho-Akt, Akt, Anti-Rb/Ms IgG HRP-linked (Cell Signaling); BrdU (Amersham Biosciences); Cytokeratin 8 [M20] (abcam) ; MAP2, β-actin and Synaptophysin (Sigma); SERT from Dr. Randy D. Blakely; Donkey anti-mouse biotinylated, peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc); Synaptic vesicle protein 2 (SV2) (Hybridoma facility, University of Iowa), S-100β (BD Biosciences). Fluorescent photomicrographs were obtained on Leica microscope.
Animals
H-2Kb-tsA58 Transgenic mice were obtained from Charles River (Wilmington, MA). The origin and characteristics of this strain have been described in detail8. In brief, the promoter of the mouse H-2Kb class I gene was fused to the SV40 tsA58 early region coding sequences. This construct was microinjected into fertilized oocytes from (CBA/Ca X C57BL/10) F1 mice, and the oocytes were reimplanted. One founder animal, H2ts6, was used to establish the strain of H-2Kb-tsA58 transgenic mice used in the present experiments. Timed pregnant female mice (at embryonic day 13) or day 2 pups were used for the isolation of enteric neurons.
C57BL/6J controls and piebald heterozygote mice (ednrbS-l/ednrbS) were obtained from the Jackson Laboratories and were used for the transplantation experiments. The coat color of heterozygotes is a composite of black and white. These are easily distinguished from the homozygotes that have an almost completely white coat.
nNOS−/− mice (B6;129S4-NOS1tm1plh/J) were obtained from Jackson Laboratories and were used for the intestinal neuronal transplantation experiments.
All animal studies were approved by the Emory University Institutional Review Board.
Isolation and In Vitro culture of p75 expressing cells
p75-expressing cells were isolated from the intestines of E13-Immorto mouse fetuses and second day post natal mice by magnetic bead immunoselection with a monoclonal antibody to low affinity NGF receptor (p75NTR, 10 μg/mL) as previously described7. Following immunoselection, trypan blue excluding cells were plated onto a 75-cm2 cell culture flask in modified N2 medium2 containing GDNF (100 ng/ml), 10% fetal bovine serum (FBS) and 20 units/ml of Recombinant Ms IFN-γ and cultured in a humidified tissue culture incubator containing 10% CO2 at permissive temperature, 33°C. The cells were observed regularly for signs of proliferation and were passaged when the flask became confluent. The cells were mechanically dislodged from the plates, dissociated with Trypsin-EDTA (0.25%) and replated at low density on 6 or 24 well plates coated with poly-D lysine and laminin (to which the cells more firmly attach) for other experiments. All experiments were performed between passages 20–40. To further differentiate into neuronal cells, when the cell confluency was about 50–60% the medium was changed to Neurobasal-A medium containing B-27 serum free-supplement, 1 mmol/L glutamine, 1% FBS and GDNF (100 ng/ml) and were transferred to an atmosphere of 5% CO2 at 39°C.
BrdU cell proliferation assay
Cells were incubated at 33°C overnight, and then incubated in parallel at either 33°C (for another 24 h) or 39°C (7 days). BrdU (10 μM) was added to the medium. Two hours later the cells were fixed, denatured and stained as described previously12. Two hundred PGP9.5 positive enteric neurons/well were scored in a blinded fashion to determine the percentage of BrdU+/PGP9.5+ cells. At least four wells were scored for each condition and the experiment was repeated three times.
RT-PCR
IM-FEN or IM-PEN were cultured in the conditioned medium at 33°C or 39°C (for 2 or 7 days). Total RNA was isolated using the RNeasy Mini Kit. One μg of total RNA was used to synthesize first strand cDNA using iScript cDNA Synthesis kit according to recommended procedure; and reverse transcription polymerase chain reaction (RT-PCR) was performed as described previously12 using the following oligonucleotide primers:
c-Ret (forward) 5′-TACCGTACACGGCTGCATGAGAAT-3′ and
c-Ret (reverse) 5′-ATGTGGAAGTGGTAGAAGGTGCCA -3′;
Peripherin (forward) 5′-ACAACCTGGTGCTCTTCCGTA-3′ and
Peripherin (reverse) 5′-TCTGGCTTCACTGTTGCCTCT -3′;
SERT (forward) 5′-AAGCGCAGGGAATGGTTTGT-3′ and
SERT (reverse) 5′-AGCATTTCCTTCACGTCGCT -3′;
5-HT4A (forward) 5′-CCAAGGCAGCCAAGACTTTA-3′ and
5-HT4A (reverse) 5′-GGCCACTGTGCAAAACTGT-3′;
Synaptophysin (forward) 5′-TCTTTGTCACCGTGGCTGTGTT-3′ and
Synaptophysin (reverse) 5′-TCCCTCAGTTCCTTGCATGTGT -3′;
GAPDH (forward) 5′-CCAGTATGATTCTACCCACGGCAA-3′ and
GAPDH (reverse) 5′-ACAGTCTTCTGAGTGGCAGTGATG-3′.
The 5-HT2A, 5-HT3A, Tau, Nestin, GFAP and α-SMA primers used for semi-quantitative PCR have been described previously13, 14, 15. Two thirds of each reaction were analyzed on a 2% agarose gel stained with ethidium bromide and the amplified products visualized by UV translumination.
Real-time PCR
For real time PCR, amplifications were detected using SYBR Green I in reactions performed as previously described12. RET or peripherin mRNA was then normalized to the respective control GAPDH amounts and presented as a ratio under different conditions.
Immunocytochemistry (double labeling)
Cells were fixed and incubated with primary antibody (Nestin, 1:200; α-SMA, 1:500; Cytokeratin 8 (M20), 1:100 and S-100β, 1:1000) for 24 h. Secondary detection was performed in conjunction with Alexa-Fluor 488 antibody. All cells were stained for peripherin, neuronal marker (1:100) and secondary detection was performed in conjunction with Alexa fluor-594. Simultaneous staining for negative controls, using an isotopic antibody (control IgG) to peripherin (1:100) was used in certain wells and the cells were stained with DAPI and appropriate secondary antibody. Caco2-BBE human intestinal epithelial cells were also stained alongside with IM-PEN cells with the neuronal marker peripherin to document specificity of the antibody. Activated hepatic stellate cells or glial cells (primary glial cells from mouse cortex) were stained for α-SMA or S-100β respectively as a positive control alongside with IM-PEN cells. In addition these cells were stained for peripherin as a negative control. At least two wells (Two hundred cells/well) were scored in a blinded fashion for each condition in each of the experiments and the experiment was repeated four times.
Western Blotting
Cell lysates were obtained from IM-FEN and IM-PEN cultured at 33°C or 39°C (7 days) and 16 μg of protein/lane were loaded onto a 12% SDS-PAGE gel. The membranes were probed for PGP9.5, HuD, SERT, α-SMA, GFAP, Synaptophysin and SV2 using respective specific antibodies. β-actin was used as a loading control. The experiment was repeated atleast 3 times. A semi-quantitative measurement of the band intensity was performed using the computer software Scion Image (Maryland, USA) and expressed as a ratio of band intensity with respect to the loading control.
Akt phosphorylation
IM-PEN were cultured at 39°C for 7 days in conditioned medium and switched to serum free medium for 48 h. Cells were incubated with Krebs buffer for 1 h and then treated with vehicle or GDNF (100 ng/ml) for 30 min in Krebs buffer. Cell lysates were obtained and Western blotting performed using phospho-Akt (Ser473) and Akt antibody.
Transfection and Transplantation
IM-FEN were cultured in the conditioned medium at 33°C for 24 h. Cells were transfected (3 μg pEGFP DNA vector/well) using Lipofectamine 2000 according to the manufacturer’s instructions. For transplantation, transfected cells were suspended at a concentration of 50,000 cells/μl in Dulbecco’s phosphate-buffered saline (PBS) containing the caspase-1 inhibitor Ac-YVAD-CMK (500 μmol/L) and kept on ice. Experimental recipient mice, piebald hetero (4–6 weeks old) or nNOS−/− mice (8 weeks old) were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally). A midabdominal incision was made and 2 μl of cell suspension was injected (2 injections) into the proximal colon of nNOS−/− mice, proximal and distal colon of Piebald heterozygous mice (1 cm from rectum) immediately subserosal using a 22-gauge needle attached to a 10-μL Hamilton syringe16. C57BL/6 control mice received only PBS.
Isometric Muscle Recording
One week following cell transplantation, longitudinal muscle strips (with myenteric plexus) from C57BL/6, nNOS−/− and nNOS−/− transplanted mice proximal colon, circular muscle strips from C57BL/6, piebald hetero and piebald hetero transplanted mice distal colon were obtained by careful dissection using a dissecting microscope. Settings for recording were done as described previously17. Colonic relaxation was measured in the C57BL/6, nNOS−/− and nNOS−/− transplanted mice proximal colon longitudinal muscle strips in response to transmural stimulation and was recorded by electrical field stimulation (EFS; 24 V, 8 Hz, 5 millisecond pulse for duration of 30 seconds) in the presence of atropine (1 μM) and guanethidine sulphate (1 μM) to block cholinergic- and adrenergic-mediated responses, respectively. Strips were pre-contracted with 5-hydroxytryptamine (10 μM) for 30 sec.
Colonic contraction was measured in the C57BL/6, piebald hetero and piebald hetero transplanted mice distal colon circular muscle strips in the presence of NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 100 μM) using EFS (24 V, 20 Hz, 5 millisecond pulse for duration of 30 seconds). Contraction or relaxation was expressed as a % change from baseline muscle tone. For quantitative determination of contraction or relaxation, intestinal strips from at least 3 mice/strain were used, and the average of contractions or relaxations was obtained from EFS.
Measuring Colonic Motility
Colonic motility was assessed as described previously18. A 2-mm glass bead was placed 2 cm proximal to the anal opening using a plastic Pasteur pipette lightly lubricated with lubricating jelly. The animals were immediately placed into a cage and monitored for the time to pass the bead. Colonic motility was assessed by measuring the amount of time between bead placement and expulsion of the bead. The time to expulsion is reported here to the nearest tenth of a minute.
Whole mount
Proximal and mid distal colons were obtained from Piebald hetero mice. Preparation for whole mount, fixation and Acetylcholinesterase staining was done as described17. Five randomly selected fields per mouse were evaluated from 3 animals of each genotype. The neurons were counted within a 0.1-mm2 grid. Total number of neurons was expressed as acetylcholinesterase positive neurons per grid.
For assessment of GFP transfected cells in the distal colon, fixed intestinal tissues were blocked with 3% BSA for 1 h and then incubated with rabbit anti-GFP polyclonal antibody (1:100) in 1% BSA. Secondary detection was performed in conjunction with Alexa-Fluor 488 antibody. Tissues were stained for Peripherin, neuronal marker (1:100) and secondary detection was performed in conjunction with Alexa-fluor 594.
Statistical Analysis
Statistics was done with the Student t-test or with the one-way analysis of variance (ANOVA) (1999 Graph Pad software Inc). Statistical tests were 2-sided. P value ≤ 0.05 was considered statistically significant.
Results
Establishment of cell lines
Fetal cell lines were established from E-13 heterozygous immortomice. Post natal cell lines were established from homozygous immortomice pups (1 or 2 day old). The entire stomach and intestine was used to generate the cell lines. In the initial stages of the establishment of the lines we used four week old either homozygous or heterozygous immortomice and were not able to obtain cells that would divide and attain confluence. The first ten mice that were used for establishment of fetal cell lines did not yield an adequate number of cells to create a cell line. Following this with adjustment of plating density and culture conditions the next six mice were used to generate four fetal and two post natal cell lines. These adjustments included increasing the plating density to 0.05 million cells/well in a twenty four well plate and improving the culture medium by adding 10% serum to the N2 medium at 33°C and using Neurobasal medium with 1% serum at 39°C. When cells are harvested from these mice initial time to confluency can vary between 2–4 weeks. Following this the cells attain a standard growth rate as shown in Figure 2.
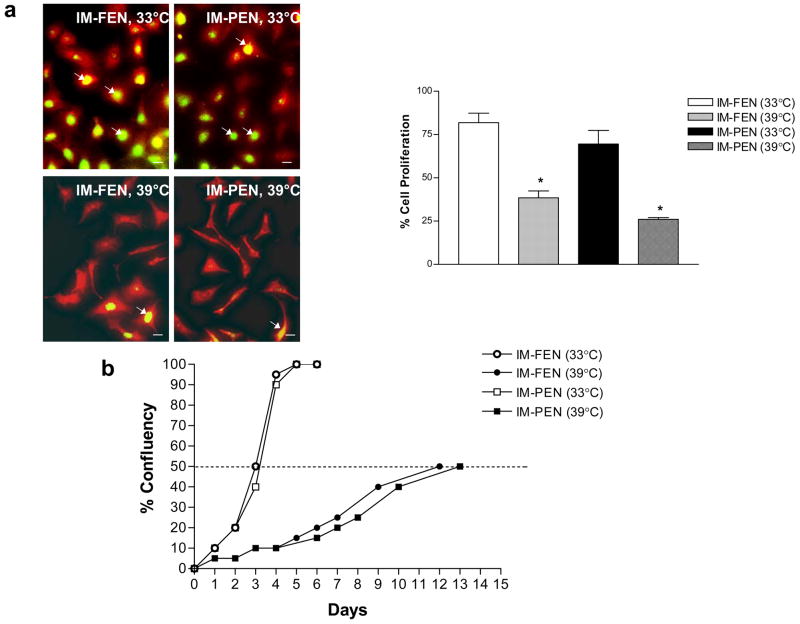
Figure 2. Proliferation rate.
(a) BrdU labeling of IM-FEN and IM-PEN grown at the permissive temperature (33°C) in medium containing interferon-γ or 39°C for 7 days in medium without interferon-γ. BrdU was added to the medium 2 h prior to fixation. The % of BrdU+ (green)/PGP9.5+ (red) neurons was determined to assess proliferation. Arrows point to the BrdU+ neurons. Results are mean + S.E., n=3, * = p<0.05. Scale bar: 20 μm. (b) The percentage confluency of the cell lines at 33°C and 39°C is shown. The rate of achieving fifty percent confluence was much faster for IM-FEN and IM-PEN (3 days) at 33°C when compared to 39°C (12–13 days).
Culture Characteristics
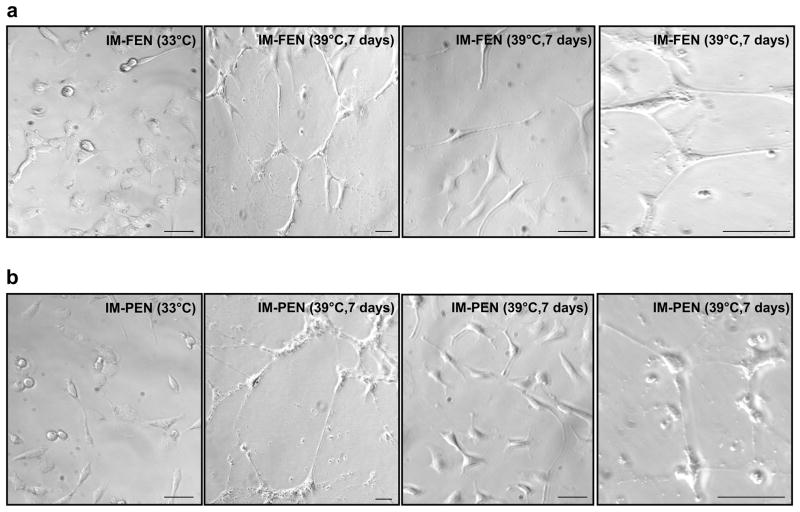
IM-FEN and IM-PEN were passaged as described in the Methods. When cultured at the permissive temperature, 33°C they proliferated until confluent monolayers were formed. Phase-contrast photomicrographs of IM-FEN and IM-PEN shows circular cells at 33°C that differentiate and form neurites after culture for 7 days at 39°C (Fig. 1). The rate of proliferation assessed by BrdU labeling was significantly higher at 33°C compared to 39°C (Fig. 2a). Double immunostaining demonstrated that BrdU+ cells were PGP9.5+ indicating that the proliferating cells were of neuronal origin.
Figure 1. Phase-contrast photomicrographs of IM-FEN and IM-PEN.
Cells cultured at the permissive conditions, 33°C and in the presence of interferon-γ, appear circular with short neuronal processes. After 7 days at the non-permissive conditions (39°C without interferon-γ), a majority of the cells get further differentiated with longer neuronal processes. Photographs at 7 days are shown at 2 different magnifications (10X and 20X). Scale bar: 100 μm.
Cells in the 75-cm2 cell culture flasks were observed daily for percentage confluency. As demonstrated by the growth curves, the rate of achieving fifty percent confluency was much faster for IM-FEN and IM-PEN at 33°C (3 days) when compared to 39°C (12–13 days) (Fig. 2b).
IM-FEN and IM-PEN demonstrate the presence of neuronal markers
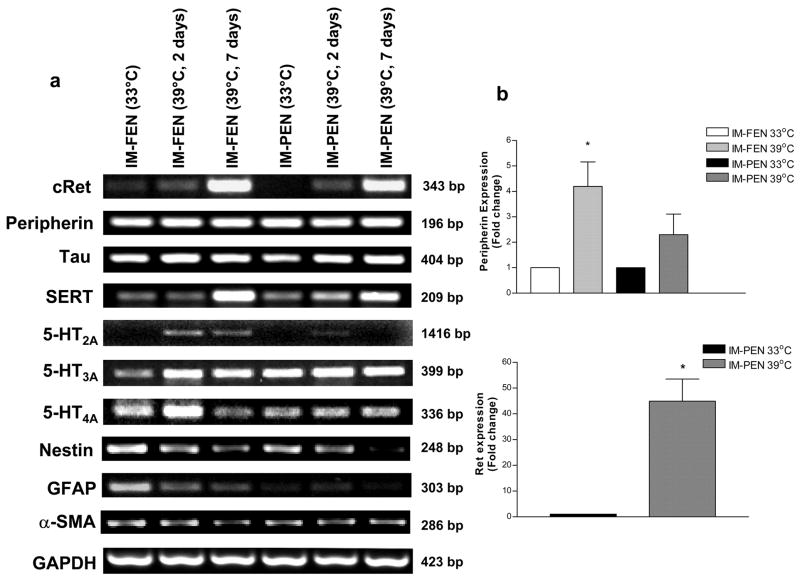
Neuronal markers peripherin and Tau, receptor cRet and transporter SERT were expressed at both 33°C and 39°C in both cell lines, IM-FEN and IM-PEN. At 39°C there was a significant increase in the expression of cRet and SERT. Consistent with the primary enteric neurons both cell lines showed the presence of 5HT2A, 5HT3A and 5HT4A receptors. 5HT2A was present mainly in the fetal neuronal cell line. Glial marker GFAP was expressed at 33°C but was minimally expressed at 39°C. This decrease in GFAP expression was due to the effect of GDNF in promoting the survival and differentiation of enteric neurons as has been previously shown2. Although there was a slight expression of smooth muscle actin at 33°C, this was reduced at 39°C. Nestin, an intermediate filament type VI, has been extensively used to identify neural stem cells. Nestin expression was higher at 33°C compared to 39°C (Fig. 3a). To assess the quantitative changes in neuronal marker and RET receptor expression, we performed Real-time PCR on fetal and post natal cell lines cultured at 33°C and 39°C. As seen in Figure 3b we found a significant increase in Peripherin expression at 39°C. Ret expression was also quantified and found to be increased in IM-PEN at 39°C compared to 33°C.
Figure 3. Expression of neuronal markers in IM-FEN and IM-PEN cultured at 39°C (2 or 7 days) compared to cells cultured at 33°C.
(a) RNA was isolated from IM-FEN and IM-PEN cultured at 33°C or 39°C (2 or 7 days) and amplified for cRet, peripherin, Tau, SERT, 5-HT2A, 5-HT3A, 5-HT4A, Nestin, GFAP, α-SMA and GAPDH (loading control), n=4. (b) Histogram for IM-FEN and IM-PEN mRNA assessed by Real Time PCR showing the relative level of expression of Peripherin or Ret (IM-PEN) after normalization to GAPDH expression. Results are mean + S.E., * = p<0.05.
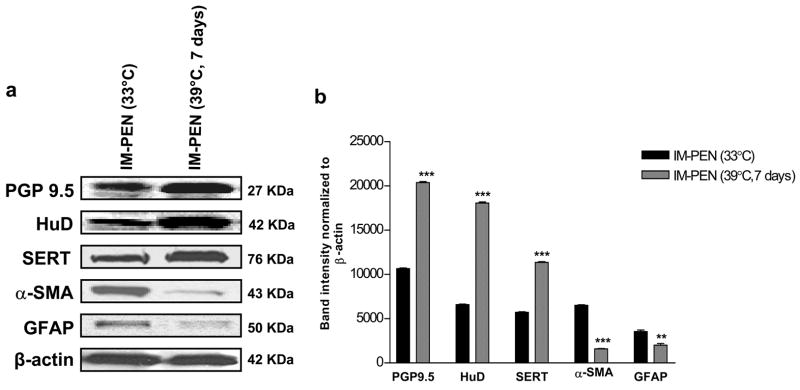
Western blot analysis of neuronal marker expression
Western blot analysis showed the expression of neuronal markers, (PGP9.5 and HuD) and transporter SERT in IM-FEN and IM-PEN cultured at 33°C. The expression of these proteins increased significantly when cultured at 39°C for 7 days. GFAP and α-SMA expression was minimal at 39°C compared to 33°C in both cell lines (Fig. 4a, b).
Figure 4. Enhanced neuronal markers expression and limited expression of α-SMA and GFAP at 39°C.
Western blot analysis for neuronal markers (PGP 9.5, HuD), transporter SERT, α-smooth muscle actin (α-SMA), Glial Fibrillary Acidic Protein (GFAP) and β-actin as a loading control. Neuronal markers were present in both IM-FEN and IM-PEN and there was very minimal expression of α-SMA and GFAP at 39°C. Results are mean + S.E., n=3, ** = p<0.01, *** = p<0.001.
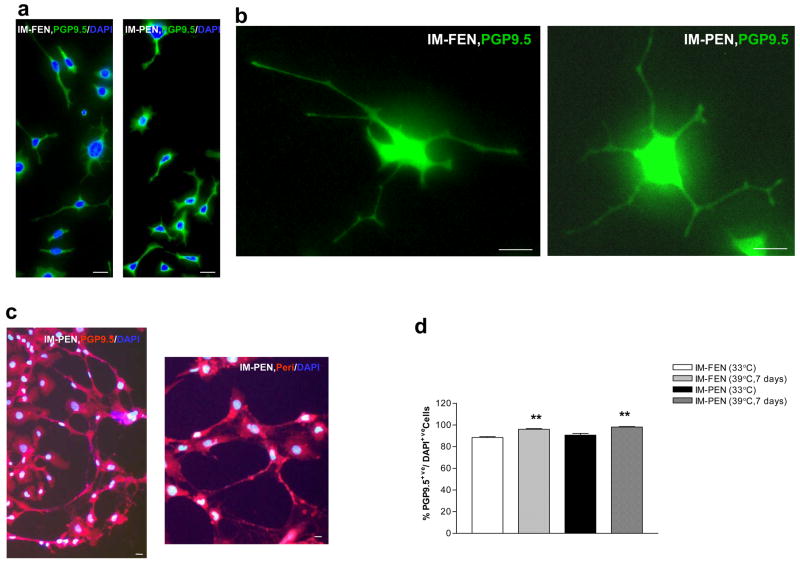
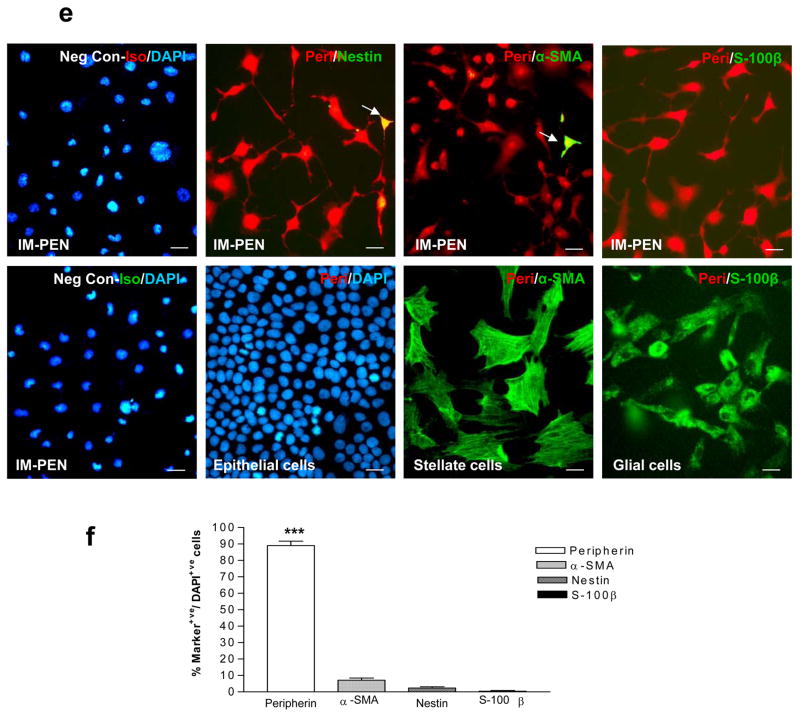
Immunocytochemical assessment of neuronal, glial and smooth muscle markers in IM-FEN and IM-PEN
Immunocytochemical analysis of cells for neuronal marker PGP9.5 showed greater than 88% of PGP9.5 positive cells at both temperatures (Fig. 5d). Double labeling was performed with neuronal marker Peripherin (red) in conjunction with other markers (green). Nestin, S-100β and α-SMA expression was very minimal at 39°C, (Fig. 5e, f). The specificity of the neuronal marker Peripherin was assessed by staining activated stellate cells, glial cells and epithelial cells alongside our neuronal cells. As shown in Fig. 5e the neuronal marker is specific for IM-PEN and is not taken up by the epithelial, stellate or glial cells. Positive controls for α-SMA and the glial marker S-100β are also included. This is consistent with RT-PCR and western blot data where we observed increased neuronal markers and decreased GFAP and α-SMA expression at 39°C compared to 33°C. Assessment for Cytokeratin to identify epithelial cells revealed no evidence of staining in the cells (data not shown).
Figure 5. Immunocytochemical assessment of neuronal, glial and smooth muscle markers in IM-FEN and IM-PEN.
(a) Representative images of fetal and post natal enteric neurons cultured for 7 days at 39°C and stained with PGP9.5 and DAPI. (b) Higher power view of a (IM-FEN and IM-PEN) neuron with neurites stained with PGP9.5 is shown. (c) Neuronal network stained with PGP9.5 (red) or Peripherin (red) and DAPI. (d) Histogram shows the relative percentages of PGP9.5 positive cells at 33°C and 39°C. (e) Using IM-FEN cells double labeling was performed with peripherin (red) in conjunction with other stated markers (arrow, green). Nuclear stain is shown with DAPI staining. Negative controls with isotopic antibody to peripherin and peripherin staining of epithelial cell line, stellate cells and glial cells are shown. Positive controls for α-SMA (activated stellate cells) and for S-100β (cortical glial cells) are shown. (f) Histogram shows the stated cell markers expressed as a percentage of DAPI+ cells. Results are mean + S.E., n=4, *** = p<0.001. Scale bar: 20μm.
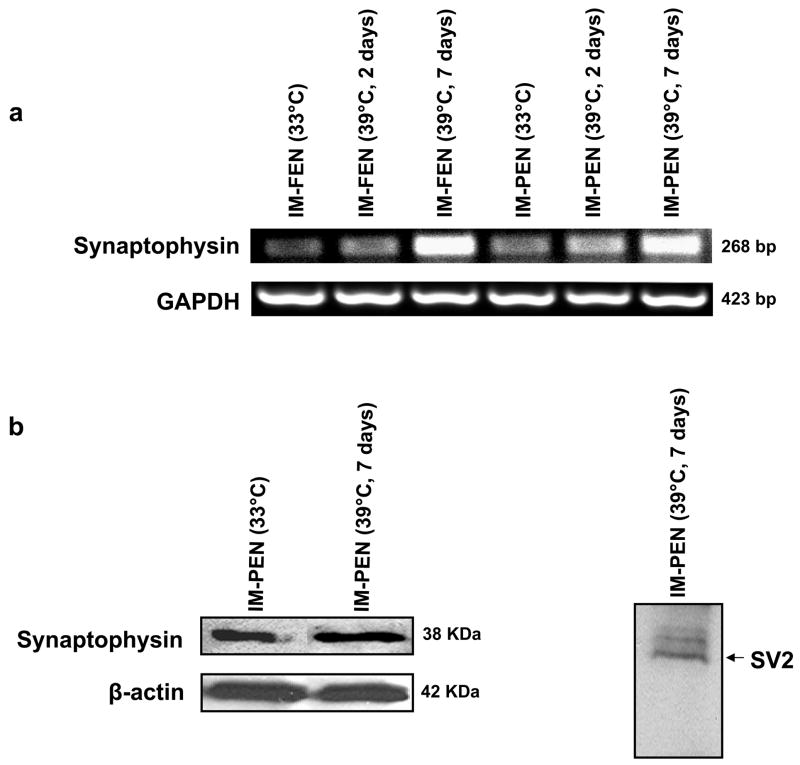
Synaptic markers are present in IM-FEN and IM-PEN
To further confirm that the cell lines are neuronal, we determined the presence of synaptic markers - Synaptophysin and SV219,20. We found the presence of these markers at both 33°C and 39°C (Fig. 6).
Figure 6. Synaptic markers are present in IM-FEN and IM-PEN.
(a) RNA was isolated from IM-FEN and IM-PEN cultured at 33°C or 39°C (2 or 7 days) and amplified for Synaptophysin and GAPDH (loading control). Synaptophysin expression was higher at 39°C compared to 33°C. (b) Western blot analysis confirmed the expression of synaptic markers, Synaptophysin and SV2. β-actin was used as a loading control.
GDNF activates Akt in IM-PEN similar to primary enteric neuronal cultures
We have previously demonstrated that GDNF activates the PI-3-kinase pathway in primary enteric neurons10. Cell lysates were obtained from IM-PEN treated with vehicle or GDNF for 30 min and Western blot was performed. Similar to primary neurons GDNF significantly increased the phosphorylation of Akt compared to vehicle in IM-PEN. Total Akt was used as a loading control (Supplemental Fig. 1).
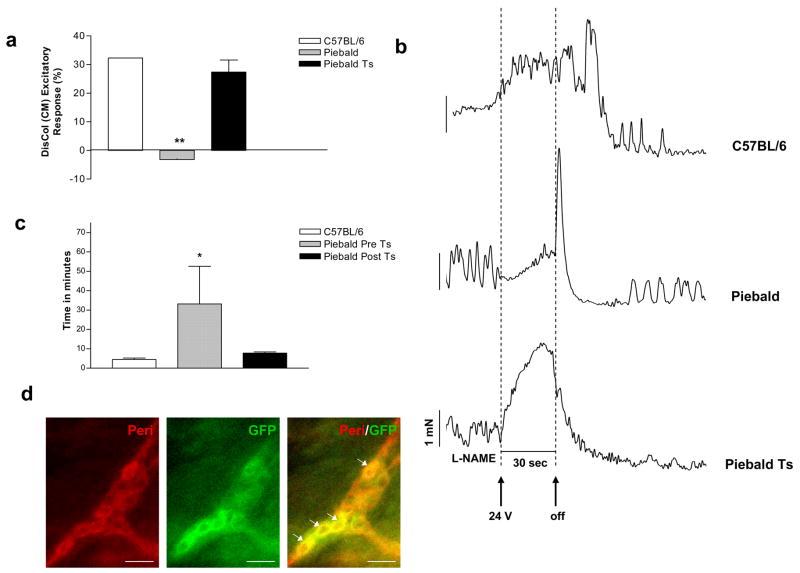
Transplantation of IM-FEN improves contraction in the piebald mice colon and relaxation in the nNOS−/− mice colon
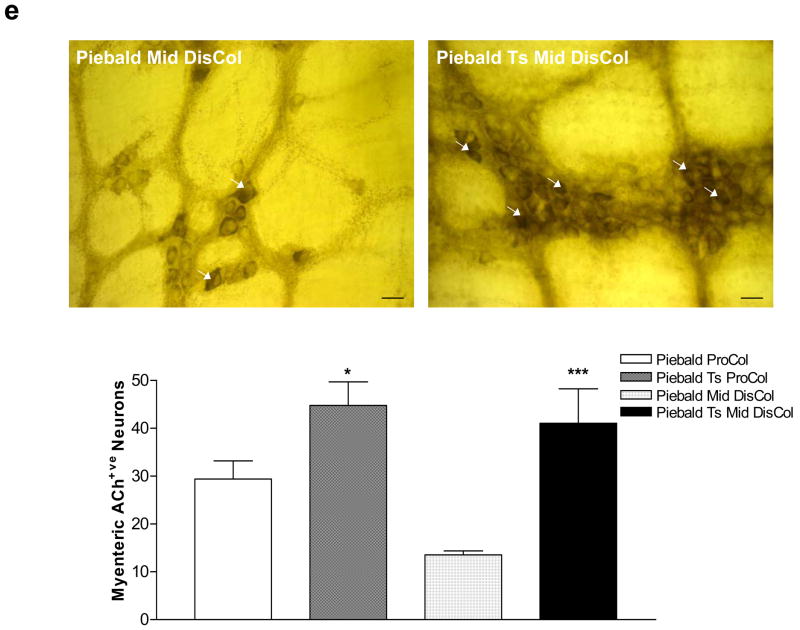
GFP labeled IM-FEN cultured at 33°C were transplanted into the proximal and distal colon of the heterozygous piebald mice (200,000 cells). These mice have a known reduction in the number of enteric neurons in the colon with the most distal 1 cm being aganglionic21. The homozygous piebald lethal mice die at 3–4 weeks of age and hence were not used for tranplantation. One week after transplantation the mice were sacrificed and the colon was assessed for immunostaining with GFP and Peripherin, acetylcholine esterase staining and isometric muscle recording. As seen in Fig. 7a there was improved contractile activity in the distal colon (P<0.01) of the transplanted piebald mice. Colonic motility was also assessed by the time taken to expel a glass bead inserted into the colon. The bead expulsion time was significantly higher in the Piebald heterozygotes pre transplant. After transplantation of the enteric neurons there was an improvement of the expulsion time and this was similar to C57BL/6 mice (Fig. 7c). The transplanted neurons were identified in vivo by double staining with GFP and Peripherin (Fig. 7d). Staining for acetylcholine esterase revealed an increase in these neurons in transplanted mice compared to non-transplanted mice (Fig. 7e).
Figure 7. Improvement of colonic neuronal function in the piebald heterozygote mice and nNOS−/− mice transplanted with GFP transfected IM-FEN.
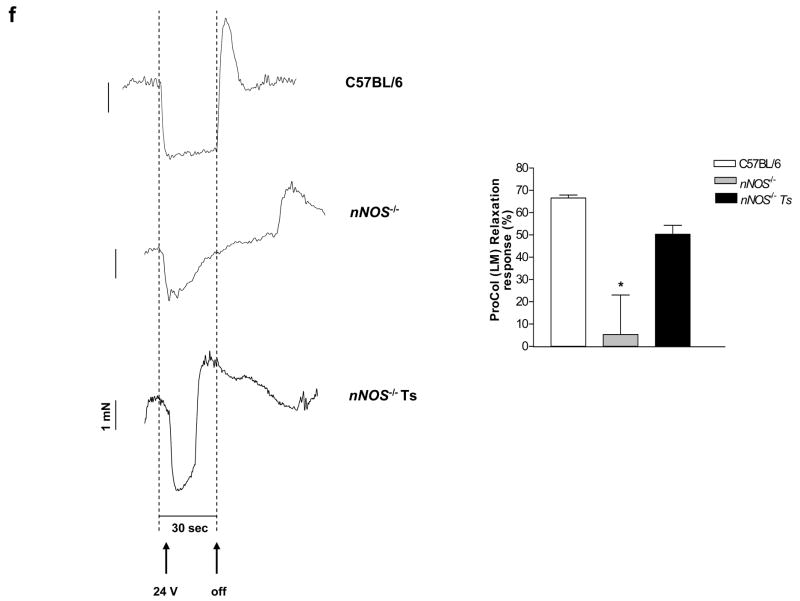
(a) Histogram showing the excitatory responses from distal colons of C57BL6, Piebald and Piebald transplanted mice. There was improvement in the contractile activity in the transplanted distal colon. Results are mean ± S.E., n=3, ** = p<0.01 (b) Representative tracing of C57BL/6, piebald and transplanted piebald mice distal colon. (c) Colonic transit time was assessed in C57BL/6, pre and post-transplanted piebald mice using the bead test as described in the materials and methods. n = at least 3 animals in each group and * = p<0.05. (d) The neurons were identified by staining with GFP (transplanted neurons, green) and peripherin (red). (e) Whole mount preparation from the Piebald heterozygote and transplanted proximal and mid-distal colon were stained for acetylcholine esterase. An increase in acetylcholine esterase staining neurons in piebald heterozygote transplanted mice colon compared to vehicle treated mice is seen. Results are mean ± S.E., n=3, * = p<0.05, *** = p<0.001. (f) Representative tracings of EFS-induced relaxation in C57BL/6, nNOS−/− and nNOS−/− transplanted mouse colons. There was reduction in relaxation in nNOS−/− mice compared to controls. This reduction was improved in the transplanted nNOS−/− mice. Histogram shows the average relaxation responses. Results are mean ± S.E., n=3, * = p<0.05.
GFP labeled IM-FEN were also transplanted into the proximal colon of the nNOS−/− mice. These mice have been shown to have major impaired relaxation in the lower esophageal sphincter and pyloric sphincter. Impaired relaxation was also observed in the ileum22. EFS of the proximal colon under NANC conditions was performed to assess neurally mediated nitrergic relaxation of the longitudinal muscle and found a reduction in relaxation compared to controls (P<0.05). This reduction was improved in the transplanted nNOS−/− mice (Fig. 7f).
Discussion
Using the H-2Kb-tsA58 transgenic mice we have developed fetal and postnatal enteric neuronal cell lines with features similar to primary enteric neurons. The IM-FEN and IM-PEN have been passaged forty times since their isolation and have shown no major changes in their cell growth characteristics, morphology, neuronal properties, or GDNF sensitivity. This homogeneity suggests that the lines will tend to remain phenotypically and biologically stable in vitro for many generations. One of the problems with introduction of immortalizing genes into cells is that these genes can alter normal cellular physiology. This problem can theoretically be overcome through the use of conditional immortalizing genes which allow the generation of continuously proliferating cell lines capable of differentiation after inactivation of the immortalizing gene. In other studies to date, H-2Kb-tsA58 transgenic mice have been used to isolate epithelial cell lines from the crypts of colon and small intestine23 and osteoclast precursor cell lines which can induce such precursors to differentiate into osteoclasts24. Cell lines derived from the immorto-mice demonstrate properties similar to primary cells25.
In the current study we demonstrate that IM-FEN and IM-PEN have neuronal characteristics demonstrated by the expression of HuD, the neurofilament markers peripherin, PGP9.5 and Tau. In the presence of interferon-γ at 33°C, the temperature at which the enteric neuronal precursors isolated from immorto-mice proliferate, we find a minimal expression of glial markers or α-smooth muscle actin. However at 39°C, the temperature which inhibits proliferation and promotes differentiation we find a reduction in glial or smooth muscle markers and further differentiation of neuronal markers. We also found expression of nestin in both IM-FEN and IM-PEN at 33°C but very limited at 39°C. Neuroblastoma cell lines have previously been demonstrated to give rise to cells expressing smooth muscle markers26. We found a small expression of α-smooth muscle actin in our cells.
The SERT transporter is very important in enteric neurons and the role of serotonin in the functioning of enteric neurons has been well established. The 5-HT receptors known to be expressed in enteric neurons include 5HT1A, 5-HT2A, 5HT2B, 5-HT3 and 5HT4. We demonstrate the presence of the SERT transporter in both IM-FEN and IM-PEN. In addition we identified the presence of the main serotonergic receptors 5-HT3, 5HT4 which have been previously demonstrated in the mouse enteric nervous system14, 27.
In order to study the similarities in signal transduction between the primary enteric neurons and our cell lines we studied the PI-3-kinase pathway. GDNF is known to promote the survival of enteric neurons through the PI-3-kinase pathway10. The PI-3 kinase/Akt/FOXO signaling pathway plays a critical role in ENS precursor survival and neurite extension10. We now demonstrate that GDNF can phosphorylate Akt in our enteric neuronal cell line. This Ret dependent activation of Akt is an important characteristic of enteric neurons9.
The use of immortalized neuronal stem cells has been previously considered28. Cells of hippocampal origin have been demonstrated to restore cognitive function in models of global ischemia. Incomplete gut colonization by neural crest cells results in Hirschsprung’s disease, characterized by aganglionosis of the distal bowel29. It has been shown that multipotent, self renewing ENS progenitor cells (ENSPC) capable of generating neurons and glia derived from the neural crest can be isolated from the gut of fetal and neonatal mice and rats, and are capable of colonizing the colon after transplantation3, 15. In our experiments we performed non-syngeneic transplantation of enteric neuronal cells. We did not observe graft rejection of neurons and this may be due to the short seven day period of observation post transplantation. Immune rejection of neurons will need to be considered if this method is used for therapeutic purposes and appropriate immunosuppression measures will need to be undertaken.
In summary, we have developed fetal and postnatal enteric neuronal cell lines which have similar characteristics as primary enteric neurons. These cells will be a valuable tool to study the neurobiology of the enteric nervous system. These cell lines can be used to study the development of the enteric nervous system as well as the effects of diseases such as diabetes and Parkinson’s disease on the enteric nervous system.
Supplementary Material
Acknowledgments
This work is supported by the NIH-KO8 DK067045 (S.S), DK062092 (FA), DK075397 (FA), DK06411 (SVS) and the DDRDC (DK064399). We would like to thank Dr. Randy Blakley, Vanderbilt University for providing us with the SERT antibody.
Abbreviations
- GDNF
Glial cell line-derived neurotrophic factor
- IM-FEN
Immorto fetal enteric neurons
- IM-PEN
Immorto postnatal enteric neurons
Footnotes
Conflict of interest: All authors have no conflict of interests to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gershon MD. Genes and lineages in the formation of the enteric nervous system. Curr Opin Neurobiol. 1997;7:101–9. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- 2.Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200:116–29. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- 3.Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126:157–68. doi: 10.1242/dev.126.1.157. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–3. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 5.Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–24. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 6.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–3. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 7.Chalazonitis A, Tennyson VM, Kibbey MC, Rothman TP, Gershon MD. The alpha1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut. J Neurobiol. 1997;33:118–38. doi: 10.1002/(sici)1097-4695(199708)33:2<118::aid-neu2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88:5096–100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–73. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Anitha M, Mwangi S, Heuckeroth RO. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol Cell Neurosci. 2005;29:107–19. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci U S A. 1997;94:7018–23. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anitha M, Chandrasekharan B, Salgado JR, Grouzmann E, Mwangi S, Sitaraman SV, Srinivasan S. Glial-derived neurotrophic factor modulates enteric neuronal survival and proliferation through neuropeptide Y. Gastroenterology. 2006;131:1164–78. doi: 10.1053/j.gastro.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu MT, Rayport S, Jiang Y, Murphy DL, Gershon MD. Expression and function of 5-HT3 receptors in the enteric neurons of mice lacking the serotonin transporter. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1398–411. doi: 10.1152/ajpgi.00203.2002. [DOI] [PubMed] [Google Scholar]
- 15.Suarez-Rodriguez R, Belkind-Gerson J. Cultured nestin-positive cells from postnatal mouse small bowel differentiate ex vivo into neurons, glia, and smooth muscle. Stem Cells. 2004;22:1373–85. doi: 10.1634/stemcells.2003-0049. [DOI] [PubMed] [Google Scholar]
- 16.Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129:1817–24. doi: 10.1053/j.gastro.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 17.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil. 2006;18:464–71. doi: 10.1111/j.1365-2982.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 19.Phelan P, Gordon-Weeks PR. Widespread Distribution of Synaptophysin, a Synaptic Vesicle Glycoprotein, in Growing Neurites and Growth Cones. Eur J Neurosci. 1992;4:1180–1190. doi: 10.1111/j.1460-9568.1992.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 20.Schivell AE, Batchelor RH, Bajjalieh SM. Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem. 1996;271:27770–5. doi: 10.1074/jbc.271.44.27770. [DOI] [PubMed] [Google Scholar]
- 21.Ro S, Hwang SJ, Muto M, Jewett WK, Spencer NJ. Anatomic modifications in the enteric nervous system of piebald mice and physiological consequences to colonic motor activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G710–8. doi: 10.1152/ajpgi.00420.2005. [DOI] [PubMed] [Google Scholar]
- 22.Mang CF, Truempler S, Erbelding D, Kilbinger H. Modulation by NO of acetylcholine release in the ileum of wild-type and NOS gene knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1132–8. doi: 10.1152/ajpgi.00192.2002. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A. 1993;90:587–91. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers TJ, Owens JM, Hattersley G, Jat PS, Noble MD. Generation of osteoclast-inductive and osteoclastogenic cell lines from the H-2KbtsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1993;90:5578–82. doi: 10.1073/pnas.90.12.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virley D, Ridley RM, Sinden JD, Kershaw TR, Harland S, Rashid T, French S, Sowinski P, Gray JA, Lantos PL, Hodges H. Primary CA1 and conditionally immortal MHP36 cell grafts restore conditional discrimination learning and recall in marmosets after excitotoxic lesions of the hippocampal CA1 field. Brain. 1999;122(Pt 12):2321–35. doi: 10.1093/brain/122.12.2321. [DOI] [PubMed] [Google Scholar]
- 26.Sugimoto T, Ueyama H, Hosoi H, Inazawa J, Kato T, Kemshead JT, Reynolds CP, Gown AM, Mine H, Sawada T. Alpha-smooth-muscle actin and desmin expressions in human neuroblastoma cell lines. Int J Cancer. 1991;48:277–83. doi: 10.1002/ijc.2910480221. [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148–63. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 28.Sinden JD, Stroemer P, Grigoryan G, Patel S, French SJ, Hodges H. Functional repair with neural stem cells. Novartis Found Symp. 2000;231:270–83. doi: 10.1002/0470870834.ch16. discussion 283–8, 302–6. [DOI] [PubMed] [Google Scholar]
- 29.Kapur RP. Hirschsprung disease and other enteric dysganglionoses. Crit Rev Clin Lab Sci. 1999;36:225–73. doi: 10.1080/10408369991239204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.