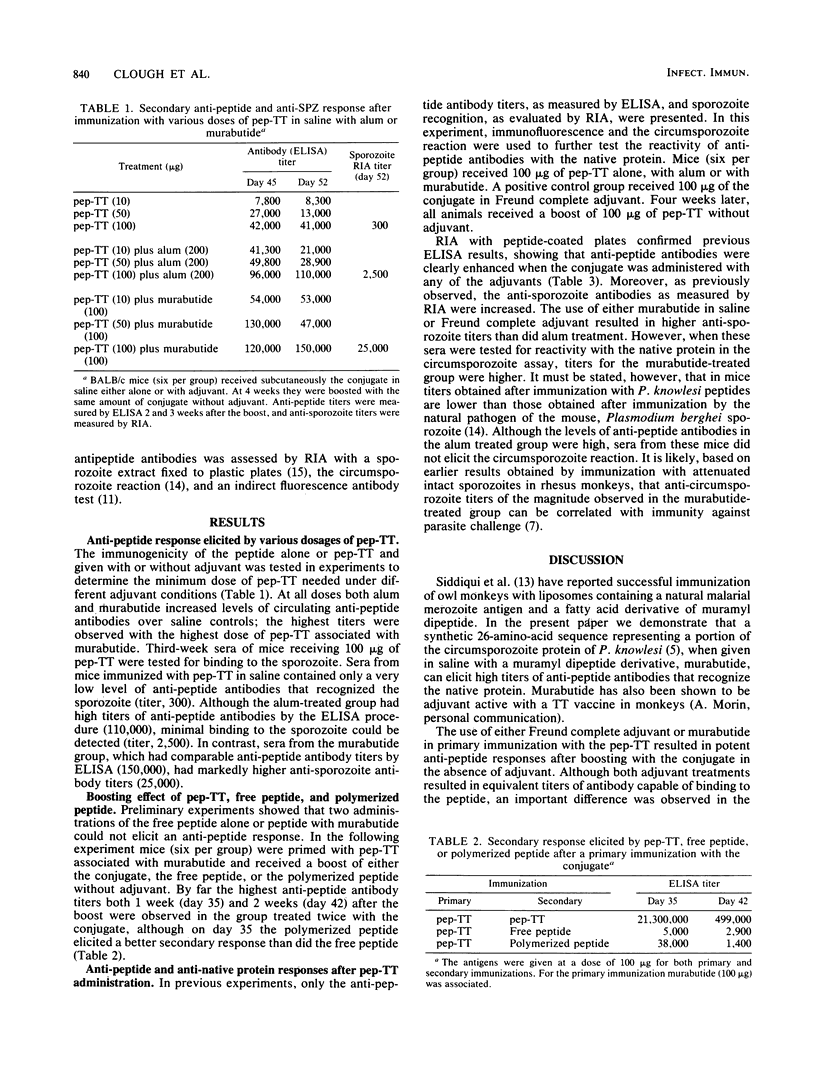
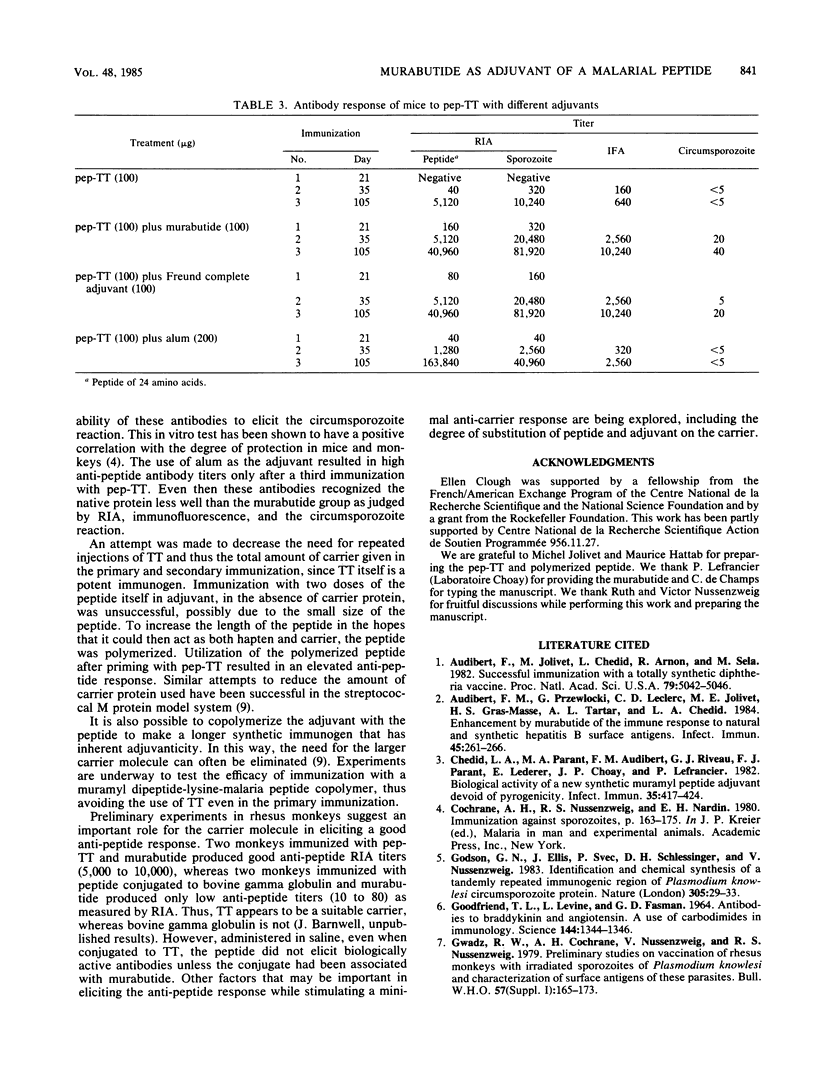
Abstract
A synthetic peptide whose sequence was derived from the circumsporozoite protein of Plasmodium knowlesi coupled to bovine gamma globulin has been shown to be immunogenic when administered with Freund complete adjuvant. The present experiments were designed to test the immunogenicity of the peptide when attached to a tetanus toxoid carrier and administered with alum or murabutide, both acceptable clinical adjuvants. In both cases, the use of an adjuvant increased the levels of circulating anti-peptide antibodies over those observed when no adjuvant was used. However, when the antisera were tested for reactivity with the native protein, animals of the group receiving the conjugate associated with murabutide always had titers greatly exceeding those observed in animals that received the conjugate with alum. Moreover, the sera of the murabutide-treated group were shown to be more active in eliciting shedding of the circumsporozoite protein than were sera of animals of the Freund complete adjuvant-treated group. The use of tetanus toxoid in secondary immunizations could be eliminated when the mice primed with peptide-tetanus toxoid and murabutide were boosted with a polymer of the peptide. The results indicate that the synthetic malarial peptide-tetanus toxoid conjugate is capable of stimulating high levels of biologically active antibodies only when administered with murabutide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audibert F. M., Przewlocki G., Leclerc C. D., Jolivet M. E., Gras-Masse H. S., Tartar A. L., Chedid L. A. Enhancement by murabutide of the immune response to natural and synthetic hepatitis B surface antigens. Infect Immun. 1984 Jul;45(1):261–266. doi: 10.1128/iai.45.1.261-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audibert F., Jolivet M., Chedid L., Arnon R., Sela M. Successful immunization with a totally synthetic diphtheria vaccine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5042–5046. doi: 10.1073/pnas.79.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L. A., Parant M. A., Audibert F. M., Riveau G. J., Parant F. J., Lederer E., Choay J. P., Lefrancier P. L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982 Feb;35(2):417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Ellis J., Svec P., Schlesinger D. H., Nussenzweig V. Identification and chemical synthesis of a tandemly repeated immunogenic region of Plasmodium knowlesi circumsporozoite protein. Nature. 1983 Sep 1;305(5929):29–33. doi: 10.1038/305029a0. [DOI] [PubMed] [Google Scholar]
- Gwadz R. W., Cochrane A. H., Nussenzweig V., Nussenzweig R. S. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ. 1979;57 (Suppl 1):165–173. [PMC free article] [PubMed] [Google Scholar]
- Gysin J., Barnwell J., Schlesinger D. H., Nussenzweig V., Nussenzweig R. S. Neutralization of the infectivity of sporozoites of Plasmodium knowlesi by antibodies to a synthetic peptide. J Exp Med. 1984 Sep 1;160(3):935–940. doi: 10.1084/jem.160.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Zavala F., Nussenzweig R. S., Nussenzweig V. Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J Immunol. 1982 Apr;128(4):1929–1930. [PubMed] [Google Scholar]
- Jolivet M., Audibert F., Beachey E. H., Tartar A., Gras-Masse H., Chedid L. Epitope specific immunity elicited by a synthetic streptococcal antigen without carrier or adjuvant. Biochem Biophys Res Commun. 1983 Dec 16;117(2):359–366. doi: 10.1016/0006-291x(83)91208-1. [DOI] [PubMed] [Google Scholar]
- Nardin E., Gwadz R. W., Nussenzweig R. S. Characterization of sporozoite surface antigens by indirect immunofluorescence: detection of stage- and species-specific antimalarial antibodies. Bull World Health Organ. 1979;57 (Suppl 1):211–217. [PMC free article] [PubMed] [Google Scholar]
- Santoro F., Cochrane A. H., Nussenzweig V., Nardin E. H., Nussenzweig R. S., Gwadz R. W., Ferreira A. Structural similarities among the protective antigens of sporozoites from different species of malaria parasites. J Biol Chem. 1983 Mar 10;258(5):3341–3345. [PubMed] [Google Scholar]
- Siddiqui W. A., Taylor D. W., Kan S. C., Kramer K., Richmond-Crum S. M., Kotani S., Shiba T., Kusumoto S. Vaccination of experimental monkeys against Plasmodium falciparum: a possible safe adjuvant. Science. 1978 Sep 29;201(4362):1237–1239. doi: 10.1126/science.99814. [DOI] [PubMed] [Google Scholar]
- Vanderberg J., Nussenzweig R., Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. V. In vitro effects of immune serum on sporozoites. Mil Med. 1969 Sep;134(10):1183–1190. [PubMed] [Google Scholar]
- Zavala F., Cochrane A. H., Nardin E. H., Nussenzweig R. S., Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983 Jun 1;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]


