Abstract
Our mechanistic understanding of progesterone’s involvement in murine mammary morphogenesis and tumorigenesis is dependent on defining effector pathways responsible for transducing the progesterone signal into a morphogenetic response. Toward this goal, microarray methods were applied to the murine mammary gland to identify novel downstream gene targets of progesterone. Consistent with a tissue undergoing epithelial expansion, mining of the progesterone-responsive transcriptome revealed the up-regulation of functional gene classes involved in epithelial proliferation and survival. Reassuringly, signaling pathways previously reported to be responsive to progesterone were also identified. Mining this informational resource for rapidly induced genes, we identified “inhibitor of differentiation 4” (Id4) as a new molecular target acutely induced by progesterone exposure. Mammary Id4 is transiently induced during early pregnancy and colocalizes with progesterone receptor (PR) expression, suggesting that Id4 mediates the early events of PR-dependent mammary morphogenesis. Chromatin immunoprecipitation assay detecting direct recruitment of ligand occupied PR to the Id4 promoter supports this proposal. Given that Id4 is a member of the Id family of transcriptional regulators that have been linked to the maintenance of proliferative status and tumorigenesis, the establishment of a mechanistic link between PR signaling and Id4 promises to furnish a wider conceptual framework with which to advance our understanding of normal and abnormal mammary epithelial responses to progestins. In sum, the progesterone-responsive transcriptome described herein not only reinforces the importance of progesterone in mammary epithelial expansion but also represents an invaluable information resource with which to identify novel signaling paradigms for mammary PR action.
EXPERIMENTAL MOUSE genetic studies have reinforced and extended the physiological importance of the progesterone receptor (PR) in murine mammary morphogenesis (1). Despite exposure to the complete spectrum of pregnancy hormones, the mammary gland of the progesterone receptor knockout (PRKO) mouse fails to undergo ductal side branching and alveologenesis, early morphological changes that are required for the establishment of a terminally differentiated mammary gland at parturition (2). In short, the PRKO mammary phenotype underscores the essential role played by PR-controlled downstream signaling pathways during parity induced mammary morphogenesis.
At the cellular level, the underlying cause of the PRKO mammary defect is an inability of the PRKO epithelium to undergo proliferation in response to hormone exposure (3). These findings support a mitogenic role for progesterone in the murine mammary epithelial compartment [a clear distinction from this steroid’s established antiproliferative effects in the uterine epithelium (1)]. Indeed, recent data suggest that progesterone (along with estrogen) may project its proliferative influence through a paracrine mechanism of action in the rodent mammary epithelium (4,5); a cellular mechanism of action that may translate to the human (6). Importantly, the inability of the PRKO mammary epithelium to launch a proliferative response to hormone exposure renders this cellular compartment significantly less susceptible to tumorigenesis (3). These observations suggest an important role for progesterone action not only in normal mammary epithelial biology but also in the promotion of mammary neoplastic progression, a proposal that concurs with conclusions drawn from more recent rodent studies (7,8) and clinical trials (9).
Notwithstanding the significant contributions that mouse genetics has made toward our current understanding of progesterone’s involvement in mammary morphogenesis and tumorigenesis, our knowledge of the molecular mechanisms that underpin the proliferative response of the mammary epithelial cell to progesterone is still rudimentary. Clearly, advancing our mechanistic understanding of progesterone’s involvement in mammary epithelial proliferation is predicated upon identification of downstream genes, pathways, and networks that transduce the progesterone-initiated morphogenetic response. From past reports it would appear that the progesterone signal might co-opt a number of seemingly divergent downstream molecular pathways to mediate its morphogenetic effects in the murine mammary epithelium. Although evidence strongly supports wingless-related mouse mammary tumor virus (MMTV) integration site (Wnt-4) and receptor of activated nuclear factor-κB ligand (RANKL) as possible effector pathways of the mammary progesterone signal (10,11,12), it is more likely that these pathways represent only a small percentage of the parallel and, perhaps, interconnected signaling cascades that execute proliferative and prosurvival programs required for progesterone-induced mammary morphogenesis.
Therefore, driven in large part by the advances in genome-wide transcriptional profiling and analysis, high-density oligonucleotide microarray methods together with a narrowly defined progesterone stimulus were used to uncover molecular targets that operate immediately downstream of the progesterone proliferative signal in the mammary gland of the normal mouse. Our studies demonstrate that at the cellular level, the mammary epithelium of the ovariectomized mouse elaborates a clear proliferative response to short-term progesterone exposure (4–72 h), and that prolonged progesterone exposure (∼2 wk) results in overt ductal side branching and alveologenesis in the absence of estrogen and prolactin (PRL). In keeping with a tissue undergoing rapid epithelial expansion, analysis of the resultant mammary transcriptome generated by acute progesterone exposure reveals signature gene families involved in cell division, survival, and/or viability. Importantly, the induction of previously known targets [Wnt-4 (10), RANKL (11,12), calcitonin (13), and amphiregulin (Areg) (14)] validates this hormone-treatment paradigm as a feasible approach with which to identify new molecular effectors of mammary PR action.
Initial mining of the aforementioned progesterone-responsive transcriptome for acutely induced targets reveals “inhibitor of differentiation 4 (Id4) to be acutely induced by progesterone in the murine mammary epithelial cell. Id4 is a member of a small family of helix-loop-helix (HLH) transcriptional regulators that block the function of basic HLH (bHLH) transcription factors required for cellular differentiation (15,16). Because of its role in the maintenance of cellular proliferation, the Id protein family has been linked to tumorigenesis [i.e. Id4 has been implicated in mammary tumorigenesis in the rat (17)]. Our spatiotemporal expression studies demonstrate that Id4 nuclear expression is only induced in a subset of luminal epithelial cells of the murine mammary gland during early pregnancy and is restricted to cells that score positive for PR expression. Although speculative, these findings suggest that during the proliferative phase of parity induced mammary morphogenesis, Id4 may act locally to prevent premature differentiation of the epithelium of the prelactating mammary gland, thereby ensuring complete expansion of the mammary epithelial ductal network and alveolar compartment before terminal differentiation. Importantly, transient transfection of the PR into murine mammary epithelial (HC-11) cells demonstrates that PR induces Id4 transcription in a ligand-dependent manner. In addition, chromatin immunoprecipitation (ChIP) assay reveals that PR-mediated Id4 induction in this cell culture system is enabled through direct recruitment of the PR to the Id4 promoter in the presence of ligand. These findings suggest that Id4 may act as a direct molecular target of the PR in the mammary epithelial cell.
In summary, our studies demonstrate that the mammary transcriptome described herein represents a powerful information resource with which to identify new molecular effectors of (and, therefore, new signaling paradigms for) the murine mammary PR. In future studies, such a molecular tool promises to furnish not only new perspectives on progesterone action during normal mammary development but may well provide unique molecular insights into progesterone’s role in mammary tumorigenesis, currently a controversial area of contemporary breast cancer research and treatment.
Materials and Methods
Mice and hormone treatments
Nulliparous Balb/c mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). For microarray studies these mice were ovariectomized at 6 wk of age and rested for 2 wk (to purge ovarian hormones) before treatment with sesame oil (vehicle control) or progesterone (Sigma Chemical Co., St. Louis, MO). In the case of progesterone treatment, mice received a daily sc injection (within the intrascapular region) of 1 mg progesterone dissolved in 100 μl sesame oil. Mice were killed 4, 16, 28, or 76 h after the first injection. For controls, an equal number of mice received sesame oil (100 μl) and were killed at the same time points as the progesterone-treated group. For a given time point and treatment group, three sets of mice (five mice in each set) were used for microarray analyses; mammary RNA pooled from each set was hybridized to one microarray chip (see Microarray analysis). Therefore, a total of 15 mice (or three microarray chips) was used per time point and treatment group to ensure statistical significance (supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). For long-term progesterone treatment, ovariectomized mice sc received a beeswax pellet (implanted in the intrascapular region), which delivered 1 mg progesterone daily (18); a control group received a beeswax pellet without hormone (six mice were killed either 1 or 2 wk after pellet implantation). For timed pregnancies the morning of observing the vaginal plug was considered 0.5 d pregnancy. The generation and characterization of the PRlacZ knock-in mouse model have been reported elsewhere (18). Although the PRlacZ knock-in is a phenocopy of normal mice, PRlacZ mice harbor one PR allele in which the lacZ reporter gene is inserted downstream (and under the control) of the endogenous PR promoter. Mammary tumors were derived from MMTV-Wnt-1 transgenic mice, which were purchased from The Jackson Laboratory (Bar Harbor, ME) [also known as FVB.Cg-Tg(Wnt1)1Hev/J (stock no. 002934)].
Mice were housed in a temperature-controlled (22 ± 2 C) room with 12-h light, 12-h dark photocycle, and provided rodent chow meal (Purina Mills, LLC, St. Louis, MO) and fresh water ad libitum. All mice were treated humanely in accordance with institutional and Institutional Animal Care and Use Committee guidelines for the care and surgical manipulation of animals.
Microarray analysis
For microarray analysis, total RNA was isolated from both inguinal mammary glands (lymph node removed) per Balb/c mouse using the RNeasy system for lipid tissues according to the manufacturer’s instructions (QIAGEN, Inc., Valencia, CA). To avoid potential contamination from muscle, only inguinal (rather than thoracic) glands were used in these studies. Physical integrity of the RNA was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA); RNA quantitation was performed using the Nanodrop ND1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). For microarray purposes, 10 μg total RNA pooled from a set of five mice per time point and treatment group was analyzed. To ensure statistical significance, three separate sets of mice (15 mice in total) per time point and treatment group were used for these microarray experiments (see Mice and hormone treatments and supplemental Table 1). The Affymetrix GeneChip Mouse Genome 430 2.0 Array (Affymetrix, Inc., Santa Clara, CA), containing 45,101 probe sets covering 39,000 transcripts on a single array, was used in these studies. RNA for microarray analysis was labeled and hybridized according to the Affymetrix protocol (Affymetrix GeneChip Expression Analysis Technical Manual, Revision 3) by the Baylor College of Medicine Microarray Core Facility. After scanning and low-level quantification using Microarray Suite (Affymetrix), the DNA Chip analyzer (19) was used to adjust arrays to a common baseline using invariant set normalization (20) and estimate expression using the PM/MM difference model described by Li and Wong (21). The 24 gene chips (supplemental Table 1) were normalized to the same baseline and were modeled together. Array data have been deposited in the public Gene Expression Omnibus database, accession no. GSE10290. Except for calcitonin/calcitonin-related polypeptide α (Calca) [a known progesterone target (13)], transcripts with present calls in less than half of the samples profiled were removed from the analysis, and gene expression values were log transformed. For each time point, two-sample t tests determined significant differences in mean mRNA levels between untreated and progesterone-treated groups. Fold changes between groups were computed by taking the ratio of the average of the untransformed expression values of the first group with that of the second. The supervised clustering analysis for differentially expressed genes was performed as follows: 1) expression values for a given time point and treatment group were transformed to sd values from the mean; 2) each pattern of interest (i.e. genes induced by 28 h but not 16 h or genes induced at 4–28 h but not 76 h) was represented as a series of 1s and 0s; 3) for each gene, Pearson’s correlation was computed between its expression values and each of the predefined patterns; 4) for each treatment group, the pattern best correlated with the expression of each gene was determined; and 5) genes were manually sorted by their patterns. Expression values were visualized as color maps (or color grams) using Cluster and Java TreeView software (22,23). Gene ontology (GO) annotation terms were obtained and searched for enrichment within gene sets as described previously (24). For color map display, the log of the expression intensity values for each time point and progesterone-treatment group were first centered on the mean values of the corresponding vehicle control.
Molecular analysis
Quantitative real-time PCR.
For quantitative real-time RT-PCR, total mammary RNA was isolated with TRIzol reagent (Invitrogen Corp., Carlsbad, CA). Expression levels were validated by real-time RT-PCR TaqMan analysis with the ABI Prism 7700 Sequence Detector System according to the manufacturer’s instructions (PE Applied Biosystems, Foster City, CA). The TaqMan gene expression assay (catalog no. 4309169; PE Applied Biosystems) with One-step RT-PCR Universal Master Mix reagent was used to perform real-time RT-PCR according to the manufacturer’s instructions. Prevalidated probes and primers for murine Areg (Mm00437583_m1), calcitonin (Calca) (Mm00801463_g1), Id4 (Mm00499701_m1), RANKL [TNF ligand superfamily member 11 (Tnfsf11)] (Mm00441908_m1), metallothionein2 (Mt2) (Mm00809556_s1), estrogen receptor (ER)-α (Esr1) (Mm00433147_m1), PR (Pgr) (Mm00435625_m1), wingless-related MMTV integration site 4 (wnt4) (Mm00437342_g1), and 18S rRNA (catalog no. 4319413E; an internal control) were purchased from PE Applied Biosystems. The reaction conditions consisted of an initial activation step of 50 C for 2 min, followed by 10 min at 95 C, and then 35 cycles of denaturation at 95 C for 15 sec, annealing and extension at 60 C for 1 min. Standard curves were generated by serial dilution of a preparation of total mammary RNA isolated from adult intact virgin mice. All experiments were performed in triplicate using three independent RNA sets in each case; mRNA quantities were normalized against 18S rRNA with ABI rRNA control reagents. Statistical analysis used one-way ANOVA followed by Tukey’s post hoc multiple range test with the Instat package from GraphPad Software Inc. (San Diego, CA). P values less than 0.05 were considered statistically significant, and values less than 0.01 highly significant; results are presented as se.
Ribonuclease (RNase) protection assay.
The RNase protection assay (RPA) was performed using the RPAIII kit according to the manufacturer’s instructions (Ambion, Inc., Austin, TX). A full description of the murine Areg, calcitonin, RANKL, and Wnt-4 riboprobes has previously been provided (12,13).
Cell culture and hormone treatments.
HC-11 mouse mammary epithelial cells were maintained at 37 C and 5% CO2 in RPMI 1640 medium supplemented with 10% bovine calf serum (HyClone, Logan, UT), 2 mm glutamine, 5 μg/ml insulin, 10 ng/ml epidermal growth factor, and 100 U/ml penicillin and streptomycin. After cells reached confluency (2 × 107 cells), they were grown for an additional 72 h in the same medium transduced with human PR-B (hPR-B) adenovirus [50 plaque-forming units (pfus) per cell], and then incubated for a further 48 h in priming medium (RPMI 1640 supplemented with 0.5 m glutamine, 5 μg/ml insulin, and 10% stripped donor horse serum). Hydrocortisone (1 μg/ml) was added to priming medium and during subsequent treatment with other hormones unless otherwise noted. After 48 h in priming medium, cells were treated in the same medium with various hormone regimens as indicated in the Results section [see the legend in Fig. 8, including the synthetic progestin R5020 (100 nm) and ovine PRL (3 μg/ml) at various periods of time (1 h for ChIP and 24 h for quantitative RT-PCR analysis)]. For quantitative RT-PCR analysis of Id-4 transcription, total RNA was isolated from untreated and hormone-treated (24 h) HC-11 cells using the RNeasy kit (no. 74104). Physical integrity of the RNA was assessed using the Agilent 2100 Bioanalyzer; RNA quantitation was obtained by measuring A260/280 of each sample on a DU 800 spectrophotometer (Beckman Coulter, Inc., Fullerton, CA).
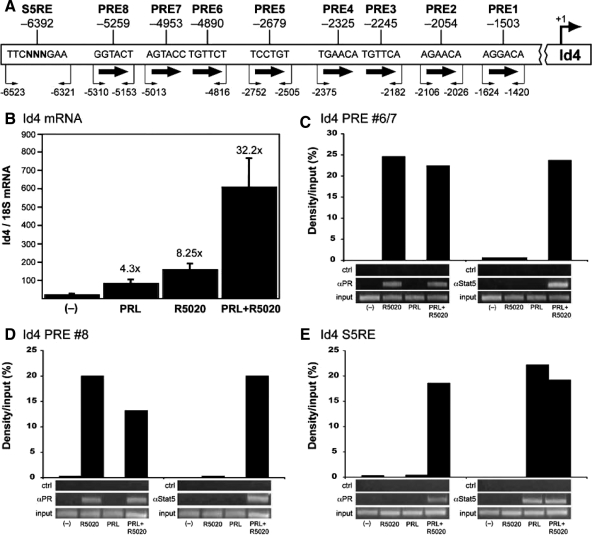
Figure 8.
Progesterone induction of Id4 gene expression is mediated by a direct interaction of PR and functionally synergizes with PRL/Stat5a signaling. A, Schematic of the predicted S5RE and PRE half-sites in the upstream promoter region of Id4, and PCR primers used for ChIP assay. The numbering represents the number of base pairs upstream of the transcription start site of the murine Id4 gene. B, HC-11 cells grown and differentiated, as described in Materials and Methods, were transduced at an multiplicity of infection of 50 pfu per cell with an adenoviral vector that encodes mouse PR-B. Cells were treated for 24 h with vehicle (−), R5020 (100 nm), PRL (3 μg/ml), or R5020 (100 nm) plus PRL (3 μg/ml), total RNA was prepared, and Id4 mRNA was measured by real-time quantitative PCR as described in Experimental procedures. The Id4 mRNA was normalized to 18S RNA, and data represent the mean ± se of triplicate biological RNA samples measured by duplicate PCRs. The fold increase by hormone treatment relative to the vehicle control is indicated over each bar. This is a single experiment that is representative of three independent experiments. C–E, Differentiated HC-11 cells transduced with an adenovirus vector encoding hPR-B were treated for 1 h with vehicle (−), R5020 (100 nm), PRL (3 μg/ml), or R5020 plus PRL (3 μg/ml), and ChIP assays were performed. Lower panels, Agarose gels of PCR products from input DNA and DNA immunoprecipitated by a control antibody, antibody specific for PR (1294), or antibody specific for Stat5a (L-20). Upper panels, Quantification of ChIP results using Syngene gel imager and Genetools software calculated as the average density of the immunoprecipitated DNA as a percentage (%) of input DNA after subtraction of signal obtained with the control antibody. Shown is a representative ChIP result of these independent experiments.
Adenovirus transduction of HC-11 cells to express PR.
Recombinant adenoviral transfer vectors containing hPR-B under the control of the cytomegalovirus (CMV) promoter were constructed by overlap recombination as described previously (25,26). These are replication-defective viruses lacking the transforming EIA and EIB genes. hPR-B cDNA was cloned into the BamHI site of a transfer plasmid (ACCMVC-1) containing the CMV promoter and adenovirus sequences to yield pCMV-PR-B. Permissive 293 cells were cotransfected using calcium phosphate precipitation with pCMV-PR-B and the adenovirus AD5d1327Bst β-gal (26). Homologous recombination of plasmid and viral sequences resulted in generation of infectious viral particles that were plaque purified. Plaques were initially examined by PCR for the presence of PR-B. Positives were picked, grown in 293 cells, and examined for PR-B protein expression by Western blot. Purified viruses encoding PR-B, termed Ad5dl327CMV-PR-B, were grown in large scale and concentrated by CsCl gradient centrifugation. The virus concentration was determined by absorbance at 260 nm, in which 1 A260 U represents 1012 viral particles. The particle to pfu ratio was determined to be close to 100 for all viruses.
ChIP assay.
The ChIP assay was performed as described previously with slight modification (27). Cell pellets were sonicated using a Branson-450 Sonifer (Branson Ultrasonics Corp., Danbury, CT) with microtip in 10-sec bursts, followed by 1 min cooling on ice for a total sonication time of 100 sec/sample. This resulted in DNA fragment sizes of 0.1–1.0 kb. Sonicated samples were centrifuged at 14,000 rpm for 15 min at 4 C, and the supernatants were diluted 10-fold in ChIP buffer [140 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% Na-deoxycholate, 0.1% sodium dodecyl sulfate, and 50 mm HEPES (pH 7.8)] containing protease inhibitors. Of the supernatant, 3% was taken for input controls and processed with immunoprecipitated samples at the point of the cross-linking reversal step. Remaining supernatants were incubated at 4 C overnight with appropriate antibodies: 10 μg anti-hPR mouse monoclonal 1294 (27); 10 μg antimouse signal transducer and activator of transcription −5 (Stat5a) (L-20) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); or 6 μg control unrelated antibody (rabbit antimouse IgG). Immunocomplexes were collected with 25 μl Protein G Sepharose-4 Fast Flow (GE Healthcare Biosciences AB, Uppsala, Sweden) (17-0618-01) for 1 h at 4 C with rotation. Beads were washed consecutively for 5 min each in ChIP buffer, high-salt wash buffer (ChIP buffer plus 500 mm NaCl), and LiCl wash buffer [20 mm Tris (pH 8.0), 1 mm EDTA, 250 mm LiCl, 0.5% Nonidet P-40, and 0.5% Na-deoxycholate]. All buffers contained protease inhibitors. Beads were then washed twice for 5 min in Tris-EDTA buffer [10 mm Tris (pH 8.0) and 1 mm EDTA]. Complexes were eluted, and formaldehyde cross-links were reversed. The DNA was PCR amplified and analyzed by electrophoresis. The relative amounts of immunoprecipitated and input DNA were determined by comparison to a standard curve generated by serial dilutions (1:100, 1:20, 1:10, 1:5, and 1:1) of input DNA. PCR amplified DNA was analyzed by electrophoresis on ethidium bromide-stained 2% agarose gels, and relative band intensities were captured using an image scanner (ChemiGenius Bioimaging System; Syngene, Frederick, MD) and system software (GeneSnap 6.08; Syngene), and band densities were quantitated using Syngene Genetools 3.07 software. DNA immunoprecipitated with specific antibody was normalized by first subtracting signals obtained with control unrelated IgG and then expressing the normalized value as a ratio to input DNA.
PCR of immunoprecipitated DNA.
PCRs were performed in a Bio-Rad DNA Engine PTC-200 (Bio-Rad Laboratories, Inc., Hercules, CA). The following primers were used: PRE 1/2 site no. 1, 5′-CAAGCATGTCTAAAGGTGGG and 3′-GTGTTGTGTGTGTGTGTGTG; PRE 1/2 site no. 2, 5′-CACGGTATTTACACACACAC and 3′-GTCTCCATCCACACTTACTG; PRE 1/2 site no. 3/no. 4, 5′-GTGGACAAGCTTAGCCTAG and 3′-GAAGCAGTCCTGGTGAGCAA; PRE 1/2 site no. 5, 5′-GCATGAACTCTAGTGTGCTC and 3′-GGTTACCACGATGGAG-TAGG; PRE 1/2 site no. 6/no. 7, 5′-CCAGGGAGAATCTGTAGAAAG and 3′-CCAGTTGTG-AAGTTCTTTCCG; PRE 1/2 site no. 8, 5′- CCATTTCCCTAGTACAAGCCC and 3′-AAG-GATCACAGGAGCAGAG; and Stat5 response element (S5RE), 5′-CACCAAGCTTCCTGTTTGTG and 3′-GACCC-TCTGGAACTGTAAGC. PCR was performed using 5 μl DNA (input DNA was diluted 1:4) using 32 cycles of: 30 sec at 95 C, 30 sec at 55 C, and 1 min at 72 C.
Western immunoblot analysis.
Protein extracts were prepared from mammary and tumor tissues as described previously (28). Mammary and tumor protein (10 μg) was resolved by 4–15% gradient SDS-PAGE before transfer to polyvinylidene difluoride membranes (Bio-Rad Laboratories). Immunoreactive bands for Id4 and cyclin D1 were detected with rabbit polyclonal antibodies to mouse Id4 [Santa Cruz Biotechnology; catalog no. L-20 (1:1000 dilution)] and human cyclin D1 [Neo Markers, Fremont, CA; catalog no. RB-9041-P (1:1000 dilution)], respectively. A goat antihuman β-actin primary polyclonal antibody [Santa Cruz Biotechnology; catalog no. SC-1616 (1:1000 dilution)] to β-actin was used as a loading control. For primary antibodies to Id4 and cyclin D1, signal intensity was amplified with horseradish peroxidase-conjugated goat antirabbit IgG [Santa Cruz Biotechnology; catalog no. SC-2004 (1:5000 dilution)] as the secondary antibody. A rabbit antigoat IgG [Santa Cruz Biotechnology; catalog no. SC-2768 (1:5000 dilution)] against the primary antibody to β-actin was used as the secondary antibody. Immunoreactive bands were visualized with an enhanced chemiluminescence substrate detection kit (Pierce, Rockford, IL).
Whole mounts and immunohistochemical analysis
Carmine-red stained inguinal (no. 4) mammary gland whole mounts [and hematoxylin and eosin (H&E) stained sections thereof] were performed as previously described (28). Mammary gland and uterine lacZ staining has been reported previously (18). For immunohistochemical analysis, tissues were fixed overnight in Bouin’s fixative or 4% paraformaldehyde, whereas for immunofluorescence detection, tissues were fixed in 4% paraformaldehyde for 2 h. Immunohistochemistry for PR and ER-α was performed as previously described (18). The rabbit anti-hPR polyclonal antibody (catalog no. A0098; 1:100 dilution) and mouse monoclonal against human ER-α (catalog no. M7047; 1:100 dilution) were purchased from the Dako Corporation (Carpinteria, CA). Immunohistochemical detection of Id4 using a rabbit polyclonal against the C terminus of mouse Id4 [Santa Cruz Biotechnology; catalog no. SC-491; (1:100 dilution)] was undertaken as previously reported (17). The antibody-antigen interaction was visualized in situ by the immunoperoxidase method by which 3, 3′-diaminobenzidine is converted by streptavidin-horseradish peroxidase conjugated to the secondary antibody to an insoluble brown stain in immunopositive cells (18,28). The percentage of immunopositive mammary epithelial cells was calculated using methods previously described (3,29). Briefly, cell counting entailed scoring the number of immunopositive cells in a random field of 1000 luminal epithelial cells. The mean number of immunopositive cells in a given tissue section was obtained by taking the mean obtained from counting four separate fields of 1000 cells per field. Final results were expressed as the percentage of epithelial cells immunopositive for the antigen under study. To detect 5-bromo-2-deoxyuridine (BrdU) incorporation, mice were injected (ip) with BrdU (Amersham Biosciences Inc., Piscataway, NJ) at 0.1 ml/10 g body weight 2 h before being killed. Mammary tissue was fixed, processed, embedded, and sectioned as previously described (2). For dual-immunofluorescence detection of PR and Id4, the tyramide signal amplification fluorescence kit (NEL701; PerkinElmer Life Sciences, Boston, MA) was used as previously described (18,28). Tetramethyl rhodamine isothiocyanate (red)-conjugated streptavidin and fluorescein isothiocyanate (green) was used to fluorescently detect Id4 and PR expression, respectively. Slides were washed and mounted in Vectashield mounting medium with 4′, 6′-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were initially captured with an Axioplan 2 microscope equipped for epifluorescence detection, and with the appropriate tetramethyl rhodamine isothiocyanate and fluorescein isothiocyanate filters (Carl Zeiss, Jena, Germany). Captured digital images were initially acquired and processed with MetaVue Software 4.6r9 (Universal Imaging, Downington, PA); final image composites were assembled with Photoshop CS (Adobe Systems, Inc., San Jose, CA).
Results
PR is expressed in the mammary epithelium of the ovariectomized mouse
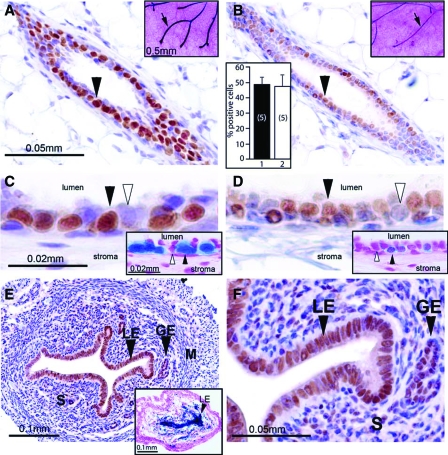
In the intact pubertal mouse (Balb/c strain), approximately 50% of mammary epithelial cells robustly express the PR (Fig. 1A). Although ovariectomy significantly reduces the level of mammary PR expression, residual PR protein is still readily detected by immunohistochemistry (Fig. 1B). Higher magnification reveals that approximately the same percentage of luminal epithelial cells express the PR in the mammary gland of the intact and ovariectomized mouse (Fig. 1, C and D). The reduction in mammary PR expression after ovariectomy is reflected by an attenuation in PR promoter activity as indicated by the PRlacZ reporter mouse (insets in Fig 1, A–D). This result supports the proposal that most of the PR expression in the mammary epithelium of the intact mouse is maintained by the activation of the PR promoter by ovarian-derived estrogen signaling. It should be noted that the staining intensities for PR promoter activity (as measured by X-gal staining) and PR protein (as measured by immunoperoxidase staining) are not equivalent due to the different half-lives between LacZ derived β-galactosidase (and its product) and PR protein. The difference may be further due to the fact that LacZ expression occurs only from one PR allele, whereas PR protein expression originates from both PR alleles. In contrast to the mammary gland, most luminal and glandular epithelial cells of the uterus strongly express the PR in the ovariectomized mouse (Fig. 1, E and F). Uterine tissue from ovariectomized mice was previously used as an RNA source to identify progesterone targets by microarray methods (30). These results demonstrate that a low (but detectable) level of PR expression is retained in the murine mammary epithelium after ovariectomy. Importantly, the retention of a low level of mammary PR expression in the ovariectomized pubertal mouse suggested to us that our recent microarray strategy to uncover uterine PR targets (30) could also be applied (with some modifications) to reveal mammary PR targets.
Figure 1.
Retention of mammary PR expression in the ovariectomized mouse. A, Immunohistochemical detection of PR is clearly evident in a transverse section of a mammary duct from an intact 8-wk-old wild-type virgin mouse (arrowhead). Inset shows robust β-gal activity (arrow) in a lacZ stained mammary whole mount from a similar aged intact PRlacZ knock-in mouse. B, Two weeks after ovariectomy, an 8-wk-old wild-type mouse exhibits a lower but detectable level of PR immunoreactivity in the mammary gland (arrowhead). Upper-right corner inset shows detectable β-gal activity (arrow) in a lacZ stained mammary gland whole mount from an ovariectomized 8-wk-old PRlacZ knock-in mouse. The histogram in the lower-left corner shows the percentage of mammary luminal epithelial cells positive for PR immunoreactivity in the intact and ovariectomized mouse (bars 1 and 2, respectively). The values represent the mean ± sem from five animals per group. C and D, Higher magnifications of regions shown in A and B, respectively. C, Note that robust PR immunoreactivity is restricted to the luminal epithelial compartment of the mammary gland (black arrowhead); cells scoring negative for PR expression are indicated by the white arrowhead. D, PR immunoreactivity is detected in the luminal epithelial compartment (black arrowhead); a subset of cells is negative for PR expression (white arrowhead). Insets in C and D show lacZ stained sections of mammary glands from the intact and ovariectomized PRlacZ knock-in mouse, respectively. Note the clear β-gal activity in both panels (black arrowhead); a cell negative for β-gal activity is indicated by a white arrowhead. E, Robust PR immunoreactivity is clearly detectable in the luminal and glandular epithelial (LE and GE, respectively) compartments of the uterus from an 8-wk-old ovariectomized mouse. Inset shows strong β-gal activity (black arrowhead) in the luminal epithelial compartment of a lacZ stained uterine transverse section from an 8-wk-old ovariectomized PRlacZ knock-in mouse. F, A higher magnification of a region shown in E; note the strong PR immunoreactivity in most cells of the luminal and glandular epithelial compartments (black arrowheads) and in a subset of stromal (S) cells. The luminal epithelial, glandular epithelial, stromal, and myometrial compartments are denoted by LE, GE, S, and M, respectively. Scale bars in A and C, apply to B and D, respectively; scale bars in insets in A and C apply to insets shown in B and D, respectively.
Progesterone-induced mammary morphogenesis in the ovariectomized mouse
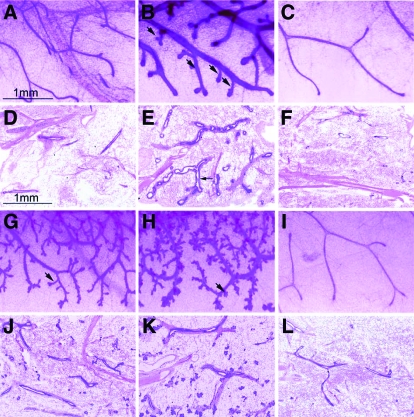
To determine whether the PR population retained in the mammary epithelium after ovariectomy can (in response to progesterone exposure alone) exhibit all or a subset of the morphological changes previously ascribed to mammary PRs in the intact mouse (2), ovariectomized mice were acutely treated for 76 h with progesterone. Whole mount analysis clearly reveals that acute progesterone exposure elicits both ductal distension and side branching, a morphological response that is not observed with vehicle alone (Fig. 2, A–F). These results demonstrate that the PR subpopulation remaining in the mammary epithelium after ovariectomy can induce morphological changes previously attributed to progesterone action in the mammary gland of the intact mouse (2). With prolonged progesterone exposure (for 1 or 2 wk), the mammary gland undergoes more pronounced ductal side branching and shows the emergence of alveolar buds (Fig. 2, G–L) (also see supplemental Fig. 1, for lower magnification images of these whole mounts). Examination of H&E stained sections of whole mounts shown in Fig. 2 reveals that acute and prolonged progesterone exposure induces significant mammary epithelial proliferation (supplemental Fig. 2). These results show that (in response to progesterone exposure alone) the PR population residing in the mammary epithelium of the ovariectomized mouse exhibits many of the functional characteristics of mammary PRs in the early parous mouse.
Figure 2.
Acute progesterone exposure elicits branching morphogenesis in the mammary gland of the ovariectomized mouse. A–C are high-magnification images of mammary gland whole mounts from untreated, progesterone-treated (for 76 h), or sesame oil-treated (for 76 h) ovariectomized wild-type mice, respectively. Note the overt dilation of the ductal network and the emergence of numerous ductal side branches (arrows) in response to 76 h progesterone exposure (compare B with A). Sesame oil treatment for 76 h fails to induce these morphological responses (compare C with B). D–F show representative H&E stained sections of the whole mounts shown in A–C, respectively. Note the significant expansion of the epithelial compartment (arrow) after progesterone treatment for 76 h (compare E with D and F). G and H show whole mounts of mammary glands from ovariectomized mice continuously treated with progesterone for 1 and 2 wk, respectively. In both panels, note the clear increase in ductal side branching and limited alveolar budding with progesterone exposure (arrows). I, Absence of these mammary morphological changes in the ovariectomized wild-type mouse after 2 wk sesame oil treatment. J–L, H&E stained sections of the whole mounts shown in G–I, respectively; a marked increase in epithelial content is observed with progesterone exposure for 1 and 2 wk (compare J and K with L). Scale bar in A applies to B, C, and G–I, respectively; scale bar in D applies to E, F, and J–L, respectively. Also see supplemental Figs. 1 and 2.
Acute progesterone exposure induces luminal epithelial proliferation and down-regulates PR expression in the mammary gland of the ovariectomized mouse
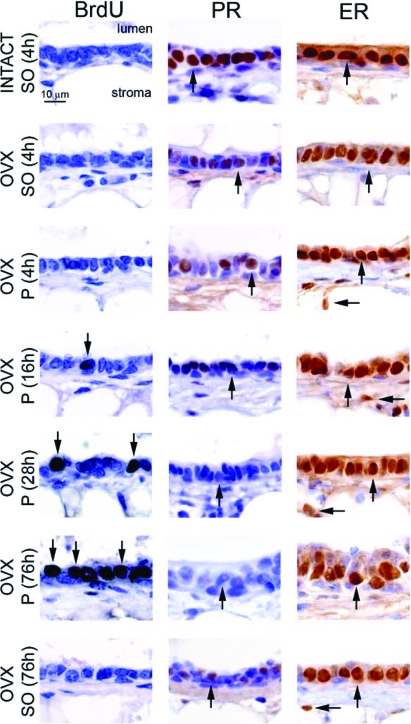
Because nascent ductal side branches are evident in the mammary gland of the ovariectomized mouse as early as 76 h after progesterone treatment, we performed a progesterone exposure time course to determine the earliest time point at which mammary epithelial cells undergo progesterone-induced proliferation. By 16 h progesterone exposure, we first detect a subset of luminal epithelial cells that score positive for BrdU incorporation in the mammary gland of the ovariectomized mouse (Fig. 3). As the number of BrdU positive cells increase with prolonged progesterone exposure, the level of PR expression significantly decreases [progesterone is known to down-regulate its own receptor (18)]. After 76 h progesterone administration, numerous luminal epithelial cells stain positive for BrdU, whereas PR expression is undetectable. In contrast to the observed progesterone-induced down-regulation of mammary PR expression during this time period, the expression level of mammary ER-α is unaffected by progesterone exposure (Fig. 3).
Figure 3.
Short progesterone exposure induces mammary epithelial proliferation in the ovariectomized mouse. High-magnification images of mammary gland sections from different hormone treatment groups [i.e. 8-wk-old intact or ovariectomized (OVX) wild-type mice treated with sesame oil (SO) or progesterone (P)] stained for BrdU incorporation, PR, and ER-α expression are shown as three sets of seven panels. Although the mammary epithelium of sesame oil-treated intact and ovariectomized mice does not exhibit BrdU incorporation, a significant increase in the number of luminal epithelial cells scoring positive for BrdU incorporation (arrow) in the mammary gland of the ovariectomized mouse is clearly evident by 16 h progesterone exposure, and this number significantly increases by 76 h progesterone exposure (arrows). Although ovariectomy significantly decreases the levels of mammary PR expression in the ovariectomized mouse [compare ovariectomized (sesame oil 4 h) with INTACT (sesame oil 4 h), and also see Fig. 1], mammary PR expression is further attenuated in the ovariectomized mouse when treated with progesterone for 16 h (arrow) and is absent by 76 h progesterone exposure (arrow). Unlike progesterone treatment, PR expression levels are not changed in the ovariectomized mouse with continuous sesame oil treatment for 76 h [arrow in ovariectomized (sesame oil 76 h)]. In contrast to PR expression, ER-α expression levels are not changed with progesterone exposure. ER-α expression levels in the luminal (vertical arrow) and stromal (horizontal arrow) compartments do not change in any hormone treatment group. Scale bar applies to all panels.
Importantly, these results indicate that the PR is required to trigger mammary epithelial proliferation in response to initial progesterone exposure but that continued epithelial proliferation is dependent on the activation and/or suppression of unidentified downstream gene networks that do not necessarily require the continued presence of ligand-activated PR for their activity.
Established PR target genes are transcriptionally induced by acute progesterone exposure in the mammary gland of the ovariectomized mouse
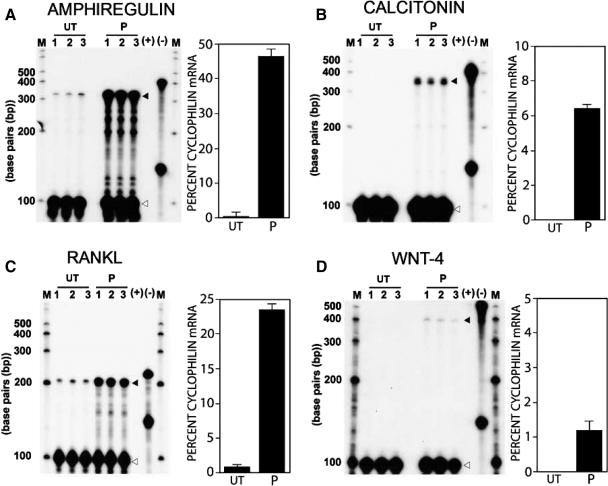
The RPA was performed to determine whether the limited number of target genes previously ascribed to PR in the mammary gland and/or uterus is transcriptionally induced within 76 h progesterone exposure in the mammary gland of the ovariectomized mouse. Figure 4 clearly shows that Areg, Calca, RANKL [also known as Tnfsf11, osteoprotegerin ligand, or TNF-related activation-induced cytokine], and Wnt-4 are transcriptionally induced by progesterone during this time period.
Figure 4.
Areg, calcitonin, RANKL, and Wnt-4 transcription is induced in the mammary gland of the ovariectomized mouse after acute progesterone exposure. A–D show RPA for Areg, calcitonin, RANKL, and Wnt-4 in the mammary gland of ovariectomized 8-wk-old mice that are untreated (UT) or progesterone (P) treated for 76 h, respectively. Lanes 1–3 in the untreated control and progesterone test group denote total mammary RNA isolated from three separate sets of mice, each set containing total RNA pooled from five mice for a total of 15 mice tested in the untreated and progesterone group for each RPA experiment. The RPA experiment was performed three times using different mice in each experiment; therefore, 45 mice in total were examined per test and control group in this study. A–D show that Areg (black arrowhead; protected fragment: 330 bp), calcitonin (black arrowhead; protected fragment: 350 bp), RANKL (black arrowhead; protected fragment: 205 bp), and Wnt-4 (black arrowhead; protected fragment: 400 bp) are induced by 76 h progesterone exposure, respectively. For each RPA experiment, cyclophilin served as a loading control (white arrowhead; protected fragment: 100 bp). Located on the right side of each RPA gel are probe controls in the presence of yeast RNA consisting of target gene and cyclophilin antisense riboprobes in the presence (+) or absence (−) of RNase; M, marker lanes on either side of each gel. The histogram in each panel quantitatively displays target gene transcript induction as a percentage of cyclophilin mRNA from triplicate experiments (error bar denotes ± sem).
Together, the RPA data demonstrate that the PR population retained in the murine mammary epithelium after ovariectomy is capable of inducing (in response to progesterone exposure alone) the transcriptional expression of genetic pathways previously proposed to mediate progesterone-induced mammary epithelial proliferation and/or survival in the mouse. Many of these signaling molecules (i.e. Areg, RANKL, and Wnt-4) are not only important for normal mammary epithelial expansion but have important roles in the etiopathogenesis of breast cancer. Interestingly, recent gene profiling of the murine mammary gland has shown that the aforementioned signaling molecules are also transcriptionally controlled by PRL signaling (31), suggesting that this subset of mammary molecular targets represent gene-network convergence points for progesterone and PRL signaling inputs.
A complex transcriptional response is induced by acute progesterone exposure in the mammary gland of the ovariectomized mouse
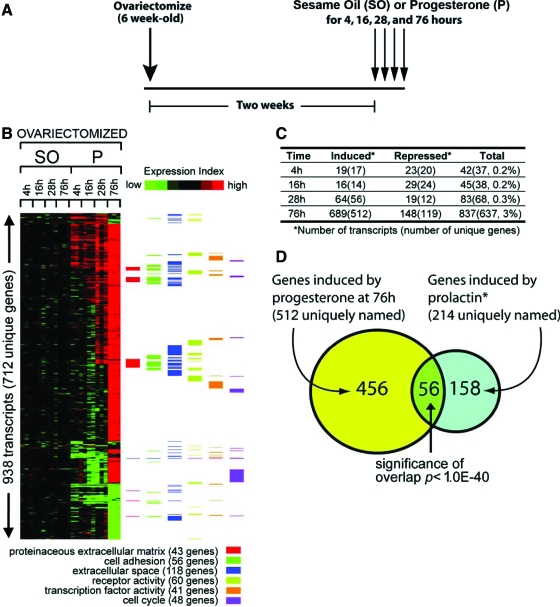
We have demonstrated that acute progesterone exposure of the ovariectomized pubertal mouse results in incipient mammary ductal side branching that is reflected at the cellular and molecular level by epithelial proliferation and the induction of molecular targets previously assigned to mammary PR action. Therefore, we used a modified version of a hormone treatment paradigm in combination with microarray methods that were recently used to identify uterine PR targets (30). As shown in Fig. 5A, microarray analysis was performed on mammary tissue total RNA obtained from ovariectomized mice previously treated with progesterone for 4, 16, 28, and 76 h. For each hormone treatment time point, mammary gland microarray data from sesame oil (vehicle) treated mice were included as controls (see Materials and Methods and supplemental Table 1 for a more detailed description of the research design). Total mammary gland RNA from each time point and treatment group was interrogated using the Affymetrix GeneChip Mouse Genome 430 2.0 Array, which contains 45,000 probe sets covering over 34,000 verified mouse genes. An expression data matrix of mammary genes [939 transcripts (712 unique genes)] showing either induction or suppression after 4, 16, 28, and 76 h progesterone exposure is shown in Fig. 5B (the complete list of genes induced and suppressed by progesterone for each time point is provided in supplemental Table 2). The expression color gram representing the time course data set reveals a progressive temporal complexity for progesterone modulation of mammary transcription (Fig. 5, B and C). In concordance with a progesterone-target tissue undergoing extensive proliferation and morphogenesis, analysis of the progesterone-responsive mammary transcriptome for significantly enriched (overrepresented) GO annotation terms revealed many functional gene classes involved in cell proliferation, survival, and turnover (Fig. 5B).
Figure 5.
Significant transcriptional activity is induced in the mammary gland of the ovariectomized 8-wk-old mouse in response to acute progesterone exposure. A, Overall hormone treatment strategy to stimulate a progesterone-induced transcriptional response in the 8-wk-old wild-type ovariectomized mouse at the times indicated. Mice were ovariectomized at 6 wk of age and rested for 2 wk to purge endogenous ovarian hormones. At 8 wk of age, ovariectomized mice were treated with 1 mg progesterone daily for 4, 16, 28, or 76 h. Ovariectomized mice treated with sesame oil (hormone vehicle) for the same time periods served as controls. B, Expression data matrix of 936 RNA transcripts (representing 711 unique genes) showing either induction or repression in the mammary gland of ovariectomized mice after progesterone (P) exposure for 4, 16, 28, or 76 h (fold change >1.8; P < 0.01). Each row represents a gene, whereas each column denotes sesame oil (SO) or progesterone treatment to a given time point in hours. The level of expression of each gene in each treatment group is represented using a red-green color scale (black: no change; and green: low expression, with bright red >2-fold change from control). To the right of the data matrix, color bars denote the corresponding annotation of genes in the matrix using selected GO terms. C, Table of the number of transcripts (with the number of unique genes in parentheses) induced or repressed by progesterone at each time point with the “Total” column listing the percentages of genes represented on the expression array. The complete list of mammary genes induced or repressed by progesterone during each of these time points is recorded in supplemental Table 2. D, Venn diagram of 512 unique progesterone-induced genes at 76 h (from panel B) and 214 PRL-induced genes as described by Harris et al. (31). The P value for the overlap between the two gene sets using one-sided Fisher’s exact test is indicated (based on the 20,964 unique named genes represented on the 430 2.0 array). The identity of 56 genes in the overlap between progesterone and PRL stimulated genes is listed in supplemental Table 3.
Harris et al. (31) recently identified 239 transcripts that decreased in expression in the mammary epithelium of the PRL receptor (PRLR) null mouse compared with wild-type mammary epithelium during pregnancy. Of the 239 molecular target genes assigned to the mammary PRLR, 214 are present on our array. Interestingly, comparison of these 214 PRLR target genes with mammary genes induced after 76 h progesterone exposure revealed 56 genes induced by both signaling pathways (a highly significant overlap; Fig. 5D). In addition to Areg, Calca, RANKL, and wnt-4, a number of genes listed in supplemental Table 3 have previously been shown to possess important roles in mammary morphogenesis and/or tumorigenesis, or have been regulated by progesterone in other physiological systems. For example, the epithelial-specific ETS transcription factor E74-like factor 5 (Elf-5) and Foxa1 (GATA3) have been shown to be essential for specification of the mammary alveolar cell lineage during pregnancy (32,33,34). Although its function is unknown, Wnt5B has been expressed in the murine mammary gland during pregnancy (35) and implicated to be involved in mammary tumorigenesis (36). One report provides evidence that Wnt5B is also induced by progesterone (37). Although not previously expressed in the mammary gland, brain-derived neutrophic factor has been induced by progesterone in other physiological systems, such as the brain (38,39). Obviously, future studies will determine whether these genes are directly controlled by mammary PR or function downstream from primary targets of mammary PR action. In addition, parallel investigations will be required to delineate the underlying mechanisms by which these genes are coordinately regulated by PR and PRLR mediated signaling.
Acute progesterone exposure induces Id4 expression in the mammary epithelium of the ovariectomized mouse
Further mining of the microarray data shown in Fig. 5 (and associated supplemental information) reveals that Id4 exhibits similar progesterone-dependent transcriptional induction kinetics as previously established PR molecular targets (Fig. 6A). Id4 is a member of a HLH family of Id proteins that modulate cellular proliferation and differentiation by functioning as dominant negative inhibitors of bHLH transcription factors required for cellular differentiation (15,16). As positive regulators of cellular proliferation, Id family members have been implicated in the etiopathogenesis of various cancers (40,41). In the rat, Id4 has been linked to mammary gland carcinogenesis (17).
Figure 6.
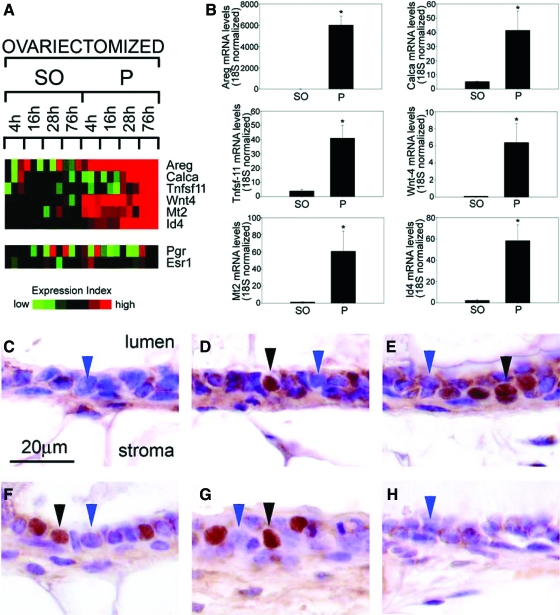
Id4 expression is markedly induced in the mammary epithelium of the ovariectomized mouse in response to acute progesterone exposure. A, Heat map analysis (from data shown in Fig. 5) for the transcriptional expression of Areg, calcitonin (Calca), RANKL (Tnfsf11), Wnt4, Mt2, Id4, PR (Pgr), and ER-α (Esr1) in mammary tissue from ovariectomized mice treated with sesame oil (SO) or progesterone (P) for the times indicated in hours. Note that Id4 transcription displays similar induction kinetics as Areg, calcitonin, RANKL, Wnt-4, and Mt2. In agreement with Fig. 3, PR transcription is predominantly repressed, and ER-α transcription remains essentially unchanged with progesterone exposure. B, Real-time PCR results for the expression of Areg, calcitonin, RANKL, Wnt-4, Mt2, and Id4 transcripts in mammary tissue from ovariectomized mice treated with sesame oil or progesterone for 76 h. Note that Id4 exhibits robust transcriptional induction in response to 76 h progesterone exposure. The results represent the mean ± se of three independent RNA sets (*, P < 0.01). Id4 immunohistochemically stained mammary sections from an untreated intact mouse (C), from an ovariectomized mouse treated with progesterone for 4 h (D), 16 h (E), 28 h (F), and 76 h (G), as well as sesame oil for 76 h (H) are shown. Note negligible Id4 nuclear staining in the luminal epithelium of the untreated intact mouse [C (blue arrowhead)]. With prolonged progesterone exposure overtime, a clear induction of nuclear staining for Id4 is observed in D–G (black arrowhead); cells scoring negative for Id4 expression are indicated by the blue arrowhead. Approximately 30% of luminal epithelial cells scored positive for Id4 expression after progesterone exposure. H shows that sesame oil treatment for 76 h fails to induce Id4 nuclear staining (blue arrowhead) in the mammary epithelium of the ovariectomized wild-type mouse.
Similar to Areg, Calca, Tnfsf-11, Wnt-4, and Mt2 [Mt2 was previously shown to be a PR molecular target (42)], significant induction of Id4 transcription in response to 76 h progesterone exposure was confirmed by real-time quantitative PCR (Fig. 6B). Furthermore, immunohistochemistry demonstrates that transcriptional induction of Id4 by progesterone is reflected at the protein level (Fig. 6, C–H). Although Id4 is negligibly expressed in the mammary epithelium of the intact virgin and vehicle-treated ovariectomized mouse, nuclear localized Id4 expression is markedly induced by progesterone in a subset of luminal epithelial cells in the mammary gland of the ovariectomized mouse (Fig. 6, D–G). Because an intrinsic nuclear localization signal is not present in the Id4 protein, we conclude that nuclear localized Id4 is due to heterodimerization with a protein partner(s) that has yet to be identified. Therefore, progesterone exposure not only induces Id4 expression but also may regulate Id4 nuclear translocation.
PR and Id4 expression localize to the same mammary cell type in the early parous mouse
To determine whether progesterone regulation of mammary Id4 expression in the hormone-treated ovariectomized murine model is physiologically relevant, immunohistochemistry was used to define the normal developmental spatiotemporal expression profile for Id4 in the mammary gland of the intact mouse. Examination of low and high-magnification fields shown in Fig. 7, A–H, clearly demonstrates that mammary Id4 expression is restricted to early pregnancy. Figure 7F reveals that Id4 immunoreactivity is localized to the nucleus of a subset of luminal epithelial cells. Because the nonuniform distribution pattern for Id4 expression is similar to the spatial expression pattern for PR in the murine mammary gland (5,18), we performed dual immunofluorescence for Id4 and PR to determine whether the subset of cells that expresses Id4 is identical to those that express the PR. Figure 7, I–L, clearly shows that Id4 and the PR are expressed in the same mammary cell type in the early parous mouse. These data provide further support for the proposal that Id4 is a molecular target of the PR during this stage of mammary gland development.
Figure 7.
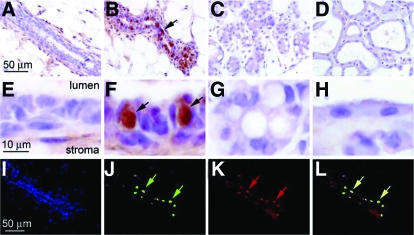
Id4 expression is induced in the epithelial compartment of the murine mammary gland during early pregnancy and colocalizes with PR expression. A–D, Mammary gland sections immunohistochemically stained for Id4 expression from adult virgin (12 wk old), early pregnant (5.5 d post coitum), late pregnant (18 dpc), and lactating (d 7) mice, respectively. Although Id4 is not detected in the epithelial compartment of the mammary gland of the virgin, late pregnant or lactating mouse (A, C, and D, respectively), note the striking increase in Id4 expression in a subset of cells in the luminal epithelial compartment of the mammary gland of the early pregnant mouse [B (arrow)]. E–H represent higher magnification images of regions shown in A–D, respectively. Note the strong nuclear expression for Id4 in a subset of luminal epithelial cells of mammary gland at early pregnancy [F (arrows)]; approximately 30% of luminal epithelial cells score positive for Id4 expression. Dual immunofluorescence clearly demonstrates that Id4 and PR expression colocalize in the mammary epithelium of the early pregnant mouse. I, A 4′, 6′-diamidino-2-phenylindole-stained section of the mammary epithelium from an early pregnant (5.5 dpc) mouse. J, The same section as in I immunofluorescently stained for Id4. Note the punctate pattern for Id4 expression (green arrows), which agrees with the immunohistochemical data shown in B and F. K shows the same section immunofluorescently stained for PR expression; again note a similar punctate pattern for PR expression in this section (red arrows). Superimposing J and K confirms that Id4 and PR expression localize to identical cells (yellow arrows in L). Scale bar in A, E, and I apply to B–D, F–H, and J–L, respectively.
Id4 is a direct PR target gene in murine HC-11 cells
The colocalization of PR and Id4 expression in luminal epithelial cells of the mammary gland during early pregnancy suggests that Id4 is a direct PR responsive target gene. To confirm whether it is a direct target, hormone induction of Id4 gene expression and binding of PR to the upstream promoter of Id4 (Fig. 8A), as detected by ChIP assay, were examined in cell culture using HC-11 mouse mammary epithelial cells. HC-11 is a well-characterized cell line that is capable of undergoing differentiation in vitro, and has been used extensively to examine hormonal regulation of mammary specific gene expression. These cells express functional PRLR and glucocorticoid receptors, and expression of the β-casein milk protein gene is induced by the synergistic actions of PRL and hydrocortisone mediated, respectively, by the PRLR/Janus kinase 2/Stat5a signaling pathway and glucocorticoid receptor (43,44,45,46). However, HC-11 cells lack expression of PR. Loss of PR (and ER-α) expression in primary mammary epithelial cell cultures and in nontransformed mammary epithelial cell lines is a common occurrence due to the loss of appropriate basement membrane interactions (47). Therefore, PR was expressed in HC-11 by transduction of cells with a recombinant adenovirus encoding the murine PR-B isoform. Conditions were used (low multiplicity of infection) to express a functional PR in a high percentage of cells at a level similar to that of endogenous PR as described (27). In HC-11 cells transduced to express mouse PR-B, an 8-fold induction of Id4 RNA was obtained by treatment for 24 h with the synthetic progestin R5020 (Fig. 8B). PRL treatment also induced Id4 gene expression, albeit by a lower 4-fold induction. Interestingly, treatment with progesterone and PRL together gave a 32-fold induction, indicating that progesterone and PRL may functionally synergize to up-regulate Id4 (Fig. 8B).
The 5′ upstream regulatory region of Id4 was analyzed by the Ensembl database (www.ensembl.org) for the presence of consensus progesterone response element (PRE) sequences as well as S5REs. Multiple hexanucleotide PRE half-sites are present between −1503 to −5259 bp from the transcription start site, and an S5RE is present at −6392 bp (Fig. 8A). ChIP assays were performed to determine whether PR or Stat5 associates with any of these sequence regions of the Id4 gene. For these experiments, hPR-B was expressed in HC-11 cells because the most suitable antibody for ChIP assay is specific for the hPR (27,48). Ectopically expressed mouse and hPR are functionally similar in HC-11 cells (27), including their ability to mediate progestin induction of Id4 (data not shown). HC-11 cells were transduced to express hPR-B, and cells were differentiated under conditions similar to that for the functional response experiment (Fig. 8B). Cells were then treated for 1 h with R5020 or PRL, fixed with formalin, lysed, and DNA was fragmented by sonication. Cross-linked DNA fragments were immunoprecipitated with an unrelated control antibody, or with an antibody specific to PR (1294) or Stat5a [Santa Cruz Biotechnology (L-20)]. The immunoprecipitated DNA fragments were amplified by PCR with seven primer pairs that cover eight potential PREs and an S5RE site in the upstream promoter region of the Id4 gene (Fig. 8B). DNA was not immunoprecipitated with a control antibody under any condition, and in the absence of hormone (vehicle), neither PR nor Stat5a associated with any of the amplified DNA regions (Fig. 8, C–E). Treatment with R5020 stimulated association of PR with the amplified DNA products that contain PRE 6/7 (Fig. 8C) and PRE 8 (Fig. 8D), whereas treatment with PRL stimulated recruitment of Stat5a to the S5RE site (Fig. 8E). No enrichment of PR was detected with PREs 1–5 (data not shown) or with the S5RE site in response to progesterone (Fig. 8E), and no enrichment of Stat5a was detected with any of the amplified DNAs containing PREs in response to PRL (Fig. 8, C and D) (data not shown). Thus, PR does not bind to all the potential PREs and is selective for only some, whereas Stat5a binds selectively to the S5RE. Interestingly a different pattern of PR and Stat5a recruitment was detected when cells were cotreated with progesterone and PRL. Stat5a was recruited to PREs 6–7 and 8 in addition to the S5RE site, whereas PR was recruited to both the S5RE site and to PREs 6–7 and 8 (Fig. 8, C–E). These results show that PR and Stat5a are recruited together to PRE and S5RE sites in the presence of both hormones, suggesting a novel mechanism of functional synergy that involves cooperative recruitment of multiple PR/Stat5a complexes to the Id4 upstream promoter.
Together, these data provide strong evidence that progesterone induced up-regulation of the Id4 expression is through a direct interaction of PR with the Id4 promoter. Furthermore, our results reveal a possible synergistic up-regulation of Id4 by progesterone and PRL mediated through a convergence of PR and Stat5a signaling at the upstream promoter of the Id4 gene.
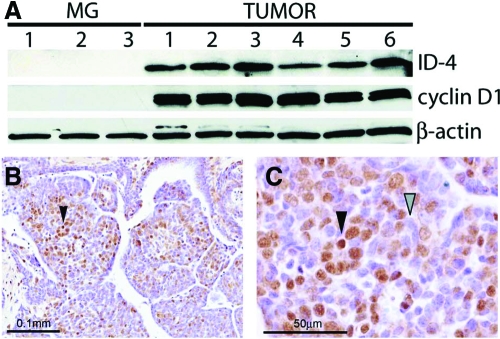
Id4 expression is up-regulated in MMTV-Wnt-1 mammary tumors
A previous study reported that Id4 protein expression is significantly elevated in mammary tumors derived from a carcinogen-induced rat mammary tumor model (17). Western analysis of mammary tumor protein derived from the commonly used MMTV-Wnt-1 transgenic mouse clearly shows that significant levels of Id4 are present in all tumors examined (Fig. 9A); follow-up immunohistochemical analysis of Id4 expression confirmed the Western data (Fig. 9B). As in the rat, these results support the argument that Id4 may have important roles in both mammary morphogenesis and tumorigenesis in the mouse.
Figure 9.
Id4 is overexpressed in MMTV-Wnt-1 mammary tumors. A, Comparative Western blot analysis of adult wild-type virgin mammary gland (MG) and MMTV-Wnt1 tumors (TUMOR) for Id4 and cyclin D1 expression (β-actin is included as a loading control). Mammary gland protein lysates from three individual wild-type mice were analyzed (lanes 1–3), whereas protein lysate from six tumors (lanes 1–6) from six individual MMTV-Wnt1 transgenic mice was analyzed by Western blot. Although not observed in the wild-type mammary gland, Id4 protein is clearly detected in all six MMTV-Wnt1 mammary tumors (TUMOR). The immunohistochemical detection of Id4 nuclear expression (black arrowhead) in MMTV-Wnt1 mammary tumors is clearly evident in B and C, which confirms the Western blot data shown in A. C is a higher magnification of a region shown in B, which shows tumor cells positive and negative for Id4 immunoreactivity (black and gray arrowheads, respectively).
Discussion
Only through disclosure of the full spectrum of genetic programs controlled by the progesterone signal can a complete molecular context be applied to progesterone’s involvement in normal mammary epithelial morphogenesis. Progesterone’s pivotal role in rodent mammary tumorigenesis (3,7,8) and the recent association made between the inclusion of progestins in postmenopausal hormone therapy and breast cancer risk (9) make identification of these downstream effectors an urgent priority.
Although progesterone-dependent global gene signatures have recently been identified in the murine uterus (30) as well as in cultured human breast cancer cells (49), gene discovery approaches have yet to be applied to the mouse mammary gland to identify its progesterone-responsive transcriptome.
To date, only a few genes have been implicated to be downstream effectors of progesterone action in the murine mammary gland, and, so far, pathway relationships between these genes have not been firmly established. Regardless of whether any or all of these genes are confirmed to operate downstream of the progesterone signal, the “one-gene-at-a-time-approach” is clearly not an effective strategy to identifying the full repertoire of immediate targets of mammary PR. To address this issue, we used microarray analysis in conjunction with an acute hormone treatment paradigm to identify those molecular pathways that operate immediately downstream of the progesterone signal during the early stages of progesterone-induced mammary morphogenesis.
We demonstrate that progesterone exposure alone can elicit mammary epithelial proliferation that leads to ductal side branching and limited alveolar budding. However, in the absence of estrogen and PRL, further alveolar expansion and differentiation (as occur during mid to late pregnancy) fail to occur. Importantly, the clear induction of mammary Areg, Wnt-4, RANKL, and calcitonin by this acute progesterone treatment protocol underscored the feasibility of using this approach with microarray approaches to uncover the progesterone-responsive transcriptome in the murine mammary gland.
Our initial analyses of the resultant mammary transcriptomes revealed the induction of a number of gene families previously shown to exert potent regulatory controls on proliferative and prosurvival pathways in the mammary epithelium. Of interest was the significant number of these genes that have also been regulated by mammary PRLR. Although speculative, these observations suggest that this gene set (or a subgroup thereof) represents a molecular convergence point for cross talk between PR and PRL-mediated signaling.
Close scrutiny of the transcriptome-induction kinetics revealed Id4 as a new target of mammary PR action. Immunohistochemical and molecular analyses show that mammary Id4 is not only induced by progesterone in PR positive cells but may well act as a direct target of mammary PR. Id4 is a member of the Id family of HLH transcription factors that also includes Id1, 2, and 3 (15,16). Through formation of nonfunctional heterodimers, Id family members inhibit bHLH transcription factors that are required for the execution of cellular differentiation programs that operate in a variety of physiological systems, including the mammary gland (50). Based on their mechanism of action, Id proteins are generally considered positive modulators of cell-cycle progression and negative regulators of terminal differentiation. In the case of Id2, knockout studies in mice have demonstrated that functional abrogation of Id2 results in a block in the developmental progression of the mammary gland during pregnancy (51,52). In a separate study, Id2 nuclear translocation and subsequent cell-cycle activation in the murine mammary epithelial cell were dependent on RANKL signaling (53). Because RANKL has been implicated as a PR mammary target (11,12), these findings raise the intriguing possibility that PR may directly regulate mammary Id4 and indirectly modulate Id2 activity, respectively.
Unfortunately, knockout mouse models for Id4 exhibit early neural proliferation impairments and associated premature differentiation abnormalities that together result in perinatal lethality (54,55). Although the phenotypes of the Id4 knockout mouse preclude studies in the adult, Id4 overexpression in the cultured murine mammary epithelial cell line, HC-11, was shown to inhibit lactogenic hormone-induced β-casein production (17). This observation suggests that Id4 may function to restrain mammary epithelial differentiation. In light of our recent finding that progesterone signaling also inhibits PRL-induced β-casein synthesis in HC-11 cells (27), determining whether Id4 serves as a downstream mediator of PR in this context represents an obvious future objective. Together, the aforementioned observations also raise the question as to the regulatory mechanisms by which Id4 expression is temporally controlled by progesterone and PRL signaling as the mammary epithelium progresses from a proliferative state (at early pregnancy) to a terminally differentiated state (at late pregnancy). Although speculative, it is not inconceivable that the loss of Id4 expression at later stages of pregnancy results from a marked reduction in PR expression as pregnancy advances to term. In support of this concept, we and others have previously shown that PR expression is significantly reduced in the mammary epithelium of the late pregnant mouse compared with early pregnancy (18,56). Unlike progesterone, PRL exerts early proliferative and later differentiative responses in the mammary epithelium of the parous animal (57). In concert with progesterone, PRL is required for early mammary alveolar expansion (57), a time window during which Id4 is induced. However, at late pregnancy, PRL alone elicits mammary epithelial differentiation [a cellular process that fails to occur in the presence of PR and Id4 signaling (17,27)]. Therefore, based on the foregoing, we suggest that progressive down-regulation of PR expression, in concert with a switch from early PRL-dependent proliferative signaling to later differentiative pathways, is an important contributory factor in Id4 suppression with pregnancy advancement.
Apart from normal mammary development, significant Id4 induction in carcinogen-induced rat mammary carcinomas (17) suggests that Id4 may have a role in the onset and/or progression of this tumor class. Our observations that Id4 is also up-regulated in murine mammary tumors indicate that this transcriptional regulator may exert a wider role in rodent mammary tumorigenesis than previously suspected. Mammary epithelial cell culture experiments clearly demonstrate that, in addition to blocking cellular differentiation, enforced Id4 expression results in a significant increase in anchorage-independent growth and colony formation in soft agar (17). Although not yet tested in an in vivo model, these observations support a causal role for Id4 in rodent mammary tumorigenesis. Studies implicating Id4 as a negative regulator of the mammary tumor suppressor, breast cancer susceptibility gene 1 (BRCA-1), further support an oncogenic role for Id4 in the mammary gland (58). Interestingly, BRCA-1 has been a negative regulator of mammary PR expression (8). Together, these independent investigations suggest that a regulatory loop may exist in the murine mammary epithelial cell that comprises BRCA-1, PR, and Id4, and suggest that Id4 may represent one molecular link between progesterone exposure and the promotion of mammary neoplastic progression. Although rodent studies provide compelling support for Id4 as a mammary gland oncogene, an oncogenic role for Id4 in the human breast is currently unclear (59,60,61). However, Id4 has been strongly implicated in other human cancers, such as prostate and bladder (40,41).
Obvious future goals in this area will be to: 1) evaluate the functional role of Id4 in mammary morphogenesis in vivo, 2) test whether Id4 signaling is causal for mammary tumorigenesis, 3) determine the mechanisms by which PR and PRLR mediated signaling coordinately regulates Id4 expression, and finally 4) identify the key-interacting partners of Id4 in the mammary epithelial cell. The achievement of the latter goal will greatly facilitate ongoing efforts to formulate new therapeutic strategies to functionally block this family of transcriptional regulators in vivo (62).
In conclusion, we have previously demonstrated that the progesterone signal is indispensable for murine mammary morphogenesis, and that this signal influences mammary tumor onset and progression. However, our understanding of the molecular mechanisms that underpin progesterone’s mammary effects is not well established. To address this knowledge gap, we have identified and characterized a temporal series of progesterone-responsive mammary transcriptomes obtained from acute progesterone exposures of the ovariectomized pubertal mouse. Revealing Id4 as a mammary target for PR action underscores the utility of this transcriptome informational resource as an investigative tool with which to identify additional effectors of mammary PR signaling in the future. Furthermore, we anticipate that this informational resource will be essential for defining genetic pathways that are commonly and differentially regulated by progesterone in the mammary gland and endometrium, as well as in the mammary epithelial cell expressing either the PRA or PRB isoform. Finally, we believe that this informational resource will not only furnish much needed molecular context to PR action in the normal mammary gland but may well provide useful molecular descriptors for breast cancer diagnosis, prognosis, and/or therapy.
Supplementary Material
Acknowledgments
We thank Jie Li, Yan Ying, and Jie Han for their technical expertise. We also thank Dr. Jaewook Jeong for initial advice on the microarray analysis.
Footnotes
These studies were supported by National Institutes of Health Grants HD-42311 (to F.J.D.), HD-038129 (to D.P.E.), and CA-77530 (to J.P.L.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online August 7, 2008
Abbreviations: Areg, Amphiregulin; bHLH, basic helix-loop-helix; BRCA-1, breast cancer 1; BrdU, 5-bromo-2-deoxyuridine; Calca, calcitonin/calcitonin-related polypeptide α; ChIP, chromatin immunoprecipitation; CMV, cytomegalovirus; ER, estrogen receptor; GO, gene ontology; H&E, hematoxylin and eosin; HLH, helix-loop-helix; hPR-B, human progesterone receptor-B; Id4, inhibitor of differentiation 4; MMTV, mouse mammary tumor virus; MT2, metallothionein 2; pfu, plaque-forming unit; PR, progesterone receptor; PRE, progesterone response element; PRKO, progesterone receptor knockout; PRL, prolactin; PRLR, prolactin receptor; RANKL, receptor of activated nuclear factor-κB ligand; RNase, ribonuclease; RPA, ribonuclease protection assay; Stat5a, signal transducer and activator of transcription −5; S5RE, signal transducer and activator of transcription −5 response element; Tnfsf11, TNF ligand superfamily member 11; Wnt,-4, wingless-related mouse mammary tumor virus integration site 4.
References
- Fernandez-Valdivia R, Mukherjee A, Mulac-Jericevic B, Conneely OM, DeMayo FJ, Amato P, Lydon JP 2005 Revealing progesterone’s role in uterine and mammary gland biology: insights from the mouse. Semin Reprod Med 23:22–37 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptors exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Lydon JP, Ge G, Kittrell FS, Medina D, O'Malley BW 1999 Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res 59:4276–4284 [PubMed] [Google Scholar]
- Russo J, Ao X, Grill C, Russo IH 1999 Pattern of distribution of cells positive for estrogen receptor-α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat 53:217–227 [DOI] [PubMed] [Google Scholar]
- Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM 2000 C/EBPβ (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol 14:359–368 [DOI] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E 1997 Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991 [PubMed] [Google Scholar]
- Efeyan A, Fabris V, Merani S, Lanari C, Molinolo AA 2004 Establishment of two hormone-responsive mouse mammary carcinoma cell lines derived from a metastatic mammary tumor. Breast Cancer Res Treat 83:233–244 [DOI] [PubMed] [Google Scholar]
- Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY 2006 Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 314:1467–1470 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative Investigators 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA 2000 Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14:650–654 [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM 2000 The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103:41–50 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail PM, DeMayo FJ, Amato P, Lydon JP 2004 Progesterone induction of calcitonin expression in the murine mammary gland. J Endocrinol 180:287–295 [DOI] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK 1995 Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 9:691–705 [DOI] [PubMed] [Google Scholar]
- Perk J, Iavarone A, Benezra R 2005 Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer 5:603–614 [DOI] [PubMed] [Google Scholar]
- Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM 2003 Id proteins in cell growth and tumorigenesis. Cancer Cell 3:525–530 [DOI] [PubMed] [Google Scholar]
- Shan L, Yu M, Qiu C, Snyderwine EG 2003 Id4 regulates mammary epithelial cell growth and differentiation and is overexpressed in rat mammary gland carcinomas. Am J Pathol 163:2495–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP 2002 A novel lacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16:2475–2489 [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH 2003 DNA-chip analyzer (dChip). In: Parmigiani G, Garrett ES, Irizarry R, Zeger SL, eds. The analysis of gene expression data: methods and software. New York: Springer; 120–141 [Google Scholar]
- Schadt EE, Li C, Ellis B, Wong WH 2001 Feature extraction and normalization algorithms for high-density oligonucleotide gene expression array data. J Cell Biochem Suppl 37:120–125 [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH 2001 Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D 1998 Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ 2004 Java Treeview—extensible visualization of microarray data. Bioinformatics 20:3246–3248 [DOI] [PubMed] [Google Scholar]
- Creighton CJ, Kuick R, Misek DE, Rickman DS, Brichory FM, Rouillard JM, Omenn GS, Hanash S 2003 Profiling of pathway-specific changes in gene expression following growth of human cancer cell lines transplanted into mice. Genome Biol 4:R46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP 2000 Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol 14:52–65 [DOI] [PubMed] [Google Scholar]
- Schaack J, Guo X, Ho WY, Karlok M, Chen C, Ornelles D 1995 Adenovirus type 5 precursor terminal protein-expressing 293 and HeLa cell lines. J Virol 69:4079–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser AC, Gass-Handel EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, Rosen JM, Watkin H, Anderson SM, Edwards DP 2007 Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the β-casein gene in mammary epithelial cells. Mol Endocrinol 21:106–125 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW 2006 Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 26:6571–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonderhaar BK, Lydon JP, Miyoshi K, Hennighausen L, Ormandy CJ, Lee AV, Stull MA, Wood TL, Rosen JM 2002 Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development. Mol Endocrinol 16:2675–2691 [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ 2005 Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146:3490–3505 [DOI] [PubMed] [Google Scholar]
- Harris J, Stanford PM, Sutherland K, Oakes SR, Naylor MJ, Robertson FG, Blazek KD, Kazlauskas M, Hilton HN, Wittlin S, Alexander WS, Lindeman GJ, Visvader JE, Ormandy CJ 2006 Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol 20:1177–1187 [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE 2007 Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9:201–209 [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z 2006 GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127:1041–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, Kazlauskas M, Pritchard MA, Chodosh LA, Pfeffer PL, Lindeman GJ, Visvader JE, Ormandy CJ 2008 The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev 22:581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC 1994 Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation 57:205–214 [DOI] [PubMed] [Google Scholar]
- Kuorelahti A, Rulli S, Huhtaniemi I, Poutanen M 2007 Human chorionic gonadotropin (hCG) up-regulates wnt5b and wnt7b in the mammary gland, and hCGβ transgenic female mice present with mammary gland tumors exhibiting characteristics of the Wnt/β-catenin pathway activation. Endocrinology 148:3694–3703 [DOI] [PubMed] [Google Scholar]
- Rudolph MC, McManaman JL, Hunter L, Phang T, Neville MC 2003 Functional development of the mammary gland: use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J Mammary Gland Biol Neoplasia 8:287–307 [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Gonzalez Deniselle MC, Guennoun R, Schumacher M, De Nicola AF 2004 Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience 125:605–614 [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M 2007 Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res 85:2441–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Hoffmann MJ, Hartmann FH, Schulz WA 2005 Amplification and overexpression of the ID4 gene at 6p22.3 in bladder cancer. Mol Cancer 4:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW 2006 Id proteins expression in prostate cancer: high-level expression of Id-4 in primary prostate cancer is associated with development of metastases. Mod Pathol 19:931–941 [DOI] [PubMed] [Google Scholar]
- Slater EP, Cato AC, Karin M, Baxter JD, Beato M 1988 Progesterone induction of metallothionein-IIA gene expression. Mol Endocrinol 2:485–491 [DOI] [PubMed] [Google Scholar]
- Doppler W, Hock W, Hofer P, Groner B, Ball RK 1990 Prolactin and glucocorticoid hormones control transcription of the β-casein gene by kinetically distinct mechanisms. Mol Endocrinol 4:912–919 [DOI] [PubMed] [Google Scholar]
- Groner B, Altiok S, Meier V 1994 Hormonal regulation of transcription factor activity in mammary epithelial cells. Mol Cell Endocrinol 100:109–114 [DOI] [PubMed] [Google Scholar]
- Kabotyanski EB, Huetter M, Xian W, Rijnkels M, Rosen JM 2006 Integration of prolactin and glucocorticoid signaling at the β-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol Endocrinol 20:2355–2368 [DOI] [PubMed] [Google Scholar]
- Stocklin E, Wissler M, Gouilleux F, Groner B 1996 Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383:726–728 [DOI] [PubMed] [Google Scholar]
- Novaro V, Roskelley CD, Bissell MJ 2003 Collagen-IV and laminin-1 regulate estrogen receptor α expression and function in mouse mammary epithelial cells. J Cell Sci 116:2975–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press M, Spaulding B, Groshen S, Kaminsky D, Hagerty M, Sherman L, Christensen K, Edwards DP 2002 Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids 67:799–813 [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB 2002 Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218 [DOI] [PubMed] [Google Scholar]
- Desprez PY, Sumida T, Coppe JP 2003 Helix-loop-helix proteins in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 8:225–239 [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Meyer B, Gruss P, Cui Y, Renou JP, Morgan FV, Smith GH, Reichenstein M, Shani M, Hennighausen L, Robinson GW 2002 Mammary epithelial cells are not able to undergo pregnancy-dependent differentiation in the absence of the helix-loop-helix inhibitor Id2. Mol Endocrinol 16:2892–2901 [DOI] [PubMed] [Google Scholar]
- Mori S, Nishikawa SI, Yokota Y 2000 Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. EMBO J 19:5772–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NS, Kim HJ, Koo BK, Kwon MC, Kim YW, Cho Y, Yokota Y, Penninger JM, Kong YY 2006 Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol 26:1002–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford L, Walker R, Kondo T, van Cruchten I, King ER, Sablitzky F 2005 Id4 is required for the correct timing of neural differentiation. Dev Biol 280:386–395 [DOI] [PubMed] [Google Scholar]
- Yun K, Mantani A, Garel S, Rubenstein J, Israel MA 2004 Id4 regulates neural progenitor proliferation and differentiation in vivo. Development 131:5441–5448 [DOI] [PubMed] [Google Scholar]
- Aupperlee MD, Smith KT, Kariagina A, Haslam SZ 2005 Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 146:3577–3588 [DOI] [PubMed] [Google Scholar]
- Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ 2008 Prolactin regulation of mammary gland development. J Mammary Gland Biol Neoplasia 13:13–28 [DOI] [PubMed] [Google Scholar]
- Beger C, Pierce LN, Kruger M, Marcusson EG, Robbins JM, Welcsh P, Welch PJ, Welte K, King MC, Barber JR, Wong-Staal F 2001 Identification of Id4 as a regulator of BRCA1 expression by using a ribozyme-library-based inverse genomics approach. Proc Natl Acad Sci USA 98:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Candia P, Akram M, Benezra R, Brogi E 2006 Id4 messenger RNA and estrogen receptor expression: inverse correlation in human normal breast epithelium and carcinoma. Hum Pathol 37:1032–1041 [DOI] [PubMed] [Google Scholar]
- Roldan G, Delgado L, Muse IM 2006 Tumoral expression of BRCA1, estrogen receptor α and ID4 protein in patients with sporadic breast cancer. Cancer Biol Ther 5:505–510 [DOI] [PubMed] [Google Scholar]
- Umetani N, Mori T, Koyanagi K, Shinozaki M, Kim J, Giuliano AE, Hoon DS 2005 Aberrant hypermethylation of ID4 gene promoter region increases risk of lymph node metastasis in T1 breast cancer. Oncogene 24:4721–4727 [DOI] [PubMed] [Google Scholar]
- Henke E, Perk J, Vider J, de Candia P, Chin Y, Solit DB, Ponomarev V, Cartegni L, Manova K, Rosen N, Benezra R 2008 Peptide-conjugated antisense oligonucleotides for targeted inhibition of a transcriptional regulator in vivo. Nat Biotechnol 26:91–100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.