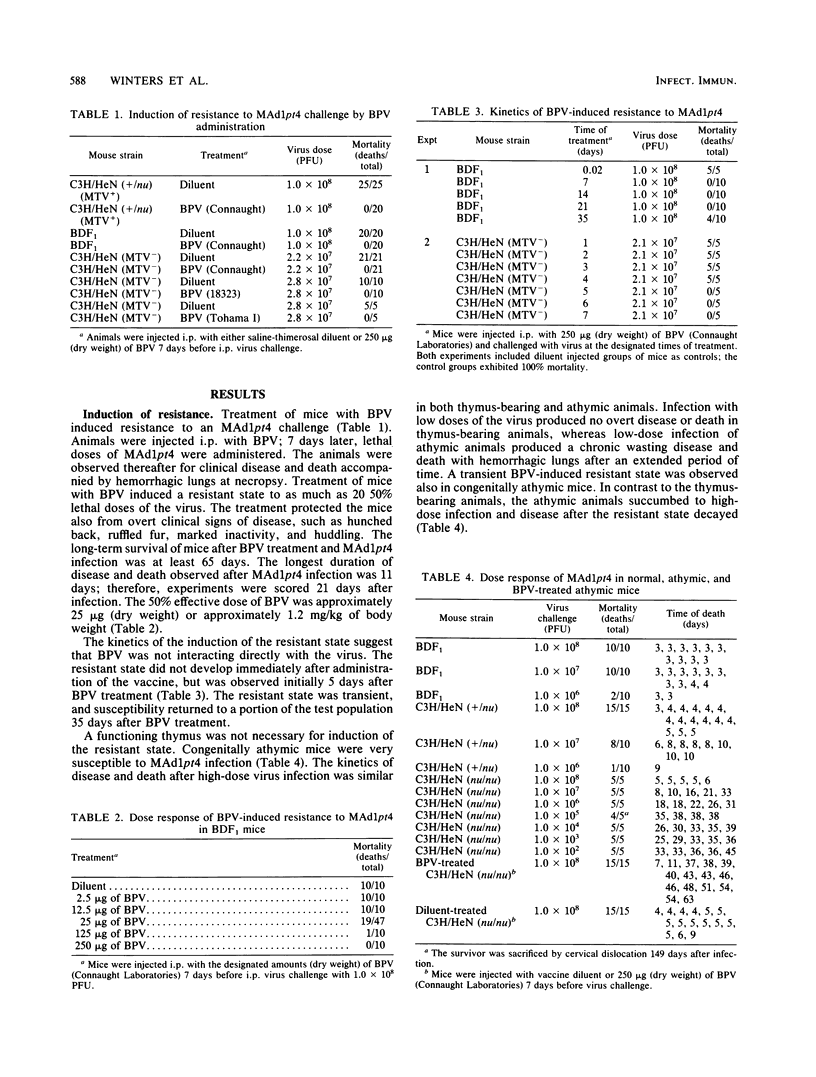
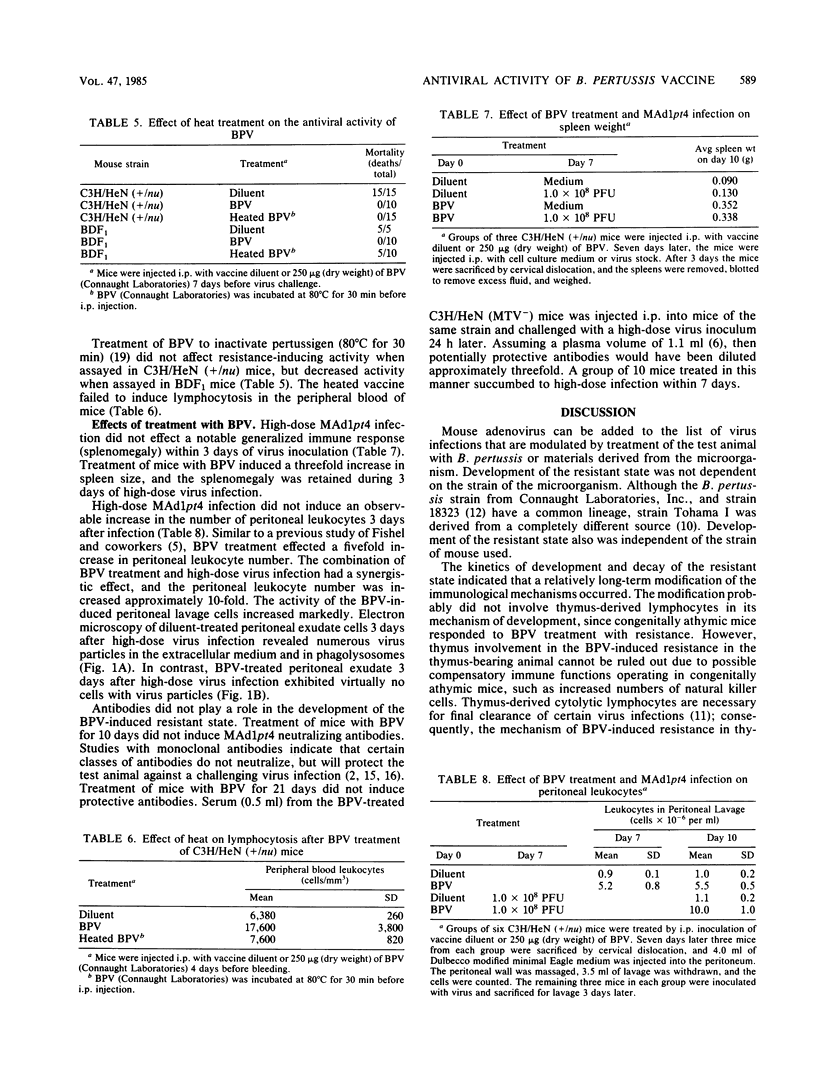
Abstract
Treatment of mice with Bordetella pertussis vaccine rendered mice resistant to mouse adenovirus infection. The resistant state took at least 5 days to develop, and susceptibility returned to a portion of the test population 35 days after treatment. Transient resistance developed in congenitally athymic mice also. Treatment with a dose of 25 micrograms (dry weight) of B. pertussis vaccine protected approximately 50% of the test population. Vaccines prepared from several different strains of B. pertussis were capable of inducing resistance, and the induction of resistance was not dependent on the mouse strain used for testing. Cross-reacting antibodies capable of neutralizing the virus or protecting against a challenging infection were not induced by treatment with B. pertussis vaccine.
Full text
PDF
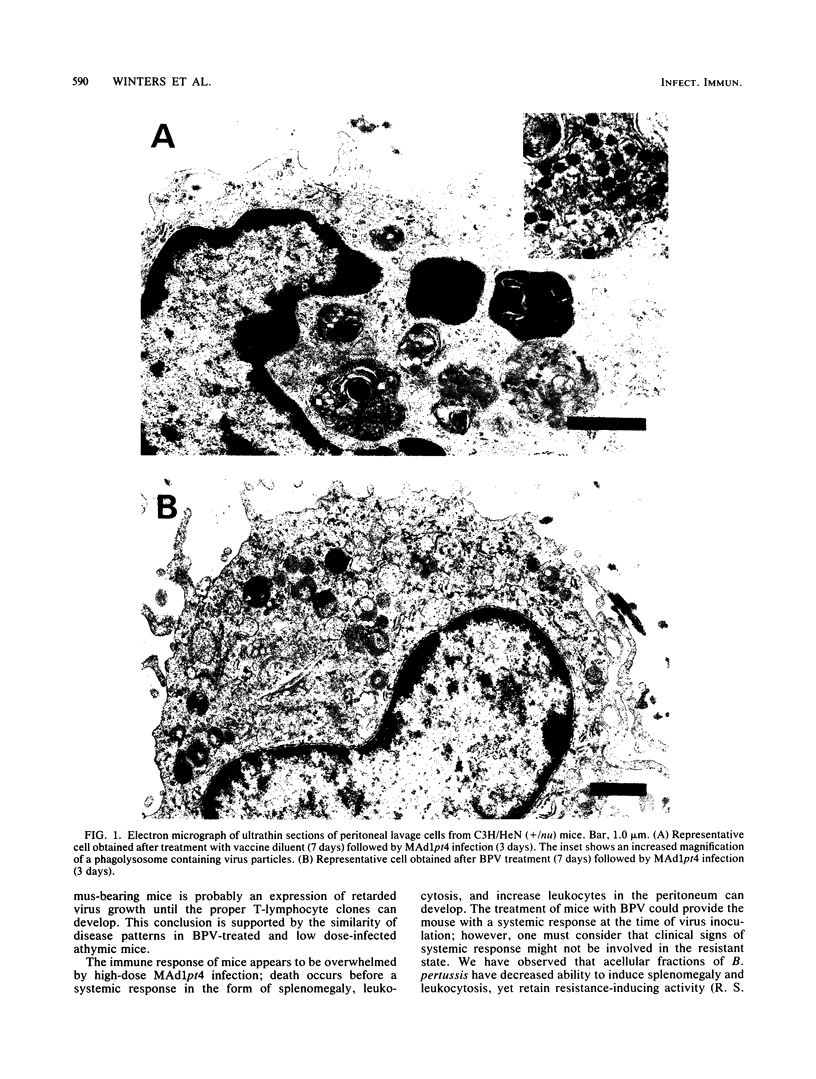

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayme G., Caroff M., Chaby R., Haeffner-Cavaillon N., Le Dur A., Moreau M., Muset M., Mynard M. C., Roumiantzeff M., Schulz D. Biological activities of fragments derived from Bordetella pertussis endotoxin: isolation of a nontoxic, Shwartzman-negative lipid A possessing high adjuvant properties. Infect Immun. 1980 Mar;27(3):739–745. doi: 10.1128/iai.27.3.739-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN J. J. Circulating and tissue hematocrits of normal unanesthetized mice. Am J Physiol. 1959 Feb;196(2):420–422. doi: 10.1152/ajplegacy.1959.196.2.420. [DOI] [PubMed] [Google Scholar]
- Fish F., Cowell J. L., Manclark C. R. Proliferative response of immune mouse T-lymphocytes to the lymphocytosis-promoting factor of Bordetella pertussis. Infect Immun. 1984 Apr;44(1):1–6. doi: 10.1128/iai.44.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel C. W., Halkias D. G., Klein T. W., Szentivanyi A. Characteristics of cells present in peritoneal fluids of mice injected intraperitoneally with Bordetella pertussis. Infect Immun. 1976 Jan;13(1):263–272. doi: 10.1128/iai.13.1.263-272.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Roberts C. O., Wolff J., Manclark C. R. Biphasic effect of pertussis vaccine on serum insulin in mice. Infect Immun. 1983 Jul;41(1):137–144. doi: 10.1128/iai.41.1.137-144.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. K., Kong A. S., Morse S. I. The in vitro effects of Bordetella pertussis lymphocytosis-promoting factor on murine lymphocytes. V. Modulation of T cell proliferation by helper and suppressor lymphocytes. J Immunol. 1980 Jan;124(1):362–369. [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch Exp Med. 1954 Sep;27(3):57–62. [PubMed] [Google Scholar]
- Kees U., Blanden R. V. A single genetic element in H-2K affects mouse T-cell antiviral function in poxvirus infection. J Exp Med. 1976 Feb 1;143(2):450–455. doi: 10.1084/jem.143.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick P. L., Eldering G., Dixon M. K., Misner J. Mouse Protection Tests in the Study of Pertussis Vaccine: A Comparative Series Using the Intracerebral Route for Challenge. Am J Public Health Nations Health. 1947 Jul;37(7):803–810. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Scott M. T., Hirt H. M., Munk K. Protection of mice against viral infection by Corynebacterium parvum and Bordetella pertussis. J Gen Virol. 1978 Oct;41(1):97–104. doi: 10.1099/0022-1317-41-1-97. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982 Dec;129(6):2763–2767. [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Bergman R. K., Sadowski P. L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981 Sep;33(3):820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARFENTJEV I. A. Bacterial allergy increases susceptibility to influenza virus in mice. Proc Soc Exp Biol Med. 1955 Nov;90(2):373–375. doi: 10.3181/00379727-90-22037. [DOI] [PubMed] [Google Scholar]
- Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect Immun. 1983 Jul;41(1):313–320. doi: 10.1128/iai.41.1.313-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M., Nakanishi Y., Otokawa M., Uchida N., Yasuda T., Sato H., Sato Y. Effect of Bordetella pertussis leukocytosis (lymphocytosis)-promoting factor (LPF) on the physical lymphoepithelial-cell association studied with the use of an in vitro model of mouse thymus. J Immunol. 1983 Jun;130(6):2767–2774. [PubMed] [Google Scholar]
- Winters A. L., Brown H. K., Carlson J. K. Interstitial pneumonia induced by a plaque-type variant of mouse adenovirus. Proc Soc Exp Biol Med. 1981 Jul;167(3):359–364. doi: 10.3181/00379727-167-41179. [DOI] [PubMed] [Google Scholar]
- Winters A. L., Brown H. K. Duodenal lesions associated with adenovirus infection in athymic "nude" mice. Proc Soc Exp Biol Med. 1980 Jul;164(3):280–286. doi: 10.3181/00379727-164-40862. [DOI] [PubMed] [Google Scholar]