Abstract
Down Syndrome (DS) results from triplication of the whole or distal part of human chromosome 21. DS subjects suffer from deficits in learning and memory and cognitive functions in general, and, starting from early development, their brains show dendritic and spine structural alterations and cell loss. These defects concern many cortical brain regions as well as the hippocampus, which is known to play a critical role in memory and cognition. Most of these abnormalities are reproduced in the mouse model Ts65Dn, which is partially trisomic for the mouse chromosome 16 that is homologous to a portion of human chromosome 21. Thus, Ts65Dn is widely utilized as an animal model of DS. To better understand the molecular defects underlying the cognitive and particularly the memory impairments of DS, we investigated whether the expression of several molecules known to play critical roles in long-term synaptic plasticity and long-term memory in a variety of species is dysregulated in either the neonatal brain or adult hippocampus of Ts65Dn mice. We found abnormal expression of the synaptic proteins synaptophysin, MAP2 and CDK5 and of the neurotrophin NT-3. Both the neonatal brain and adult hippocampus revealed significant abnormalities. These results suggest that a dysregulation in the expression of neurotrophins as well as proteins involved in synaptic development and plasticity may play a potential role in the neural pathology of DS in humans.
Keywords: Down Syndrome, Ts65Dn, memory, plasticity, synaptic protein, neurotrophin
Down syndrome (DS), the most common genetically based mental retardation disorder, is caused by the presence of an extra copy of chromosome 21 (trisomy 21). DS is characterized by a variety of deficits that affect many systems including skeletal, skin, immune and nervous. Although a high degree of variability among individuals has been described, the nervous system dysfunctions include severe impairments in learning, memory, speech and motor behavior (Roubertoux and Kerdelhue, 2006; Vicari, 2006; Raz et al., 1995).
Autopsy studies have revealed that the brains of DS subjects both at early developmental ages as well as in adults carry several synaptic abnormalities including dendritic and spine structural alterations and loss of choline acetyltransferase in several cortical regions including the hippocampus (Purpura 1973; Purpura 1975; Wisnienski 1990; Perry et al., 1986). Moreover, DS subjects have reduced hippocampal and cerebellar volumes (Wisniewski et al., 1984; Kaufmann and Moser, 2000; Fiala et al., 2002; Pinter et al., 2001a, b). These findings imply that DS pathology is associated with abnormal synaptic function, and consequently, abnormal cognition, which may result from alterations in biochemical substrates of plasticity either during development or in adult brain regions involved in cognitive processes. One of these regions is the hippocampus (Pinter et al., 2001a; Sylvester, 1983; Krasuski et al., 2002), which is known to play a critical role in memory formation (Eichenbaum, 2004). In agreement, high-resolution magnetic resonance imaging (MRI) has confirmed that subjects with DS have smaller overall brain volumes and, in particular, disproportionately smaller hippocampal regions (Pealson et al., 1998). The presence of these abnormalities from an early age suggests that fetal or early postnatal developmental differences may contribute to the morphological, cognitive and developmental deficits of DS (Raz et al., 1995; Pinter et al., 2001a, b). Despite the fact that the morphometric and histological studies show clear and progressive deficits in the brains of DS subjects, little is known about the molecular alterations that underlie these defects.
Because of difficulties in obtaining human samples to study DS, many molecular and behavioral studies have used genetic mouse models of trisomy 21 that reflect some critical phenotypic aspects of the human disorder. One of the most widely utilized models is the Ts65Dn mouse, which carries a partial trisomy of the mouse chromosome 16 (Davisson et al., 1990; Reeves et al., 1995) that is homologous to a portion of the human chromosome 21. This mouse model shows several histological defects of DS, including decreased cell number in subregions of the hippocampus (Insausti et al., 1998; Lorenzi and Reeves, 2006), reduction in asymmetric synapses in the temporal cortex (Kurt et al., 2000), reduced volume and neuronal density in the cerebellum (Baxter et al., 2000), age-related degeneration of basal forebrain cholinergic neurons (Cooper et al., 2001) and diminished responsiveness of acetylcholine (ACh) release in the hippocampus to behavioral testing (Chang and Gold, 2007). Moreover, Ts65Dn mice, like DS subjects, are cognitively impaired, especially in tasks that involve the hippocampus (Reeves et al., 1995; Escorihuela et al., 1995; Demas et al., 1998; Seregaza et al., 2006; Fernandez et al., 2007). Ts65Dn mice also exhibit a variety of hippocampal, electrophysiological and synaptic dysfunctions (Hanson et al., 2007; Kurt et al., 2000; Belichenko et al., 2004).
To better understand the molecular defects underlying the cognitive impairments of DS, in this study, we determined whether the expression of molecules known to play critical roles in long-term synaptic plasticity and long-term memory is dysregulated in either the developing brain and/or the hippocampus of adult Ts65Dn mice. The classes of proteins investigated include pre- and post-synaptic proteins, neurotrophins, transcription factors and membrane receptors, which were all previously shown to be critically implicated in long-term synaptic plasticity and long-term memory.
Specifically, we studied: 1) the synaptic proteins synaptophysin, synapsin, MAP2, PSD95, spinophilin, gephyrin and the cyclin-dependent kinase 5 (CDK5) and its cofactors p35 and p25, 2) the neurotrophins neurotrophin 3 (NT-3) and brain-derived neurotrophic factor (BDNF), 3) the transcription factors phosphorylated cAMP response element binding protein (pCREB) and CCAAT enhancer binding protein β (C/EBPβ) and 4) the membrane receptors muscle-specific tyrosine kinase receptor (MuSK) and subunits of the glutamate receptors NMDA and AMPA.
Experimental Procedures
Mouse brain and hippocampal samples
Ts65Dn mice and littermate (Lm) controls were offspring of Ts65Dn females (stock # 001924) and B6EiC3SnF1 males (stock # 001875), purchased from the Jackson Laboratory. Each breeding pair was housed together for the duration of breeding in 28.5 × 23.8 × 12.5 cm polycarbonate cages. The offspring were weaned at 21–28 days of age, and housed by gender and litter in groups of four mice. Tail samples from each offspring were genotyped by PCR for partial trisomy (Liu et al., 2003). The mice for the adult group were ear-marked at the time the tail samples were taken under brief isofluorane anesthesia. All cages were located in ventilated Thoren racks. The bottom of each cage was lined with Alpha-dri bedding (Shephard Specialty Papers), which was changed weekly. Food (Lab Diet Mouse Chow 20 #5008) and autoclaved water were always available. The mouse room was kept at 71 ± 3° F, and on a 12 hour lights on (at 6 am)/off cycle. The brains were extracted from the skull without perfusion under pentobarbital (50 mg/kg) anesthesia and kept on dry ice. Animal breeding, maintenance and brain extraction procedures were approved by the Animal Care and Use Committee of the Institute for Basic Research. Total brain extracts were obtained for 1-day-old mice and hippocampal extracts were obtained for 4-month-old mice.
Western blot analysis
Quantitative western blot analyses were carried out as previously described (Garcia-Osta et al., 2006; Taubenfeld et al., 2002). Briefly, tissues were homogenized in lysis buffer (0.2M NaCl, 5mM EDTA, 10% glycerol, 100mM HEPES, 2mM sodium phosphate) containing a protease inhibitor cocktail (Sigma, Saint Louis, Missouri, used as recommended by the manufacturer), 0.5 mM phenylmethylsulphonyl fluoride (PMSF), 2mM dithiothreitol (DTT), the phosphatase inhibitors contained in phosphatase inhibitor cocktail 1 (Sigma, used as recommended by the manufacturer), 2mM NaF, 1µM microcystin LR and 1 mM sodium orthovanadate (SOV). Protein concentration was determined using the BioRad protein assay (BioRad Laboratories, Hercules, California). Twenty µg/lane of total protein extract was resolved on 7.5%, 10%, or 15% polyacrylamide gels, according to each marker’s molecular weight, and then transferred to Immobilon-P membranes (Millipore, Billerica, Massachusetts). Membranes were incubated with primary antibodies in Tris-buffered saline overnight at 4°C. Primary antibodies: anti-synaptophysin (1:1,000), anti-MAP2 (1:1,000), anti-synapsin (1:1,000), anti-BDNF (1:500); anti-GluR2 (1:1,000); anti-PSD95 (1:50,000) and anti-pCREB (1:1,000) (Millipore, Billerica, Massachusetts); anti-MuSK (1: 300) (R&D Systems, Minneapolis, Minnesota); anti-GluR1 (1:1,000), anti-GluR3 (1:2,000), anti-NT-3 (1:1,000), anti-C/EBPβ (1:5,000), anti-CDK5 (1:1,500) and anti-p35 (1: 1,000) (Santa Cruz Biotechnology, Santa Cruz, California); anti-NR1 (1:500) (a generous gift from Dr. Wolfe, Luo et al., 1997); anti-spinophilin (1:1,000) (a generous gift from Drs. Greengard, Allen and Morrison, Allen et al., 1997); anti-gephyrin (1:250) (BD Biosciences, San Jose, California). After the primary antibody incubation, the membranes were washed and treated with a secondary HRP-labeled goat anti-rabbit (1:4,000), goat anti-mouse (1:4,000) (Amersham, Arlington Heights, Illinois), or donkey anti-goat (1: 5,000) (Santa Cruz Biotechnology), as required, for 1 hour at room temperature. Antibodies against actin (1:5,000) (Santa Cruz Biotechnology) or nuclear pore complex proteins (1:5,000) (Covance Innovative Antibodies, Princeton, NJ) were used to account for loading variation. Membranes were washed and incubated with enhanced chemiluminescence (ECL) detection reagents (Amersham) and exposed to HyBlot CL Autoradiography Film (Denville Scientific, Inc., Metuchen, NJ). Quantitative densitometric analysis was performed using NIH Image software. Statistical significance analysis was calculated using a student t-test.
Results
Quantitative western blot analysis was employed to determine the expression level of several proteins known to play an important role in long-term synaptic plasticity and memory in the brains of Ts65Dn and littermate (Lm) control mice. We investigated two distinct brain preparations at two different ages: neonatal brain and adult hippocampus. Thus, 1-day-old whole brain extracts (Lm: n = 11, Ts65Dn: n = 6) and 4-month old hippocampal extracts (Lm and Ts65Dn: n= 8 each) were used. Whole neonatal brain extracts were used to test potential developmental dysregulations, whereas adult hippocampal extracts were employed to investigate potential alterations critically involved in learning and memory.
MAP2 and NT-3 are significantly increased in the neonate brain of Ts65Dn mice
We investigated five classes of proteins. First, we determined the expression of synaptic proteins known to critically participate to synaptic plasticity and learning and memory (Gong et al. 2006; Tartaglia et al. 2001; Sarrouilhe et al. 2006; Li et a. 20004; Chhatwal et al. 2005). These included: i) synaptophysin, a pre-synaptic vesicle membrane protein known to mediate vesicle release (Valtorta et al., 2004), ii) synapsin, a pre-synaptic protein which binds synaptic vesicles to the cytoskeleton and regulates synaptic vesicle release (Hilfiker et al., 1999), iii) MAP2, a microtubule-associated protein highly concentrated in the neuronal soma and dendrites that participates in several morphological and regulatory changes (Dehmelt and Halpain, 2005), iv) PSD95, a scaffolding protein present in the post-synaptic density implicated in glutamatergic synaptic functions (Elias and Nicoll, 2007), v) spinophilin, a multifunctional scaffold protein that regulates both membrane and cytoskeletal functions and is involved in spine morphology and density regulation, synaptic plasticity and neuronal migration (Sarrouilhe et al., 2006) and vi) gephyrin, a scaffolding protein which is essential for synaptic clustering of inhibitory neurotransmitter receptors (Fritschy et al., 2003).
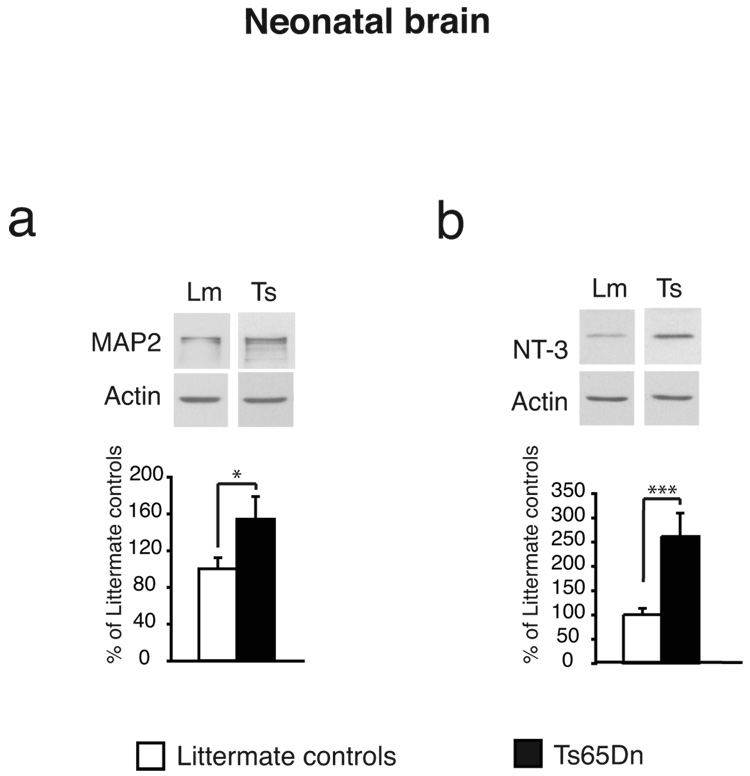
As shown in Table 1, in Ts65Dn neonate brains, the levels of synaptophysin, synapsin, PSD95, spinophilin and gephyrin were found to be similar to those of littermate controls. In contrast, as shown in both Table 1 and Fig. 1a, MAP2 levels were found to be significantly increased (p < 0.05) in the Ts65Dn brains compared to those of littermate controls.
Table 1.
Densitometric analyses of western blot of extracts from neonatal brain. Data are expressed as mean percentage±SEM off the littermate controls mean value
| Littermate controls | Ts65Dn | |
|---|---|---|
| Synaptic Proteins | ||
| Synaptophysin | 100.0 ± 9.3% | 98.9 ± 6.0% |
| Synapsin | 100.0 ± 8.2% | 111.1 ± 13.9% |
| MAP2 | 100.0 ± 12.1% | 153.9 ± 24.6% * |
| PSD95 | 100.0 ± 11.4% | 86.8 ± 10.7% |
| Spinophilin | 100.0 ± 8.9% | 90.5 ± 17.7 % |
| Gephyrin | 100.0 ± 13.3% | 83.3% ± 9.5% |
| Neurotrophins | ||
| NT3 | 100.0 ± 12.9 % | 260.9 ± 47.9 % * |
| BDNF | 100.0 ± 11.5 % | 75.4 ± 6.6 % |
| CDK5 and cofactors | ||
| CDK5 | 100.0 ± 5.3% | 108.9 ± 13.1% |
| p35 | 100.0 ± 13.0% | 87.3 ± 8.2 % |
| p25 | 100.0 ± 11.9 % | 120.5 ± 11.9 % |
| Transcription Factors | ||
| pCREB | 100.0 ± 12.1 % | 87.2 ± 7.2 % |
| c/EBPβ | 100.0 ± 11.0 % | 103.9 ± 14.7 % |
| Membrane Receptors | ||
| MuSK | 100.0 ± 11.8 % | 98.9 ± 11.8% |
| NR1 | 100.0 ± 9.1% | 82.1 ± 12.4% |
| GluR1 | 100.0 ± 9.9% | 105.4 ± 10.8% |
| GluR2 | 100.0 ± 8.2% | 99.6 ± 14.4% |
| GluR3 | 100.0 ± 14.2% | 83.3% ± 7.7% |
Significant differences
Fig. 1.
Representative examples and quantitative densitometric analyses of western blot of extracts from 1-day-old (neonatal brain) Ts65Dn whole brain and littermate age-matched controls. Protein levels were normalized using actin or nuclear pore complex protein to account for differences in loading. Data are expressed as mean percentage ±SEM of the littermate control mean values. (a) MAP2 is significantly increased in neonatal day 1 Ts65Dn. (b) NT-3 is significantly increased in neonatal day 1 Ts65Dn.
The second class of protein investigated included the neurotrophins neurotrophin-3 (NT-3) and brain derived neurotrophic factor (BDNF), two factors known to support the survival of existing neurons, the growth and differentiation of new neurons and synapses and found implicated in synaptic plasticity and memory (Chao, 2000; Shimazu et al. 2006; Yamada et al. 2002). As shown in Table 1 and Fig.1b, the levels of NT-3 resulted significantly increased in brain extracts of neonate Ts65Dn compared to their littermates (p < 0.001). On the other hand, BDNF levels did not any significant change (Table 1).
Third, the expression levels of synaptic kinase CDK5 and relative co-factors p35 and p25 were examined. CDK5 is a key regulator of neuronal function. It modulates cell adhesion and cytoskeletal dynamics, processes that are essential during development and in the adult nervous system. CDK5 is activated by p35 or p39, both of which may be cleaved to more stable and potent fragments, p25 and p29 (Cruz and Tsai, 2004). As shown in Table 1, in the neonate brains of Ts65Dn mice, the levels of CDK5, p35 and p25 were unchanged compared to those of littermate control brains.
In the fourth group of markers, we quantified the expression levels of two transcription factors known to play an evolutionarily critical role in long-term synaptic plasticity and memory formation: the phosphorylated form of CREB (Bailey et al., 1996) and C/EBPβ, a transcription factor whose expression is regulated downstream of CREB (Alberini, 1999; Alberini et al., 2005). As depicted in Table 1, we found that both pCREB and C/EBPβ were expressed at similar levels compared to their respective controls.
Finally, the fifth group of proteins that were quantified included the membrane receptor MuSK and the subunits of NMDA and AMPA receptors. MuSK has been found to play a critical role in neuromuscular junction formation (Burden, 2002) and was recently reported to be essential in the hippocampus during memory consolidation (Garcia-Osta et al., 2006). Subunits of both the NMDA and AMPA ionotropic glutamate receptors are known to be critically involved in synaptic plasticity and fast synaptic transmission, respectively (Riedel et al., 2003). As shown in Table 1, in the neonate brains of Ts65Dn, the levels of MuSK, NMDA receptor subunit NR1, GluR1 and GluR2 were all similar to those of their littermate controls. Only a trend towards down-regulation, although not statistically significant, was found for GluR3.
In the hippocampus of adult Ts65Dn, the expression of synaptophysin is significantly decreased, whereas the expressions of NT-3 and CDK5 are significantly increased
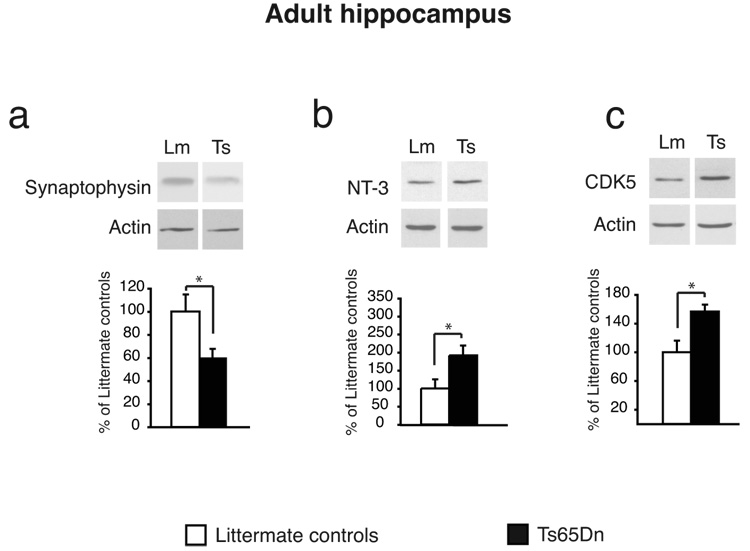
In the hippocampal extracts of adult Ts65Dn, the quantitative western blot analyses of the five classes of proteins revealed the following: the first class, that is, synaptic proteins, showed that whereas synapsin, MAP2, PSD95, spinophilin and gephyrin were all expressed at levels similar to those of littermate controls, the expression levels of synaptophysin were significantly decreased (p < 0.05, Fig. 2a and Table 2).
Fig. 2.
Representative examples and quantitative densitometric analysis of western blot of extracts from hippocampi of 4-month-old Ts65Dn (adult hippocampus) and those of littermate age-matched controls. Protein levels were normalized using actin to account for differences in loading. Data are expressed as mean percentage ±SEM of the littermate control mean values. (a) Synaptophysin is significantly decreased in Ts65Dn hippocampi. (b) NT-3 is significantly increased in Ts65Dn hippocampi. (c) CDK5 is significantly increased in the hippocampi of Ts65Dn.
Table 2.
Densitometric analyses of western blot of extracts from adult hippocampi. Data are expressed as mean percentage±SEM off the littermate controls mean value
| Littermate controls | Ts65Dn | |
|---|---|---|
| Synaptic Proteins | ||
| Synaptophysin | 100.0 ± 12.3% | 61.0 ± 7.1% * |
| Synapsin | 100.0 ± 5.4% | 99.0 ± 8.2 % |
| MAP2 | 100.0 ± 20.0% | 140.8 ± 13.9% |
| PSD95 | 100.0 ± 11.9% | 71.2 ± 12.7% |
| Spinophilin | 100.0 ± 17.3% | 112.8 ± 11.8% |
| Gephyrin | 100.0 ± 14.4% | 103.8 ± 9.8% |
| Neurotrophins | ||
| NT3 | 100.0 ± 24.9 % | 191.4 ± 27.6 % * |
| BDNF | 100.0 ± 7.5 % | 105.4 ± 6.0 % |
| CDK5 and cofactors | ||
| CDK5 | 100.0 ± 16.1% | 156.5 ± 9.7 % * |
| p35 | 100.0 ± 14.1% | 76.5 ± 30.3% |
| p25 | 100.0 ± 10.9% | 135.5 ± 30.4% |
| Transcription Factors | ||
| pCREB | 100.0 ± 14.0 % | 160.0 ± 34.6 % |
| c/EBPβ | 100.0 ± 8.8 % | 91.2 ± 10.1 % |
| Membrane Receptors | ||
| MuSK | 100.0 ± 9.3 % | 90.1 ± 6.0 % |
| NR1 | 100.0 ± 15.6% | 99.8 ± 8.7% |
| GluR1 | 100.0 ± 19.0% | 99.1 ± 13.1% |
| GluR2 | 100.0 ± 17.0% | 86.0 ± 17.6 |
| GluR3 | 100.0 ± 3.9% | 78.3 ± 14.3 |
Significant differences
The second class of proteins investigated revealed that, similarly to what was found in the neonatal brains, the expression of NT-3 was significantly increased in the hippocampal extracts of the adult Ts65Dn (p < 0.05, Table 2 and Fig. 2b). These results suggest that an increase in NT-3 might represent a general defect throughout the lifespan. On the other hand, no significant change was found in the expression levels of BDNF.
In the third class of proteins, we found a statistically significant up-regulation of CDK5 in adult Ts65Dn hippocampus compared to controls (Table 2 and Fig. 2c). In agreement with this observation, there was a trend towards decreased expression of p35 and increased expression of p25 (Table 2), although the differences were not statistically significant.
Moreover, with the forth class of proteins, like in the neonatal brain, the expression levels of the transcription factors pCREB and C/EBPβ were found expressed at levels comparable to those of littermate controls.
Finally, again in agreement with the results found in the neonatal brains, the investigation of the fifth class of proteins showed that MuSK, the NMDA receptor subunit NR1, GluR1 and GluR2 were found expressed at levels similar to those of their littermate controls, whereas a trend towards a down-regulation, although not statistically significant, was found for GluR3 (Table 2).
Discussion
Our study revealed that the expression levels of both MAP2 and NT-3 are significantly increased in the neonatal brain of Ts65Dn compared to littermate controls. Furthermore, we found that synaptophysin is significantly decreased whereas both NT-3 and CDK5 are significantly increased in the hippocampus of adult Ts65Dn compared to that of littermate controls. These results suggest that a dysregulation in the expression of synaptic proteins and neurotrophins in the brain at early developmental ages as well as in the adult hippocampus is associated with DS.
Our results are in agreement with, and biochemically extend, the observation that morphological and functional synaptic deficits are characteristic landmarks of DS. Indeed, a number of developmental synaptic abnormalities have been described in both brains of individuals with DS and mouse models of DS. By 18 months, DS subjects have dendritic spine deficits (Kaufmann and Moser, 2000), and mice models of DS show an increase in spine size throughout the brain and a decrease in spine density in the hippocampus (Belichenko et al., 2004). Our results provide biochemical support for these observations by showing that the expression of synaptic proteins that are critical for normal spine development, synapse formation and synaptic function, in both the developing brain and the adult hippocampus, is significantly dysregulated in Ts65Dn mice.
Specifically, we found that there was a significant increase in both MAP2 and NT-3 in the neonate brain of Ts65Dn. A significant increase in NT-3 was also observed in the hippocampus of adult Ts65Dn. The increase in MAP2 in the neonatal brain might reflect a compensatory overexpression that attempts to regulate the synaptic defects found at early post-natal developmental stages. Moreover, since MAP2 seems to play a critical role in neuromorphogenic processes, such as neurite initiation and recruitment of signaling proteins that may regulate microtubule mediated transport (Dehmelt and Halpain, 2005), an excess of MAP2 may underlie defective neurite/synaptic development.
A significant increase of NT-3, but not BDNF, found in both neonatal brains and hippocampi of adult Ts65Dn mice compared to littermates, suggests that this alteration is maintained from development through adulthood. Unaltered levels of hippocampal BDNF were also reported by previous studies on trisomic mice, which, however, also reported a significant decrease of BDNF expression in the frontal cortex (Bimonte-Nelson et al., 2003).
The expression of NT-3, like that of BDNF, is known to be widespread throughout the adult brain (Maisonpierre et al., 1990; Katoh-Semba et al., 1996) and both factors appear to play similar roles in brain development, survival and differentiation of neuronal progenitor cells. We speculate that the significant increase of NT-3 in the Ts65Dn mouse model represents a protective response to the synaptic and neuronal loss known to occur in DS throughout development (Wisniewski et al., 1984; Kaufmann and Moser, 2000; Becker et al., 1991). An additional intriguing hypothesis is that an increase in NT-3 might reflect an attempt to restore the cholinergic deficit described in DS (Cooper et al., 2001; Chang and Gold, 2007). In fact, previous data reported that the expression of NT-3 by cortical neurons serves to attract basal forebrain cholinergic projections to their target cells in the cerebral cortex (Robertson et al., 2006). Hence, a cholinergic deficit could indeed stimulate an increase in NT-3 expression.
Interestingly, another neurotrophin, the nerve growth factor (NGF) has been previously found to be augmented in the hippocampi of adult Ts65Dn mice. Despite the increase in concentration, the retrograde transport of this factor from the hippocampus to the basal forebrain was found to be significantly impaired (Cooper et al., 2001). In conclusion, several lines of evidence, including ours, show altered neurotrophin expression. Thus, it is tempting to speculate that, although the direction of the change (increase or decrease) may reflect different impairments (direct or compensatory) in different brain regions, the DS pathology is associated with, and perhaps in part caused by, a dysregulation in neurotrophin expression. This is also supported by the compelling evidence that neurotrophins like NGF are able to reverse pathological changes in DS mouse models (Cooper et al., 2001). Given the fact that neurotrophins may be functionally overlapping, it is also possible that, in DS, the increase of some neurotrophins in certain brain regions, such as the hippocampus, may represent a compensatory response to a decreased availability (expression or transport) of other neurotrophins in the same or other brain regions.
A significant decrease in synaptophysin and a significant increase of CDK5 were found in the hippocampi of adult Ts65Dn. The decrease in synaptophisin is in agreement with the findings that DS is characterized by a deficit in synapse number and morphology, particularly in the hippocampus (Kaufmann and Moser, 2000; Pinter et al., 2001a, b). Hence, the deficit in synaptophysin expression likely reflects a lower overall density of synapses.
A significant increase in CDK5 expression level in the hippocampus of adult Ts65Dn was accompanied by a trend toward a decrease and increase, respectively, of the CDK5 co-factors p35 and p25, which were, however, not significant. What could a CDK5 increase in the hippocampus of adult DS signify? CDK5 is a serine/threonine kinase with a multitude of functions, including neural development, dopaminergic function, neurodegeneration, learning and memory, adult synaptic plasticity and neurotransmitter release and endocytosis in the adult synapse (Ayala et al., 2007, Hawasli and Bibb, 2007, Cheng and Ip, 2003; Evans and Cousin, 2007; Graham et al., 2007, Fischer et al., 2003; Angelo et al., 2006). Thus, the significant CDK5 augmentation in the hippocampi of Ts65Dn may represent a compensatory mechanism in response to a developmental neuronal or synaptic alteration resulting from the trisomy. This would be in agreement with the abnormalities found in synaptophysin and MAP2.
Interestingly, CDK5 and its cofactors contribute to the pathogenesis of several neurodegenerative diseases such as Alzheimer’s disease (Patrick et al., 1999), amyotrophic lateral sclerosis (Nguyen et al., 2001) and Niemann–Pick type C disease (Bu et al., 2002) as well as psychiatric illnesses such as addiction (Cruz et al., 2003; Bibb, 2003). Thus, DS seems to represent an additional pathology that manifests a CDK5 dysregulation.
The expression levels of other synaptic markers such as synapsin, PSD95, spinophilin and gephyrin as well that of neurotransmitter receptors including the glutamate receptors AMPA (subunits GluR1, GluR2, GluR3) and NMDA (subunit NR1) were unchanged in both the neonate brains as well as hippocampi of adult Ts65Dn compared to their littermate controls. If, as indicated above, synapse density is dysregulated, why are all these proteins expressed at normal levels? One possibility is that, because our quantitative expression analysis has been carried out on total extracts, only relatively large changes have been detected, while leaving smaller (e.g. local) alterations undetecteble. It is also possible that the dysregulation of only certain synaptic proteins and not others occurs as a downstream effect of increased gene dose.
In conclusion, dysregulation in the expression of neurotrophins and proteins involved in synaptic development and plasticity functions at both early developmental stages and in adulthood seem to be a hallmark of the DS mouse model Ts65Dn, suggesting a potential role for these abnormalities in the neural pathology of DS in humans.
Acknowledgements
We thank Peter Needle (Mount Sinai) and Agnes Heaney (IBR) for technical assistance, Dillon Chen, Stephen Taubenfeld and Dhananjay Bambah-mukku for helpful suggestions on the manuscript. This work was supported by San Raffaele Pisana e San Raffaele Cassino; Tosinvest Sanità (to G.A.); the National Institute of Mental Health (R01 MH65635 to C.M.A.); the Hirschl Trust Foundation (to C.M.A.).
Abbreviations
- DS
Down Syndrome
- AD
Alzheimer’s disease
- MAP2
Microtubule-associated protein 2
- PSD95
Postsynaptic density 95
- CDK5
Cyclin-dependent kinase 5
- NT-3
Neurotrophin-3
- BDNF
Brain-derived neurotrophic factor
- pCREB
Phosphorylated cAMP response element binding protein
- C/EBPβ
CCAAT enhancer binding protein β
- MuSK
Muscle-specific tyrosine kinase receptor
- NMDA
N-methyl-D-aspartic acid
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4- propionic acid
- NGF
Nerve growth factor
- ACh
Acetylcholine
- PCR
Polymerase chain reaction
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HRP
Horseradish peroxidase
- ECL
Enhanced chemiluminescence
- MRI
Magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM. Genes to remember. J Exp Biol. 1999;202:2887–2891. doi: 10.1242/jeb.202.21.2887. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Taubenfeld SM, Garcia-Osta A. CREB and the CREB-C/EBP-dependent Gene Expression Cascade in Long-term memory. Cellscience. 2005 [Google Scholar]
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem. 2006;99:353–370. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- Ayala R, Shu T, Tsai LH. Trekking across the brain: The journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LL, Moran TH, Richtsmeier JT, Troncoso J, Reeves RH. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- Becker L, Mito T, Takashima S, Onodera K. Growth and development of the brain in Down syndrome. Prog Clin Biol Res. 1991;373:133–152. [PubMed] [Google Scholar]
- Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J Comp Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- Bibb JA. Role of Cdk5 in neuronal signaling, plasticity, & drug abuse. Neurosignals. 2003;12:191–199. doi: 10.1159/000074620. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J Neurosci. 2002;22:6515–6525. doi: 10.1523/JNEUROSCI.22-15-06515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden SJ. Building the vertebrate neuromuscular synapse. J Neurobiol. 2002;53:501–511. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Age-related changes in memory and in acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. Neurobiol Learn Mem. 2007;89:167–177. doi: 10.1016/j.nlm.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Trophic factors: An evolutionary cul-de-sac or door into higher neuronal function? J Neurosci Res. 2000;59:353–355. [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Ip NY. Cdk5: A new player at synapses. Neurosignals. 2003;12:180–190. doi: 10.1159/000074619. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, Kilbridge JF, Carlson EJ, Epstein CJ, Mobley WC. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci USA. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer's disease. Trends Mol Med. 2004;10:452–458. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ, Krueger BK, Yarowsky PJ. Impaired spatial working and reference memory in segmental trisomy (Ts65Dn) mice. Behav Brain Res. 1998;90:199–201. doi: 10.1016/s0166-4328(97)00116-2. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Fernandez-Teruel A, Vallina IF, Baamonde C, Lumbreras MA, Dierssen M, Tobena A, Florez J. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–146. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Cousin MA. Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5. J Neurosci. 2007;27:401–411. doi: 10.1523/JNEUROSCI.3809-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Spiess J, Radulovic J. Cdk5: a novel role in learning and memory. Neurosignals. 2003;12:200–208. doi: 10.1159/000074621. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Schweizer C, Brunig I, Luscher B. Pre- and post-synaptic mechanisms regulating the clustering of type A gamma-aminobutyric acid receptors (GABAA receptors) Biochem Soc Trans. 2003;31:889–892. doi: 10.1042/bst0310889. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A, Tsokas P, Pollonini G, Landau EM, Blitzer R, Alberini CM. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. J Neurosci. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Graham ME, Anggono V, Bache N, Larsen MR, Craft GE, Robinson PJ. The in vivo phosphorylation sites of rat brain dynamin I. J Biol Chem. 2007;282:14695–14707. doi: 10.1074/jbc.M609713200. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Blank M, Valenzuela RA, Garner CC, Madison DV. The functional nature of synaptic circuitry is altered in area CA3 of the hippocampus in a mouse model of Down's syndrome. J Physiol. 2007;579:53–67. doi: 10.1113/jphysiol.2006.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Bibb JA. Alternative roles for Cdk5 in learning and synaptic plasticity. Biotechnol J. 2007;2:941–948. doi: 10.1002/biot.200700093. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti AM, Megias M, Crespo D, Cruz-Orive LM, Dierssen M, Vallina IF, Insausti R, Florez J. Hippocampal volume and neuronal number in Ts65Dn mice: a murine model of Down syndrome. Neurosci Lett. 1998;253:175–178. doi: 10.1016/s0304-3940(98)00641-7. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Kaisho Y, Shintani A, Nagahama M, Kato K. Tissue distribution and immunocytochemical localization of neurotrophin-3 in the brain and peripheral tissues of rats. J Neurochem. 1996;66:330–337. doi: 10.1046/j.1471-4159.1996.66010330.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Krasuski JS, Alexander GE, Horwitz B, Rapoport SI, Schapiro MB. Relation of medial temporal lobe volumes to age and memory function in nondemented adults with Down's syndrome: implications for the prodromal phase of Alzheimer's disease. Am J Psychiatry. 2002;159:74–81. doi: 10.1176/appi.ajp.159.1.74. [DOI] [PubMed] [Google Scholar]
- Kurt MA, Davies DC, Kidd M, Dierssen M, Flórez J. Synaptic deficit in the temporal cortex of partial trisomy 16 (Ts65Dn) mice. Brain Res. 2000;858:191–197. doi: 10.1016/s0006-8993(00)01984-3. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LDP, Schmidt C, Billings T, Davisson M. Quantitative PCR genotyping assay for the Ts65Dn mouse model of Down syndrome. Biotechniques. 2003;35:1170–1180. doi: 10.2144/03356st02. [DOI] [PubMed] [Google Scholar]
- Lorenzi HA, Reeves RH. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 2006;1104:153–159. doi: 10.1016/j.brainres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B) Mol Pharmacol. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Breiter SN, Aylward EH, Warren AC, Grygorcewicz M, Frangou S, Barta PE, Pulsifer MB. MRI brain changes in subjects with Down syndrome with and without dementia. Dev Med Child Neurol. 1998;40:326–324. [PubMed] [Google Scholar]
- Perry EK, Perry RH, Smith CJ, Purohit D, Bonham J, Dick DJ, Candy JM, Edwardson JA, Fairbairn A. Cholinergic receptors in cognitive disorders. Can J Neurol Sci. 1986;13:521–527. doi: 10.1017/s0317167100037240. [DOI] [PubMed] [Google Scholar]
- Pinter JD, Brown WE, Eliez S, Schmitt JE, Capone GT, Reiss AL. Amygdala and hippocampal volumes in children with Down syndrome: a high-resolution MRI study. Neurology. 2001a;56:972–974. doi: 10.1212/wnl.56.7.972. [DOI] [PubMed] [Google Scholar]
- Pinter JD, Eliez S, Schmitt JE, Capone GT, Reiss AL. Neuroanatomy of Down's syndrome: a high-resolution MRI study. Am J Psychiatry. 2001b;158:1659–1665. doi: 10.1176/appi.ajp.158.10.1659. [DOI] [PubMed] [Google Scholar]
- Purpura DP. Normal and aberrant development of synaptic pathways in human hippocampus. Trans Am Neurol Assoc. 1973;98:224–226. [PubMed] [Google Scholar]
- Purpura DP. Normal and aberrant neuronal development in the cerebral cortex of human fetus and young infant. UCLA Forum Med Sci. 1975;18:141–169. doi: 10.1016/b978-0-12-139050-1.50014-8. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, Gunning FM, McQuain JD, Driesen NR, Acker JD. Selective neuroanatomic abnormalities in Down's syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology. 1995;45:356–366. doi: 10.1212/wnl.45.2.356. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Robertson RT, Baratta J, Yu J, Guthrie KM. A role for neurotrophin-3 in targeting developing cholinergic axon projections to cerebral cortex. Neuroscience. 2006;143:523–539. doi: 10.1016/j.neuroscience.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Kerdelhue B. Trisomy 21: from chromosomes to mental retardation. Behav Genet. 2006;36:346–354. doi: 10.1007/s10519-006-9052-0. [DOI] [PubMed] [Google Scholar]
- Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–1113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Seregaza Z, Roubertoux PL, Jamon M, Soumireu-Mourat B. Mouse models of cognitive disorders in trisomy 21: a review. Behav Genet. 2006;36:387–404. doi: 10.1007/s10519-006-9056-9. [DOI] [PubMed] [Google Scholar]
- Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester PE. The hippocampus in Down's syndrome. J Ment Defic Res. 1983;27:227–236. doi: 10.1111/j.1365-2788.1983.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem. 2001;276:37585–37593. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Stevens KA, Pollonini G, Ruggiero J, Alberini CM. Profound molecular changes following hippocampal slice preparation: loss of AMPA receptor subunits and uncoupled mRNA/protein expression. J Neurochem. 2002;81:1348–1360. doi: 10.1046/j.1471-4159.2002.00936.x. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- Vicari S. Motor development & neuropsychological patterns in persons with Down syndrome. Behav Genet. 2006;36:355–364. doi: 10.1007/s10519-006-9057-8. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Laure-Kamionowska M, Wisniewski HM. Evidence of arrest of neurogenesis and synaptogenesis in brains of patients with Down's syndrome. N Engl J Med. 1984;311:1187–1188. doi: 10.1056/NEJM198411013111818. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE. Down syndrome children often have brain with maturation delay, retardation of growth, and cortical dysgenesis. Am J Med Genet. 1990 Suppl 7:274–281. doi: 10.1002/ajmg.1320370755. [DOI] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]