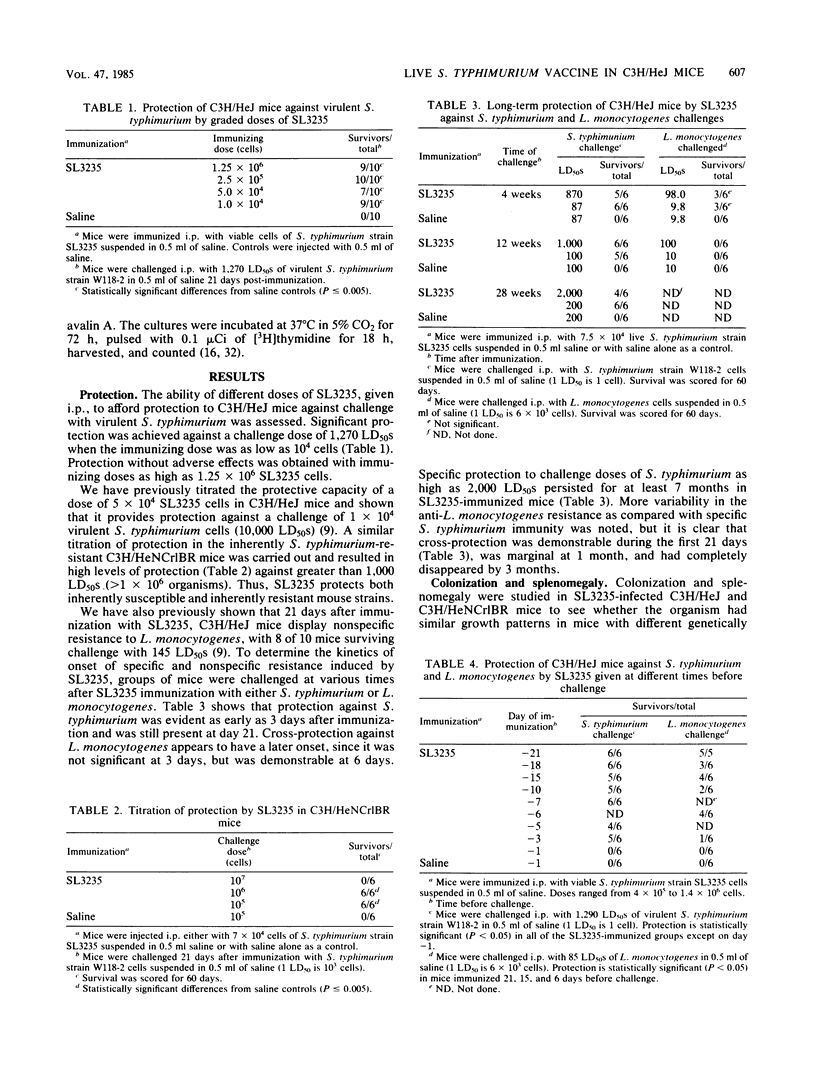
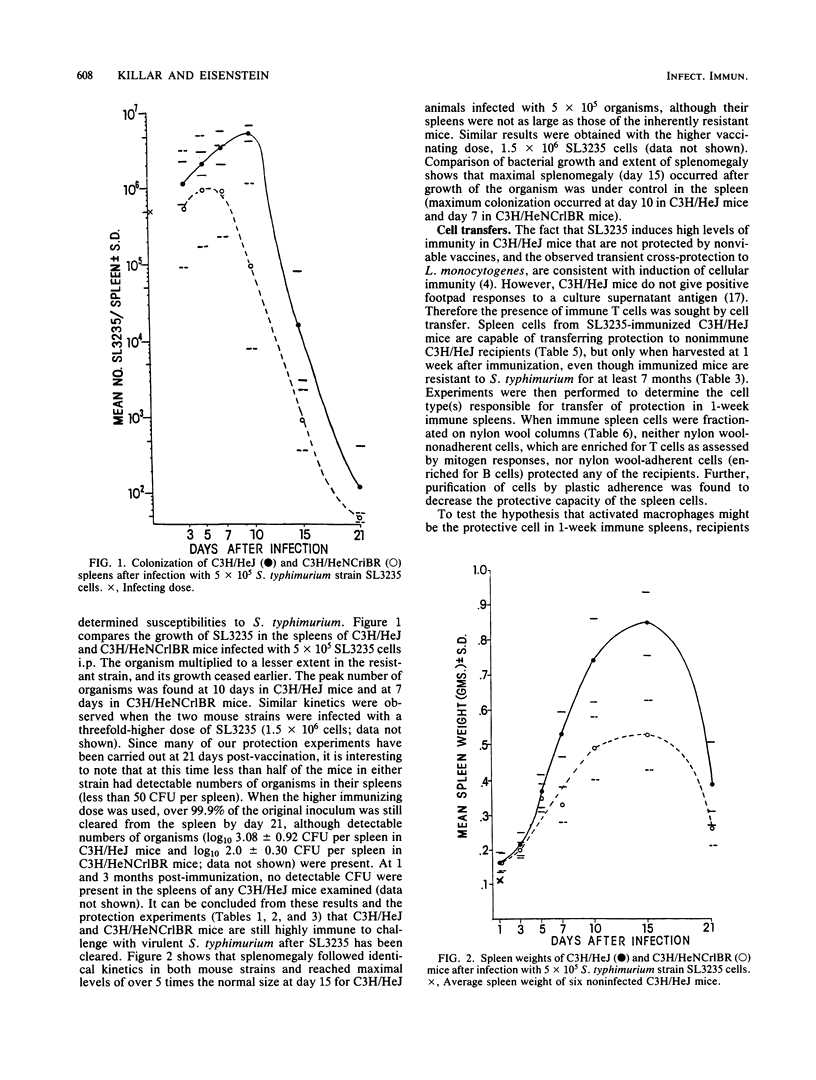
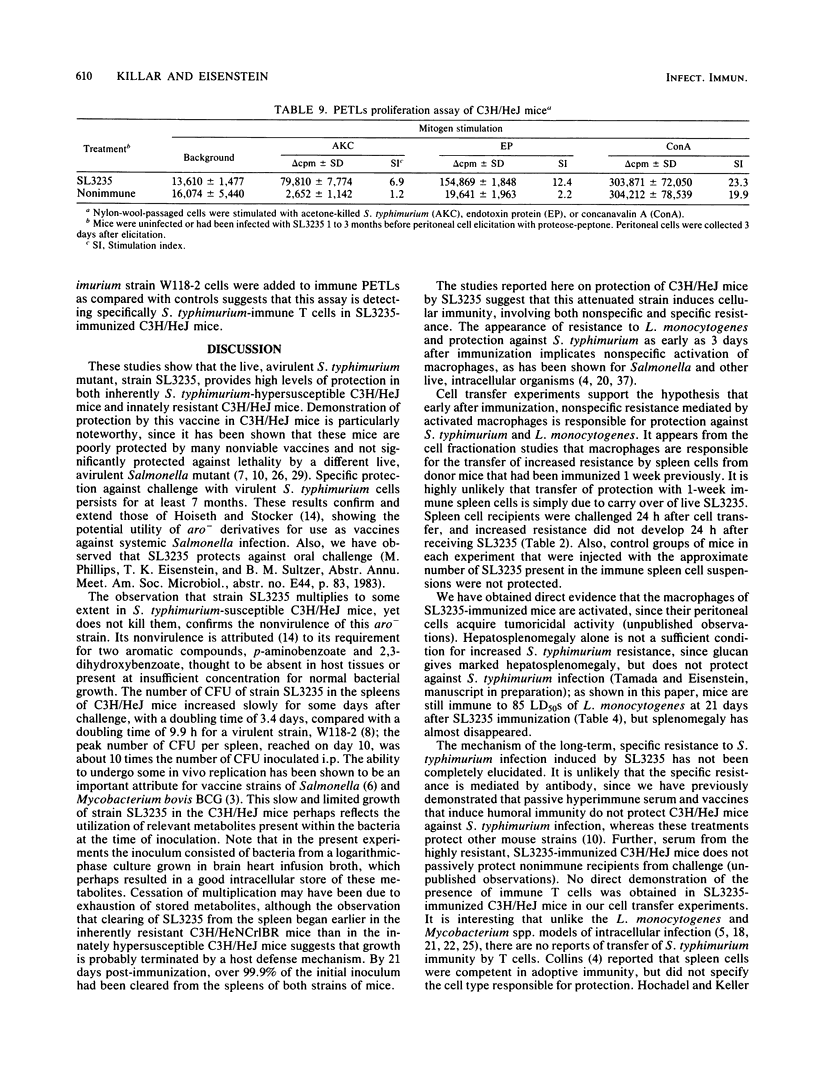
Abstract
Immunization with avirulent Salmonella typhimurium strain SL3235, a smooth, aroA- derivative, was shown to induce high levels of resistance to challenge with virulent S. typhimurium in innately hypersusceptible C3H/HeJ mice and inherently resistant C3H/HeNCrlBR mice. Strain SL3235 is one of a class of avirulent aroA- derivatives made from various strains and species of Salmonella that are being considered as vaccine candidates for cattle and humans. This paper supports their efficacy and potential utility in this regard. In C3H/HeJ mice, immunity against over 1,000 50% lethal doses of virulent S. typhimurium was evident as early as 3 days after immunization and persisted for at least 7 months. Further, the vaccine was effective over a broad spectrum of doses, ranging from 10(4) to 10(6) organisms. Infection with SL3235 led to marked splenomegaly in both mouse strains. The relationship of splenomegaly to the growth kinetics and colonization by SL3235 in the spleens of infected C3H/HeJ and C3H/HeNCrlBR mice was followed. SL3235 initially multiplied slowly in the spleens of both mouse strains and then was rapidly cleared. Less multiplication was seen in the resistant C3H/HeNCrlBR mice than in C3H/HeJ mice. Maximum splenomegaly occurred after clearance of the organism had begun. Protection against virulent S. typhimurium persisted after virtually all of the SL3235 vaccine strain had been cleared from the spleen. Cross-protection against Listeria monocytogenes was evident, but had a later onset, waned by 21 days, and was not detectable by 1 month after vaccination. Demonstration of this cross-protection is consistent with the interpretation that SL3235 induces cellular immunity. One-week immune spleen cells adoptively transferred anti-S. typhimurium and anti-L. monocytogenes immunity. T cell-enriched fractions were ineffective in adoptive transfer, as were spleen cells taken 2 weeks or later after immunization. Protective capacity was in the adherent cell fraction and seemed to be associated with macrophages. Evidence for induction of a population of sensitized T cells was obtained by using a peritoneal exudate T-lymphocyte proliferation assay on peritoneal T lymphocytes collected 1 to 3 months after SL3235 infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Ulczak O. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: demonstration and characterization of suppressor T cells in noncure mice. Infect Immun. 1984 Apr;44(1):97–102. doi: 10.1128/iai.44.1.97-102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Cellular mechanisms of anti-mycobacterial immunity. Adv Exp Med Biol. 1983;162:157–182. doi: 10.1007/978-1-4684-4481-0_16. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Eisenstein T. K., Deakins L. W., Killar L., Saluk P. H., Sultzer B. M. Dissociation of innate susceptibility to Salmonella infection and endotoxin responsiveness in C3HeB/FeJ mice and other strains in the C3H lineage. Infect Immun. 1982 May;36(2):696–703. doi: 10.1128/iai.36.2.696-703.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Stocker B. A., Sultzer B. M. Cellular immunity induced by avirulent Salmonella in LPS-defective C3H/HeJ mice. J Immunol. 1984 Aug;133(2):958–961. [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Sultzer B. M. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984 Sep;150(3):425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Graham L., Jr, Navalkar R. G. Evaluation of Mycobacterium leprae immunogenicity via adoptive transfer studies. Infect Immun. 1984 Jan;43(1):79–83. doi: 10.1128/iai.43.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochadel J. F., Keller K. F. Protective effects of passively transferred immune T- or B-lymphocytes in mice infected with Salmonella typhimurium. J Infect Dis. 1977 May;135(5):813–823. doi: 10.1093/infdis/135.5.813. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Regulatory interactions between macrophages and T-cell subsets in Listeria monocytogenes-specific T-cell activation. Infect Immun. 1982 Dec;38(3):907–913. doi: 10.1128/iai.38.3.907-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Differences in delayed-type hypersensitivity responses in various mouse strains in the C3H lineage infected with Salmonella typhimurium, strain SL3235. J Immunol. 1984 Sep;133(3):1190–1196. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Suppression of adoptive antituberculosis immunity by normal recipient animals. Infect Immun. 1983 Jul;41(1):257–263. doi: 10.1128/iai.41.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHISON N. A. Adoptive transfer of immune reactions by cells. J Cell Physiol Suppl. 1957 Dec;50(Suppl 1):247–264. doi: 10.1002/jcp.1030500416. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Crum E. D., Jungi T. W., Bell R. G. Transfer of immunity against Listeria monocytogenes by T cells purified by a positive selection technique. Infect Immun. 1978 Oct;22(1):209–218. doi: 10.1128/iai.22.1.209-218.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Wray C., Sojka W. J. The effect of T and B lymphocyte depletion on the protection of mice vaccinated with a Gal E mutant of Salmonella typhimurium. Br J Exp Pathol. 1976 Jun;57(3):354–360. [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Induction of suppressor T cells in delayed-type hypersensitivity to Mycobacterium bovis BCG in low-responder mice. Infect Immun. 1980 May;28(2):331–335. doi: 10.1128/iai.28.2.331-335.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S., Rosenstreich D. L. Defect in macrophage effector function confers Salmonella typhimurium susceptibility on C3H/HeJ mice. Cell Immunol. 1982 Mar 1;67(2):325–333. doi: 10.1016/0008-8749(82)90224-6. [DOI] [PubMed] [Google Scholar]
- Robertsson J. A., Lindberg A. A., Hoiseth S., Stocker B. A. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983 Aug;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S., Rosenstreich D. L. Macrophage activation for tumor cytotoxicity: control of macrophage tumoricidal capacity by the LPS gene. J Immunol. 1978 Aug;121(2):543–548. [PubMed] [Google Scholar]
- Scott M. T. Delayed hypersensitivity to Trypanosoma cruzi in mice: specific suppressor cells in chronic infection. Immunology. 1981 Oct;44(2):409–417. [PMC free article] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Smith R. A., Esa A., Stiff M. Transfer of Salmonella resistance and delayed hypersensitivity with murine-derived transfer factor. Infect Immun. 1982 Apr;36(1):271–276. doi: 10.1128/iai.36.1.271-276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. Cell-mediated immune response to Salmonella typhimurium infection in mice: development of nonspecific bactericidal activity against Listeria monocytogenes. Infect Immun. 1976 Apr;13(4):1069–1073. doi: 10.1128/iai.13.4.1069-1073.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


