Abstract
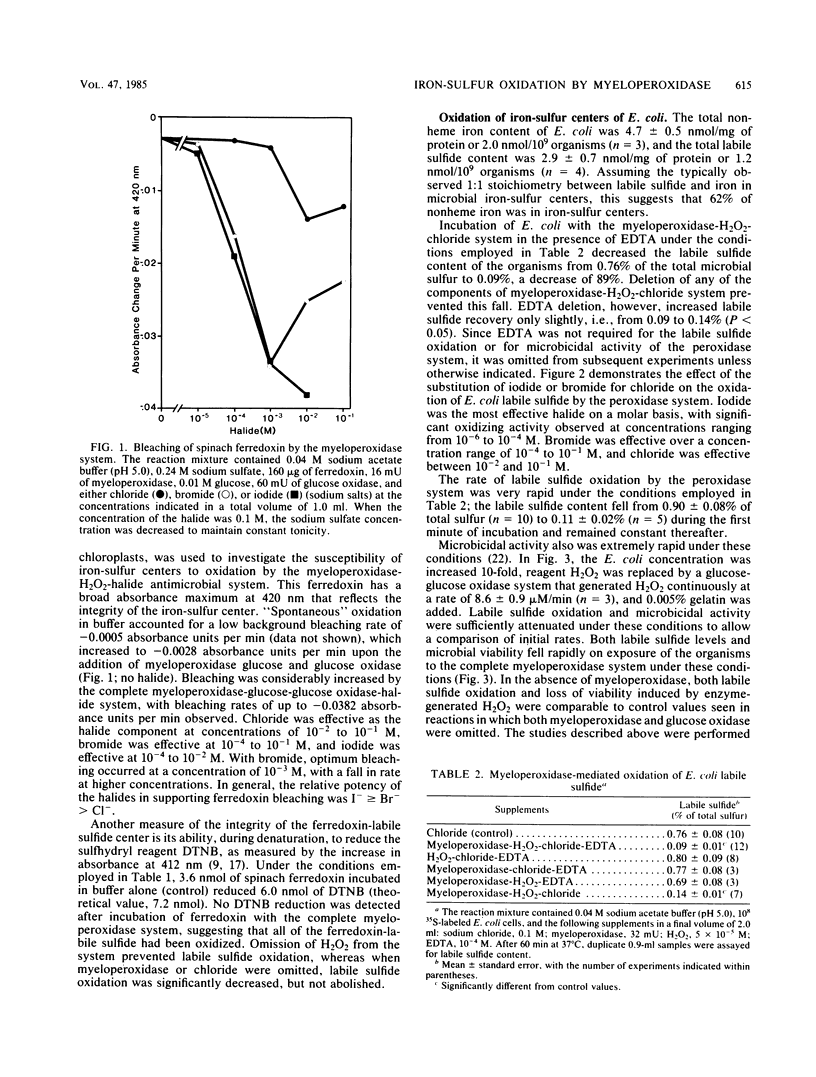
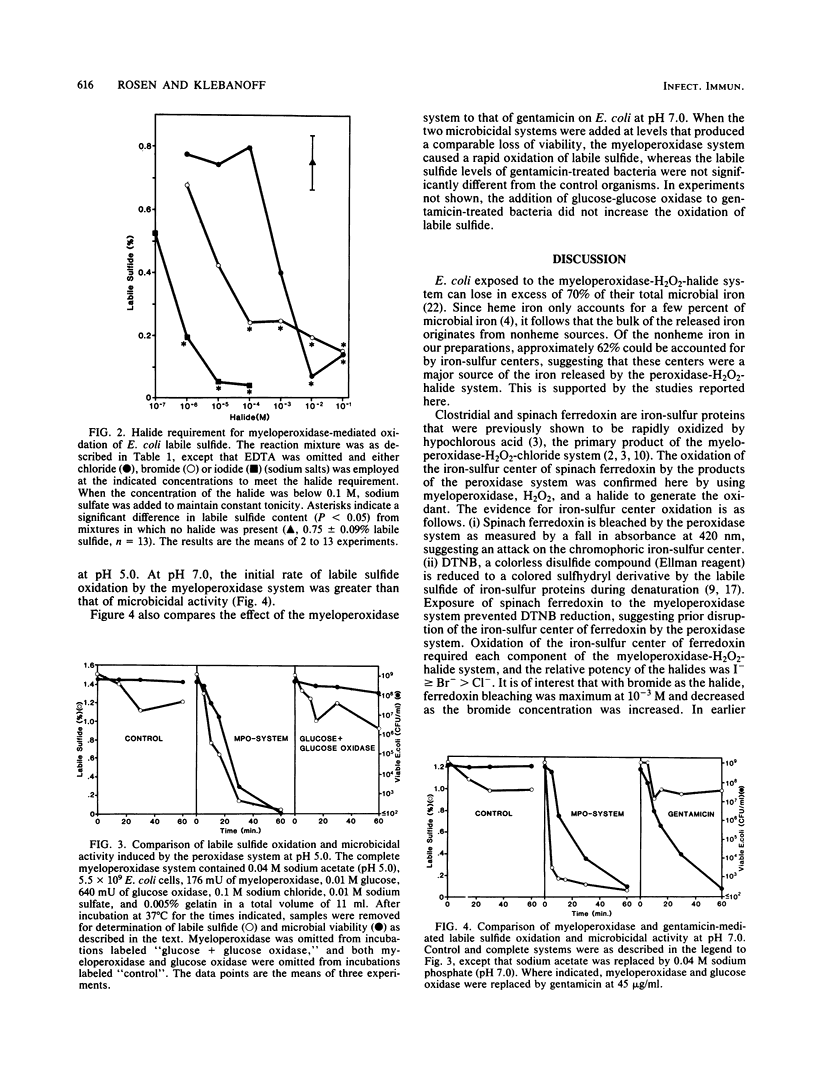
Myeloperoxidase, H2O2, and a halide (chloride, bromide, or iodide) form a potent microbicidal system that contributes to the antimicrobial activity of neutrophils. The mechanism of toxicity is not completely understood. Powerful oxidants are formed that presumably attack the microbe at a variety of sites. Among the consequences of this attack is the release of a large proportion of 59Fe of prelabeled organisms. We report here that the myeloperoxidase-H2O2-halide system oxidizes the iron-sulfur centers of model compounds (spinach ferredoxin) and intact microorganisms (Escherichia coli) with the loss of labile sulfide. The oxidation of the iron-sulfur centers of ferredoxin was measured by the fall in absorbance at 420 nm (bleaching) and by the loss of 5,5'-dithiobis-(2-nitrobenzoic acid) reducing activity. The latter compound is a sulfhydryl reagent that is reduced by ferredoxin labile sulfide during denaturation. The oxidation of E. coli iron-sulfur centers by the peroxidase system was determined by the loss of labile sulfide content, as measured by the release of H2S by acid and its reaction with zinc acetate to form ZnS. The halides were effective as components of the peroxidase system in the order I greater than Br greater than Cl. The oxidation of E. coli iron-sulfur centers by the peroxidase system was rapid and preceded the loss of viability. Gentamicin, at a concentration which produced a loss of viability comparable to that of the peroxidase system, did not cause a loss of labile sulfide from E. coli, suggesting that labile sulfide loss is not a nonspecific reflection of the loss of viability, but a direct consequence of the action of the myeloperoxidase system. The oxidation of iron-sulfur centers in microorganisms by the myeloperoxidase-H2O2-halide system may contribute to the death of the organism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A., Garland P. B. Non-haem iron and the dissociation of piericidin A sensitivity from site 1 energy conservation in mitochondria from Torulopsis utilis. Biochem J. 1971 Aug;124(1):135–151. doi: 10.1042/bj1240135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Hasumi H., Nakamura S., Koga K., Yoshizumi H. Effects of neutral salts on thermal stability of spinach ferredoxin. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1095–1101. doi: 10.1016/s0006-291x(79)80020-0. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXVII. The nature of nonheme iron in Mycobacterium phlei. J Biol Chem. 1967 Jun 25;242(12):2909–2916. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malkin R., Rabinowitz J. C. The reactivity of clostridial ferredoxin with iron chelating agents and 5,5'-dithiobis-2-nitrobenzoic acid. Biochemistry. 1967 Dec;6(12):3880–3891. doi: 10.1021/bi00864a034. [DOI] [PubMed] [Google Scholar]
- Petering D., Fee J. A., Palmer G. The oxygen sensitivity of spinach ferredoxin and other iron-sulfur proteins. The formation of protein-bound sulfur-zero. J Biol Chem. 1971 Feb 10;246(3):643–653. [PubMed] [Google Scholar]
- Rabinowitz J. C. Analysis of acid-labile sulfide and sulfhydryl groups. Methods Enzymol. 1978;53:275–277. doi: 10.1016/s0076-6879(78)53033-4. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Oxidation of Escherichia coli iron centers by the myeloperoxidase-mediated microbicidal system. J Biol Chem. 1982 Nov 25;257(22):13731–13735. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Role of iron and ethylenediaminetetraacetic acid in the bactericidal activity of a superoxide anion-generating system. Arch Biochem Biophys. 1981 May;208(2):512–519. doi: 10.1016/0003-9861(81)90539-7. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Oxidation of Escherichia coli sulfhydryl components by the peroxidase-hydrogen peroxide-iodide antimicrobial system. Antimicrob Agents Chemother. 1978 Jun;13(6):1006–1010. doi: 10.1128/aac.13.6.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


