Abstract
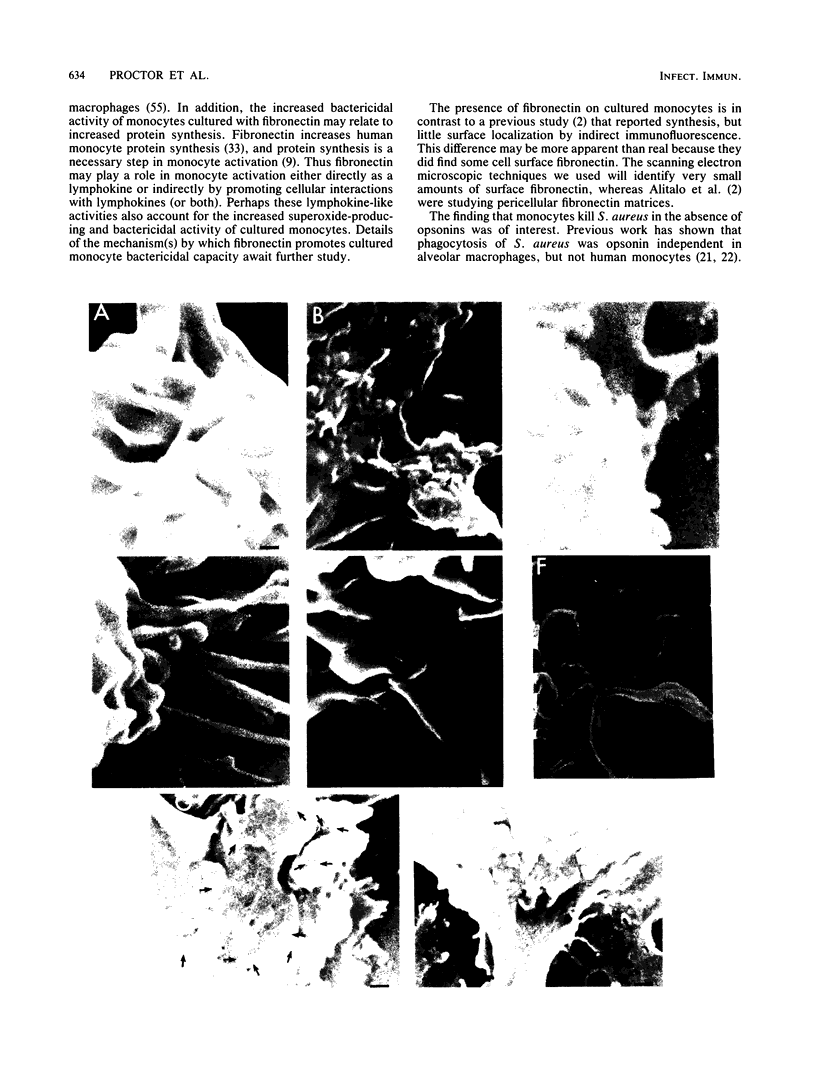
Fibronectin is a high-molecular-weight glycoprotein found as a soluble dimer in plasma and as an insoluble multimer in tissues. It has been proposed that plasma fibronectin facilitates phagocytic removal of lysed cells and damaged tissues. Fibronectin binds avidly to several species of gram-positive bacteria and enhances staphylococcal and streptococcal attachment to cultured cells. Determination of whether fibronectin will enhance the bactericidal activity of monocytes and macrophages has not been reported. The bactericidal activity of freshly isolated monocytes, cultured monocytes, or lymphokine-activated macrophages was tested in the presence of either dimeric or multimeric fibronectin. Freshly isolated monocytes and lymphokine-activated macrophages killed Staphylococcus aureus effectively in the absence of fibronectin or whole serum. In contrast, monocytes cultured for 7 to 10 days had diminished staphylocidal capacity. When the monocytes were cultured with either dimeric or multimeric fibronectin, however, bactericidal capacity was maintained. Thus, although fibronectin did not enhance the bactericidal activity of mononuclear phagocytes, both multimeric and dimeric fibronectin were effective at maintaining the bactericidal capacity.
Full text
PDF

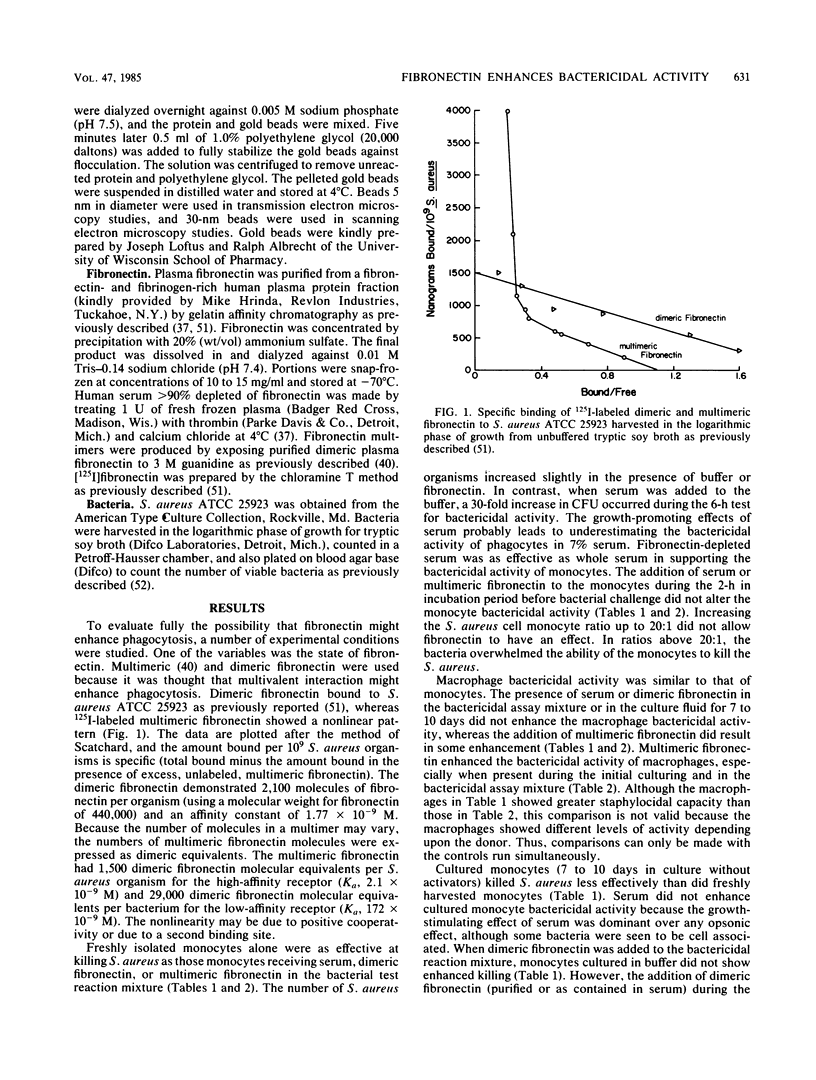
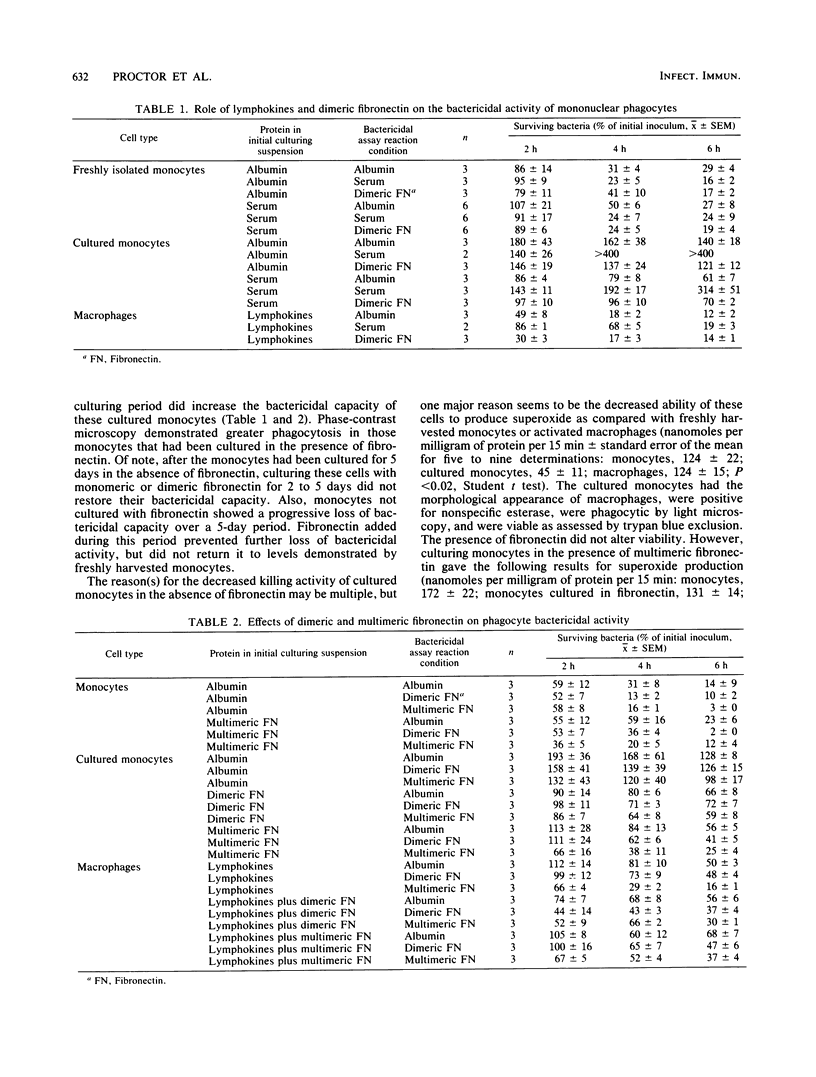
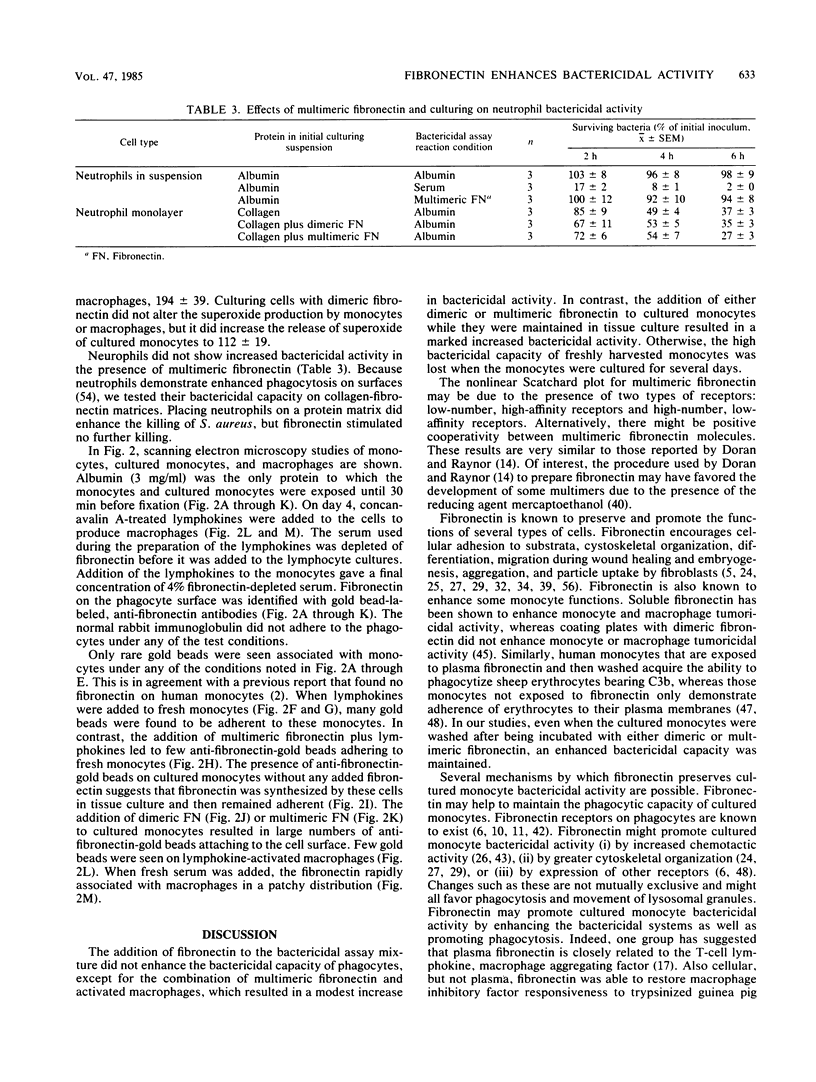



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton B. T., Hynes R. O. A difference between plasma and cellular fibronectins located with monoclonal antibodies. Cell. 1981 Jul;25(1):133–141. doi: 10.1016/0092-8674(81)90237-3. [DOI] [PubMed] [Google Scholar]
- Babu J. P., Simpson W. A., Courtney H. S., Beachey E. H. Interaction of human plasma fibronectin with cariogenic and non-cariogenic oral streptococci. Infect Immun. 1983 Jul;41(1):162–168. doi: 10.1128/iai.41.1.162-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley S. A., Tralka T. S. Fibronectin-mediated attachment of hematopoietic cells to stromal elements in continuous bone marrow culture. Exp Hematol. 1983 Feb;11(2):129–138. [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Check I. J., Wolfman H. C., Coley T. B., Hunter R. L. Agglutination assay for human opsonic factor using gelatin-coated latex particles. J Reticuloendothel Soc. 1979 Apr;25(4):351–362. [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Cosio F. G. Human fibronectin is an opsonin for IgG antibody-coated sheep erythrocytes. J Lab Clin Med. 1984 Apr;103(4):613–619. [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Augmentation of phagocytosis by a specific fibronectin fragment that links particulate activators to the fibronectin adherence receptor of human monocytes. J Immunol. 1982 Dec;129(6):2678–2681. [PubMed] [Google Scholar]
- Czop J. K., Kadish J. L., Austen K. F. Augmentation of human monocyte opsonin-independent phagocytosis by fragments of human plasma fibronectin. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3649–3653. doi: 10.1073/pnas.78.6.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Kadish J. L., Austen K. F. Purification and characterization of a protein with fibronectin determinants and phagocytosis-enhancing activity. J Immunol. 1982 Jul;129(1):163–167. [PubMed] [Google Scholar]
- Doran J. E., Raynor R. H. Fibronectin binding to protein A-containing staphylococci. Infect Immun. 1981 Sep;33(3):683–689. doi: 10.1128/iai.33.3.683-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. I., Krushell J. S., Blumenstock F. A., Kaplan J. E. Depression of phagocytosis by plasmin degradation products of plasma fibronectin. J Lab Clin Med. 1981 Aug;98(2):263–271. [PubMed] [Google Scholar]
- Fukuda M., Levery S. B., Hakomori S. Carbohydrate structure of hamster plasma fibronectin. Evidence for chemical diversity between cellular and plasma fibronectins. J Biol Chem. 1982 Jun 25;257(12):6856–6860. [PubMed] [Google Scholar]
- Godfrey H. P., Purohit A. Reversible binding of a guinea-pig lymphokine to gelatin and fibrinogen: possible relationship of macrophage agglutination factor and fibronectin. Immunology. 1982 Jul;46(3):515–526. [PMC free article] [PubMed] [Google Scholar]
- Gudewicz P. W., Molnar J., Lai M. Z., Beezhold D. W., Siefring G. E., Jr, Credo R. B., Lorand L. Fibronectin-mediated uptake of gelatin-coated latex particles by peritoneal macrophages. J Cell Biol. 1980 Nov;87(2 Pt 1):427–433. doi: 10.1083/jcb.87.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Differences in domain structures between plasma and cellular fibronectins. J Biol Chem. 1981 Nov 10;256(21):11292–11300. [PubMed] [Google Scholar]
- Hof D. G., Repine J. E., Peterson P. K., Hoidal J. R. Phagocytosis by human alveolar macrophages and neutrophils: qualitative differences in the opsonic requirements for uptake of Staphylococcus aureus and Streptococcus pneumoniae in vitro. Am Rev Respir Dis. 1980 Jan;121(1):65–71. doi: 10.1164/arrd.1980.121.1.65. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis. 1981 Jul;144(1):61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. Relationships between fibronectin (LETS protein) and actin. Cell. 1978 Nov;15(3):875–886. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarstrand C., Ahlgren T., Berghem L. Fibronectin increases the motility, phagocytosis and NBT (nitroblue tetrazolium)-reduction of granulocytes. J Clin Lab Immunol. 1982 May;8(1):59–63. [PubMed] [Google Scholar]
- Keski-Oja J., Sen A., Todaro G. J. Direct association of fibronectin and actin molecules in vitro. J Cell Biol. 1980 Jun;85(3):527–533. doi: 10.1083/jcb.85.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klainer A. S., Betsch C. J. Scanning electron microscopy of the attachment of human polymorphonuclear leukocytes to staphylococcus aureus. J Infect Dis. 1973 Jun;127(6):686–688. doi: 10.1093/infdis/127.6.686. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Wartiovaara J., Vaheri A. Cytochalasin B releases a major surface-associated glycoprotein, fibronectin, from cultured fibroblasts. Exp Cell Res. 1978 Jan;111(1):127–137. doi: 10.1016/0014-4827(78)90243-4. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Lanser M. E., Saba T. M. Fibronectin as a co-factor necessary for optimal granulocyte phagocytosis of Staphylococcus aureus. J Reticuloendothel Soc. 1981 Nov;30(5):415–424. [PubMed] [Google Scholar]
- Marquette D., Molnar J., Yamada K., Schlesinger D., Darby S., Van Alten P. Phagocytosis-promoting activity of avian plasma and fibroblastic cell surface fibronectins. Mol Cell Biochem. 1981 May 26;36(3):147–155. doi: 10.1007/BF02357031. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Majeau G. R., Unanue E. R., Cotran R. S. Stimulation of human monocyte/macrophage-derived growth factor (MDGF) production by plasma fibronectin. Am J Pathol. 1983 Jun;111(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- McAbee D. D., Grinnell F. Fibronectin-mediated binding and phagocytosis of polystyrene latex beads by baby hamster kidney cells. J Cell Biol. 1983 Nov;97(5 Pt 1):1515–1523. doi: 10.1083/jcb.97.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar J., Gelder F. B., Lai M. Z., Siefring G. E., Jr, Credo R. B., Lorand L. Purification of opsonically active human and rat cold-insoluble globulin (plasma fibronectin). Biochemistry. 1979 Sep 4;18(18):3909–3916. doi: 10.1021/bi00585a010. [DOI] [PubMed] [Google Scholar]
- Molnar J., McLain S., Allen C., Laga H., Gara A., Gelder F. The role of an alpha2-macroglobulin of rat serum in the phagocytosis of colloidal particles. Biochim Biophys Acta. 1977 Jul 22;493(1):37–54. doi: 10.1016/0005-2795(77)90258-6. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J Biol Chem. 1976 Mar 25;251(6):1639–1645. [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Mosher D. F., Furcht L. T. Fibronectin: review of its structure and possible functions. J Invest Dermatol. 1981 Aug;77(2):175–180. doi: 10.1111/1523-1747.ep12479791. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Johnson R. B. In vitro formation of disulfide-bonded fibronectin multimers. J Biol Chem. 1983 May 25;258(10):6595–6601. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Schad P. E. Cross-linking of fibronectin to collagen by blood coagulation Factor XIIIa. J Clin Invest. 1979 Sep;64(3):781–787. doi: 10.1172/JCI109524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Perri R. T., Kay N. E., McCarthy J., Vessella R. L., Jacob H. S., Furcht L. T. Fibronectin enhances in vitro monocyte-macrophage-mediated tumoricidal activity. Blood. 1982 Aug;60(2):430–435. [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., Inada S., Fries L. F., Takahashi T., Frank M. M., Brown E. J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983 Jun 1;157(6):1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., O'Shea J., Chused T., Yancey K., Frank M. M., Takahashi T., Brown E. J. Studies on the fibronectin receptors of human peripheral blood leukocytes. Morphologic and functional characterization. J Exp Med. 1984 Jan 1;159(1):137–151. doi: 10.1084/jem.159.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A. Endotoxin in vitro interactions with human neutrophils: depression of chemiluminescence, oxygen consumption, superoxide production, and killing. Infect Immun. 1979 Sep;25(3):912–921. doi: 10.1128/iai.25.3.912-921.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Hamill R. J., Mosher D. F., Textor J. A., Olbrantz P. J. Effects of subinhibitory concentrations of antibiotics on Staphylococcus aureus interactions with fibronectin. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):85–95. doi: 10.1093/jac/12.suppl_c.85. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Proctor R. A., Olbrantz P. J., Mosher D. F. Subinhibitory concentrations of antibiotics alter fibronectin binding to Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Nov;24(5):823–826. doi: 10.1128/aac.24.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Prendergast E., Mosher D. F. Fibronectin mediates attachment of Staphylococcus aureus to human neutrophils. Blood. 1982 Apr;59(4):681–687. [PubMed] [Google Scholar]
- Raynor R. H., Doran J. E., Reese A. C. A polymorphonuclear leukocyte monolayer system for studies of phagocytosis. J Reticuloendothel Soc. 1981 Jun;29(6):441–449. [PubMed] [Google Scholar]
- Remold H. G., Shaw J. E., David J. R. A macrophage surface component related to fibronectin is involved in the response to migration inhibitory factor. Cell Immunol. 1981 Feb;58(1):175–187. doi: 10.1016/0008-8749(81)90159-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Hasty D. L., Mason J. M., Beachey E. H. Fibronectin-mediated binding of group A streptococci to human polymorphonuclear leukocytes. Infect Immun. 1982 Aug;37(2):805–810. doi: 10.1128/iai.37.2.805-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Smith D. E., Nguyen B. Y., Hoidal J. R., Wilkinson B. J., Verhoef J., Furcht L. T. Human fibronectin binding to staphylococcal surface protein and its relative inefficiency in promoting phagocytosis by human polymorphonuclear leukocytes, monocytes, and alveolar macrophages. Infect Immun. 1981 Sep;33(3):811–819. doi: 10.1128/iai.33.3.811-819.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- WOOD W. B., Jr Studies on the cellular immunology of acute bacterial infections. Harvey Lect. 1951;Series 47:72–98. [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979 Feb;80(2):492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Water L., 3rd, Schroeder S., Crenshaw E. B., 3rd, Hynes R. O. Phagocytosis of gelatin-latex particles by a murine macrophage line is dependent on fibronectin and heparin. J Cell Biol. 1981 Jul;90(1):32–39. doi: 10.1083/jcb.90.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]