Abstract
Plasmodium falciparum malaria during pregnancy is an important cause of maternal and infant morbidity and mortality. Accumulation of large numbers of P. falciparum-infected erythrocytes in the maternal blood spaces of the placenta may be mediated by adhesion of infected erythrocytes to molecules presented on the syncytiotrophoblast surface. In this study, isolates from placentas and peripheral blood of infected pregnant women and from children were tested for binding to purified receptors and for agglutination with adult sera. Results suggest that adhesion to chondroitin sulfate A may be involved in placental parasite sequestration in most cases, but other factors are also likely to be important. Agglutination assay results suggest that parasites infecting pregnant women are antigenically distinct from those common in childhood disease. The prevalence of agglutinating antibodies to pregnancy isolates was generally low, but it was highest in multigravidae who are likely to have had the greatest exposure.
In regions where Plasmodium falciparum is endemic, the prevalence and severity of infection is greater in pregnant women than in nonpregnant adults [1, 2]. The impact of malaria during pregnancy depends on levels of preexisting malarial immunity, the intensity and stability of malaria transmission, and parity. Primigravid women typically experience higher rates of infection than multigravidae, and the consequences of infection are greater [1, 2]. In areas of high endemicity, malaria during pregnancy predisposes to maternal anemia and low birth weight of newborns, important risk factors for infant and maternal mortality [3-5]. Stillbirths, spontaneous abortions, and severe maternal disease can occur when malaria occurs in epidemics and in areas of low endemicity, but these events are uncommon in areas with high malaria transmission, perhaps because of significant malarial immunity acquired prior to pregnancy [1, 2].
A characteristic of infection with P. falciparum is sequestration, a process involving the accumulation of large numbers of parasitized erythrocytes in various organs. In the brain, this process has been associated with cerebral malaria [6]. Sequestration appears to be mediated by adhesive interactions between parasite ligands on the surface of infected erythrocytes (IEs) and host molecules present on microvascular endothelium [7]. Pregnant women infected with P. falciparum typically have large numbers of parasitized erythrocytes accumulated in the intervillous spaces of the placenta [8] and may show placental infection in the absence of peripheral parasitemia [9]. Placental parasites show a predominance of mature forms not seen in the periphery [8]. These features suggest that placental infection involves a cytoadherence-sequestration phenomenon.
P. falciparum IEs adhere to a range of host molecules in vitro, including chondroitin sulfate A (CSA) [10], intercellular adhesion molecule 1 (ICAM-1) [11], and CD36 [12, 13], a molecule to which most isolates from nonpregnant persons adhere [14, 15]. CSA is a glycosaminoglycan that appears to mediate adhesion through specific sulfation sequences within the polysaccharide molecule [16]. It has been identified on the surface of placental syncytiotrophoblasts [17-19] and cultured brain and lung endothelial cells [20], where it appears as chains attached to the proteoglycan thrombomodulin [21]. P. falciparum IEs from infected placentas of Kenyan women bind more to CSA than isolates from the peripheral blood of pregnant women or those from nonpregnant hosts [18]. ICAM-1 has also been identified on the syncytiotrophoblast surface where it could act as a receptor for parasite adhesion in placental sequestration [19, 22].
Clinical isolates of P. falciparum demonstrate enormous antigenic diversity [15, 23]. The principal variant antigen on the surface of IEs is the P. falciparum erythrocyte membrane protein 1 (PfEMP1) [24, 25], encoded by a large multigene family termed var [26-28]. PfEMP1 can mediate adhesion of IEs to CD36, ICAM-1 [29], and CSA [30]. Switching expression of different var genes results in different antigenic and adhesive phenotypes, a process that appears important for immune evasion and parasite survival. Variant-specific antibodies against P. falciparum correlate with protection against clinical disease [31], and adults in highly endemic areas typically possess a large repertoire of antibodies against different P. falciparum variants [15, 23].
In this study, the ability of P. falciparum isolates infecting pregnant women and children in Malawi to adhere to CSA, ICAM-1, and CD36 was examined in order to explore the possible roles of these host molecules in the sequestration of P. falciparum IEs. Variant-specific antigens on the surface of IEs of the same isolates were examined by agglutination assays, using sera from women of different parities and from adult men to investigate antigenic differences between childhood and pregnancy isolates and to evaluate the presence of antibodies to infecting isolates.
Methods
Study population
The study was conducted at the Queen Elizabeth Central Hospital, Blantyre, Malawi, from July 1997 to July 1998, in an area of year-round malaria transmission with seasonal variation [32]. All women attending the Antenatal Clinic for routine care and women presenting for delivery at the Labour Ward were screened for peripheral parasitemia in thick and thin blood films prepared from fingerprick blood samples stained by the Field method. Placentas and cord blood were collected immediately after delivery from women admitted to the Labour Ward and screened for parasitemia in a similar way. Women with high peripheral or placental parasitemias were recruited into the study. Blood samples were also collected from children with high parasitemias who either presented to outpatient clinics with uncomplicated malaria or were admitted with severe malaria. Children with both mild and severe malaria were recruited to the study to include a representative sample of isolates causing disease during childhood. In all cases in this report, the infecting species was P. falciparum. Among women attending the Antenatal Clinic, the prevalence of malaria averaged 42%; 47% in primigravidae and 38% in multigravidae [32]. The prevalence of malaria in women attending the Labour Ward averaged 34% for peripheral blood smears and 27% for placental blood smears with no difference in the parasite prevalence between primi- and multigravidae.
Parasite culture and preparation
P. falciparum was cultured in sealable flasks using RPMI-HEPES medium at pH 7.4 supplemented with hypoxanthine 50 μg/mL, NaHCO3 25 mM, gentamicin 2.5 μg/mL, and either Albumax II (Gibco BRL, Grand Island, NY) 0.5% wt/vol or 10% human AB serum vol/vol in an atmosphere of 1% O2, 4% CO2, 95% N2, as previously described [15, 33].
Laboratory P. falciparum lines FAF-EA8 [25] and CS2 [34] were used as controls for testing binding to CD36, ICAM-1, and CSA and to check conditions for parasite cultures. Patient blood samples for culturing were collected in tubes containing lithium heparin anticoagulant, centrifuged to remove plasma and buffy coat, and washed three times in PBS (pH 7.4) prior to culture.
For harvest of parasites from freshly delivered infected placentas, several biopsies of placental tissue (∼5-10 cm3) were cut from the maternal side of the placenta and put immediately into 50-mL tubes containing PBS with EDTA 50 mM and then placed on a tube roller for 60 min. Medium containing IEs and blood cells was separated from the placental tissue and centrifuged. The pellet was washed three times in PBS (pH 7.4) before use in assays or cultures. Placental parasites predominantly consisted of mature stages and could be tested for adherence or used in agglutination assays the same day without prior culture.
On some occasions, parasites harvested from culture or placentas were enriched for higher parasitemias by passage over Percoll (Pharmacia Biotechnology, Uppsala, Sweden) density gradients as described [35]. This also facilitated the separation of IEs from white blood cells and other cells, such as those from placental tissue.
Purified receptors
The following receptors were used in cytoadherence assays: CSA (from porcine rib cartilage; Sigma-Aldrich, Sydney) at a concentration of 100 μg/mL and/or CSA covalently linked to phosphatidylethanolamine, 50 μg/mL; CD36 (gift of M. Berndt, Baker Institute, Melbourne), 1 μg/mL; and soluble ICAM-1 from transfected Chinese hamster ovary cells (gift of A. Boyd, Walter and Eliza Hall Institute of Medical Research, Melbourne).
Cytoadherence assays
Assays were performed using P. falciparum trophozoite IEs at a parasitemia of ≥1% in 150-mm-diameter plastic petri dishes (Falcon 1058; Becton Dickinson, Lincoln Park, NJ) [16]. With an immunohistochemistry pen (Dako, Carpenteria, CA), multiple ∼10-mm diameter circles were drawn on the base of the petri dish. Purified receptors were then spotted (5-μL volume) into the center of each circle after being randomly allocated to different positions for each assay and allowed to adsorb overnight at 4°C in a humid box. Wells were blocked with 1% bovine serum albumin (BSA) in PBS for ≥30 min; the plate was subsequently washed with PBS before the assay was done.
Parasites, harvested from cultures or directly from placental tissue, were washed first in PBS (pH 7.4) and then resuspended in RPMI-HEPES medium containing 1% BSA (pH 6.9, 37°C) at a 5% hematocrit or a 2.5% hematocrit when using Percoll-enriched preparations. The parasite suspension (50 μL) was added to each receptor circle, and cytoadherence was allowed to occur for 30 min at 37°C. Unbound cells were washed off with PBS (pH 6.9, 37°C) using gentle agitation. Bound cells were fixed with 2% glutaraldehyde in PBS, stained with Giemsa, and counted microscopically. Numbers of bound cells were recorded as parasitized erythrocytes per square millimeter before the coating code was broken. This approach enabled the testing of adhesion to all receptors in triplicate in the same petri dish for each isolate used. BSA was used as a control to assess nonspecific binding. All results shown are averages of triplicate spots of binding above levels recorded for the BSA control.
Agglutination assays
Assays were done as previously described [15] using P. falciparum trophozoite IEs at a parasitemia of 1% or higher. Sera were collected from malaria smear-negative primigravid and multigravid women (in third and fourth pregnancies only) at midpregnancy who attended the Antenatal Clinic for routine care and were matched approximately for age and gestation. Sera from adult men were collected from parents of children with malaria who attended outpatient clinics. Sera were used in assays at a 1/10 dilution and were randomized and coded before testing. Results were read blinded. A four-point scoring system was used to grade results: 0, <3 agglutinates of 3-5 IEs; 1, ≥3 agglutinates of 3-5 IEs; 2, agglutinates of 6-20 IEs; 3, agglutinates of >20 IEs.
Results
Cytoadherence to purified receptors
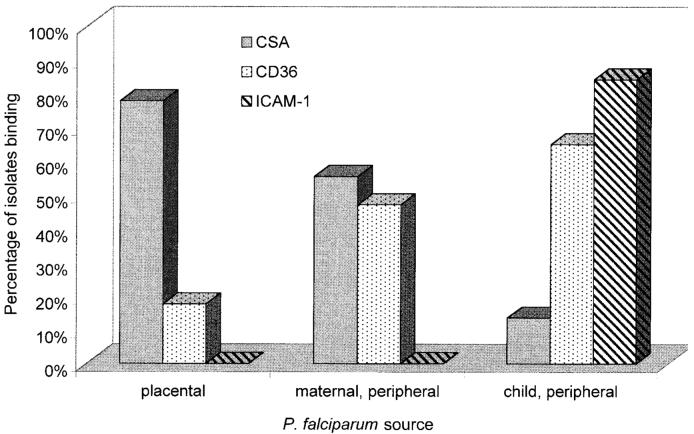
Adhesion of isolates to CSA was most common among the placental group in which 14 (77.8%) of 18 isolates tested bound at significant levels, whereas 10 (55.6%) of 18 maternal peripheral isolates and only 3 (13.6%) of 22 peripheral child isolates bound at ≥5 IE/mm2 under equivalent conditions (P < .001 for differences, χ2 test; table 1). Adhesion to CD36 was common among child isolates (13 [65%] of 20 tested), less common among peripheral maternal isolates (8 [47.1%] of 17), and uncommon among placental isolates (3 [17.6%] of 17; P < .02 for differences, χ2 test). Two maternal peripheral isolates bound to CD36 alone at significant but low levels, whereas all other pregnancy isolates that bound to CD36 also bound to CSA. A striking finding was the absence of significant adhesion to ICAM-1 by any of the isolates from pregnant women. In contrast, most (16 [84.2%] of 19) child isolates tested during the same period using equivalent conditions bound to ICAM-1. Figure 1 shows the proportion of isolates in each group that bound to a given receptor (at ≥5 IE/mm2).
Table 1.
Adhesion to chondroitin sulfate A (CSA), CD36, and intercellular adhesion molecule 1 (ICAM-1) of Plasmodium falciparum isolates from infected placentas and from peripheral blood of pregnant women and of children
| Isolate | Parasitemia (%) |
Adherence receptor |
||
|---|---|---|---|---|
| CSA | CD36 | ICAM-1 | ||
| Placenta | ||||
| P004P | 1.5 | 0 | 0 | 0 |
| P041P | 10 | 0 | 1 | NT |
| P042P | 30 | 0 | 0 | NT |
| P050P | 18 | 13 | 0 | NT |
| P081P | 15 | 933 | 0 | 0 |
| P087P | 40 | 59 | 0 | 0 |
| P048Pa | 93 | 386 | NA | NT |
| P054Pa | 90 | 56 | 0 | 0 |
| P055Pa | 8 | 31 | 25 | 0 |
| P058Pa | 57 | 17 | 18 | 0 |
| P059Pa | 64 | 0 | 1 | 0 |
| P063Pa | 97 | 13 | 0 | 0 |
| P069Pa | 94 | 64 | 1 | 0 |
| P072Pa | 65 | 140 | 0 | 0 |
| P078Pa | 96 | 1100 | 87 | 0 |
| P082Pa | 90 | 19 | 4 | 1 |
| P090Pa | 60 | 40 | 1 | 1 |
| P095Pa | 96 | 6 | 2 | 0 |
| Mean ± SD | 56.9 ± 35.6 | 160 ± 326 | 8.2 ± 21.6 | 0.2 ± 0.4 |
| Maternal peripheral blood | ||||
| AN002 | 1 | 139 | 0 | 0 |
| AN062 | 2 | 23 | 0 | NT |
| AN067 | 3 | 9 | 34 | 3 |
| AN069 | 2 | 0 | 0 | 0 |
| AN070 | 3.5 | 0 | 0 | 0 |
| AN089 | 1.5 | 127 | 27 | 0 |
| P004M | 2 | 0 | 0 | 0 |
| P048M | 2 | 2 | NA | 0 |
| P055M | 4 | 0 | 0 | NT |
| P085M | 2 | 0 | 6 | 0 |
| P069M | 1 | 42 | 0 | NT |
| P042Ma | 5 | 0 | 0 | 0 |
| P095Ma | 63 | 2305 | 10 | 4 |
| AN090a | 15 | 567 | 6 | 0 |
| AN091a | 40 | 384 | 5 | 0 |
| AN098a | 77 | 1 | 24 | 4 |
| AN099a | 82 | 2440 | 536 | 0 |
| AN109a | 2 | 222 | 1 | 2 |
| Mean ± SD | 17.1 ± 28.0 | 347 ± 753 | 38.2 ± 129 | 0.9 ± 1.5 |
| Children’s peripheral blood | ||||
| C053 | 5.5 | 0 | NA | 178 |
| C094 | 4.5 | 7 | 1126 | 167 |
| C070 | 5.5 | 1 | 1 | NT |
| C069 | 14 | 0 | 51 | NT |
| C091 | 7.5 | 0 | 48 | 1 |
| C092 | 40 | 2 | 641 | 33 |
| MP1127 | 1.5 | 3 | 145 | 4 |
| MP1074 | 6.5 | 0 | 0 | 0 |
| MP1000 | 5 | 2 | 180 | 81 |
| MP1049 | 5.5 | 1 | 1 | 7 |
| MP1035 | 5 | 0 | 2 | 34 |
| MP 1073 | 4 | 2 | 0 | NT |
| MP1046 | 5.5 | 0 | 12 | 6 |
| MP1082FU | 8 | 3 | 3 | 14 |
| MP1067FU | 18 | 46 | 333 | 68 |
| MP1146 | 46 | 0 | 202 | 252 |
| MP1124a | 77 | 0 | 0 | 89 |
| MP1140a | 80 | 0 | NA | 206 |
| MP1119a | 95 | 0 | 6503 | 58 |
| MP1134a | 83 | 0 | 3917 | 1441 |
| C096a | 97 | 0 | 150 | 682 |
| C095a | 86 | 37 | 1799 | 1969 |
| Mean ± SD | 31.8 ± 36.1 | 4.7 ± 12.1 | 756 ± 1644 | 278 ± 534 |
NOTE. Data are no. of bound infected erythrocytes/mm2 above that recorded for bovine serum albumin controls (mean of triplicate spots; SD excluded for clarity). Results not adjusted relative to parasitemia. NA, not assessable; NT, not tested.
Parasitemia was enriched by passage over Percoll gradients.
Figure 1.
Proportion (%) of P. falciparum isolates with significant binding (≥5 infected erythrocytes/mm2) obtained from placentas or from peripheral blood of pregnant women or from children. CSA, chondroitin sulfate A; ICAM-1, intercellular adhesion molecule 1.
The level of binding to CD36 of isolates from pregnant women was lower than to CSA. For placental isolates, mean binding (±SD) was 159.8 ± 325.7 to CSA and 8.2 ± 21.6 to CD36 (P < .01 for difference, Mann-Whitney U test). Among peripheral maternal isolates, mean binding was 347.4 ± 753.3 to CSA and 38.2 ± 128.7 to CD36 (difference of borderline significance for α = .05, Mann-Whitney U test). Adhesion of child isolates to CD36 (mean ± SD = 755.7 ± 1644.1) and ICAM-1 (278.4 ± 534.2) was significantly higher than to CSA (4.7 ± 12.1; P < .01, Mann-Whitney U test).
A direct comparison of adherence phenotypes of matched peripheral and placental isolates was possible in a number of cases (P004, P042, P048, P055, P069, P095; table 1). Clear differences in binding phenotype can be seen in 2 cases, for example, with P055 which demonstrated adhesion to CSA and CD36 in the placental sample only, and P095 which showed high levels of binding to CSA in the peripheral but not the placental sample.
Little or no binding to the receptors tested was observed with some pregnance isolates. Of the peripheral isolates, parasite cultures appeared healthy and grew normally prior to testing. Of the placental isolates, all appeared viable when harvested, there was no history of antimalarial drug use by donors in the week prior to collection of the sample, and P041P, P042P, and P059P demonstrated subsequent reinvasion of uninfected erythrocytes at low parasitemia when cultured (P004P was not commenced in culture). In addition, P004M, P004P, and P042P recorded positive agglutinations with some sera tested, suggesting that although binding to receptors could not be demonstrated in our adherence assays, variant antigens associated with cell adhesion were present on the surface of parasitized erythrocytes.
The average ages (±SD) of donors from which placental and peripheral maternal isolates were obtained were 21.1 ± 2.7 and 22.3 ± 4.2 years, respectively. The average gravidity (±SD) of placental isolate donors was 1.4 ± 0.8 (14 primi-, 1 secundi-, and 3 multigravidae) and 1.5 ± 0.7 for peripheral maternal isolate donors (11 primi-, 5 secundi-, and 2 multigravidae). There was no significant association between donor age or gravidity and isolate binding. Among peripheral maternal isolates, a greater proportion collected from midpregnancy (mean gestation ± SD = 20.4 ± 6.0 weeks) bound at significant levels to CSA than those collected at term (35.5 ± 3.2 weeks): 8 of 10 versus 2 of 8, respectively (P = .02, χ2 test). The binding density of these isolates (mean ± SD = 391.2 ± 743.7) was also higher than of those collected at term (293.8 ± 812.8; P < .05, Mann-Whitney U test). The proportion of midpregnancy peripheral isolates binding to CSA (80%) was very similar to that of placental isolates (78%).
Evaluation and comparison of methods
Low or absent binding of some isolates prompted an evaluation of methods used and of the effects of alternative methods and procedures on adherence results. In this study, P. falciparum IEs were gently extracted from pieces of placental tissue by washing in PBS-EDTA. Fine mincing of placental tissue before placement in PBS-EDTA for extraction of parasitized erythrocytes followed by Percoll enrichment (similar to a previously described method [18]) caused little change in adherence profiles when samples were directly compared by the two methods (data not shown). No adhesion to ICAM-1 was seen. Comparison of subgroups of Percoll-treated and untreated isolates in each category of isolates (table 1) suggests that this procedure generally increases the number of cells adhering to specific receptors, presumably due to higher parasitemias. However, for 2 of 4 isolates tested before and after Percoll enrichment, binding was not detected despite a >10-fold increase in parasitemia.
Most placental isolates were commenced in culture and grew initially at low parasitemia. In only 4 of 15 cases did parasitemia reach ≥1% and allow retesting of adhesion. After varying times in culture (2-28 days), there was no clear trend, but significant shifts in adherence phenotypes were observed (data not shown). In some cases, isolates that demonstrated no binding when first tested did bind to CD36 and/or CSA after culture, whereas others bound initially when first harvested but subsequently showed no binding after culture. None of the cultured placental isolates demonstrated adhesion to ICAM-1.
Agglutinating antibodies to infecting isolates
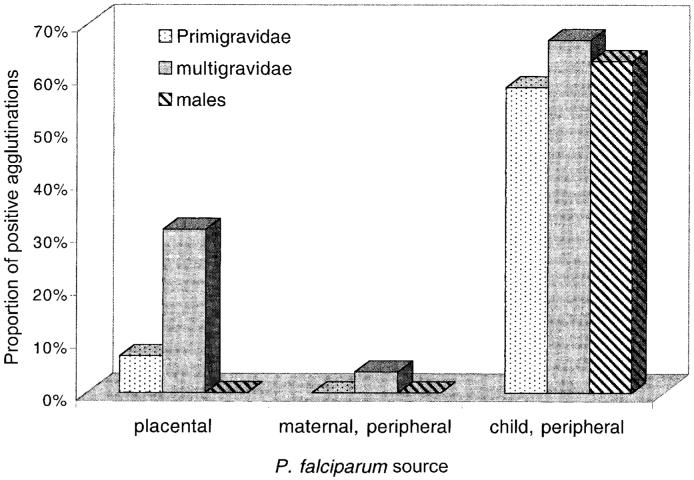
Agglutination assays were performed on placental, peripheral maternal, and peripheral child isolates from the same groups as those tested for adhesion by using small sets of sera from uninfected pregnant women (primigravidae and multigravidae) collected at midpregnancy (mean weeks of gestation ± SD, 21.6 ± 3.8 for primigravidae and 22.8 ± 2.3 for multigravidae) and from adult men. All multigravid sera were from women in their third or fourth pregnancies (mean gravidity ± SD = 3.2 ± 0.5). The mean ages ± SD for each group were 20.7 ± 2.0, 24.0 ± 2.4, and 25.2 ± 1.2 years for primigravidae, multigravidae, and adult men, respectively. Three different sets of 4 primigravid sera and 4 multigravid sera were tested separately against 2-3 different placental isolates (total = 8 different isolates), 2-4 different peripheral maternal isolates (total = 7), and 1-3 different peripheral child isolates (total = 6). Two or 3 male sera (6 different sera in total) were also tested in some assays. It was not possible to test all isolates against all sera. Results from all assays were pooled to allow statistical evaluation and are summarized in figure 2.
Figure 2.
Proportion (%) of positive agglutination assays from sera of primigravid and multigravid women and adult men against P. falciparum isolates obtained from infected placentas or from peripheral blood of pregnant women or from children.
As a group, isolates from infected children were commonly agglutinated by adult sera (26 positive assays [62%] of 42 performed), whereas agglutinating antibodies against pregnancy isolates, particularly peripheral isolates, were rare. The agglutination scores were also higher for child isolates (mean of positives = 2.03) than for pregnancy isolates (mean of positives = 1.07; P < .01, Mann-Whitney U test).
The results for placental isolates (table 2) indicate that agglutination by different sera was isolate-specific and no pan-agglutinating sera were observed. Of assays using multigravid sera, 31% (9 of 29) were positive, significantly higher (P < .05, Mantel-Haenszel equation) than with primigravid sera (2 [7%] of 29) or male sera (0 of 8). Among assays using peripheral maternal isolates, only 1 positive agglutination was recorded (multigravid serum) from the 56 performed. The differences in the proportion of positive agglutinations recorded for each group of adult sera when testing child isolates were not significant. No positive agglutinations were recorded with some pregnancy isolates, and 5 of 8 of these isolates showed significant binding to CSA and/or CD36, suggesting that variant antigens were present on the surface of IEs in these cases but not recognized by the sera.
Table 2.
Agglutination of placental isolates by sera from primigravidae and multigravidae
| Sera | Placentalisolates |
Sera | Placental isolates |
Sera | Placenta isolates |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| P004P | P041P | P048P | P050P | P055P | P058P | P063P | P069P | |||
| PG1 | − | − | PG5 | − | − | − | PG9 | − | − | − |
| PG2 | + | − | PG6 | − | − | − | PG10 | − | − | − |
| PG3 | − | PG7 | − | − | + | PG11 | − | − | − | |
| PG4 | − | PG8 | − | − | PG12 | − | − | − | ||
|
| ||||||||||
| MG1 | − | + | MG5 | + | − | − | MG9 | − | − | − |
| MG2 | − | + | MG6 | + | − | + | MG10 | − | + | − |
| MG3 | − | MG7 | − | − | + | MG11 | + | − | ||
| MG4 | − | MG8 | − | − | − | MG12 | + | − | − | |
NOTE. PG1−12, sera from different primigravidae; MG1−12, sera from different multigravidae. −, negative agglutination assay; +, positive agglutination assay. P < .05 for difference between no. of positive agglutination reactions using primigravid vs. multigravid sera (Mantel-Haenszel equation).
Discussion
We found that most placental P. falciparum isolates adhere to CSA in vitro. The proportion of isolates binding to CSA was higher in this group than among isolates from the peripheral blood of the same group of women or isolates from children. However, a significant number of placental isolates in this study did not bind to any of the receptors tested or bound at very low levels. Fried and Duffy [18] reported that all 14 placental isolates tested from Kenyan women bound at significant levels to CSA, leading them to conclude that adhesion to CSA may be a universal feature of parasites sequestered in the placenta. In the present study, a higher proportion of peripheral maternal isolates bound to CSA, indicating that this phenotype may not be exclusively localized to the placenta. We could not identify any methodologic explanations for the differences of our findings; in each case, parasites appeared viable and in 2 cases where no binding was seen, positive agglutinations were recorded, suggesting that parasite molecules associated with adhesion were present on the erythrocyte surface.
A number of factors may have influenced adhesion results, including inhibition by antibodies or leukocytes, which were often seen bound to parasitized erythrocytes (J.G.B. and S.J.R., unpublished data) or by soluble inhibitors such as CSA or CD36. Isolates from the peripheral blood were required to be cultured prior to testing adhesion, whereas placental isolates could be tested without intervening culture. Culture conditions may favor the growth of subpopulations of parasites, and infections in the study population often consist of >1 genotype (S.J.R., unpublished data), which may have different adhesive or antigenic properties. It should also be noted that in vitro adhesion assays do not quantify the proportion of all harvested parasites that can bind. When isolates consist of a mixture of parasite populations, the binding characteristics of some populations may be missed.
Our results generally support a role for specific adhesion to CSA present on the syncytiotrophoblast surface in placental sequestration of P. falciparum; however, other factors appear likely to be important for the accumulation of IEs in the placenta. These may include adhesion to molecules other than CSA or low placental blood flow [36], which together with reduced cell deformability of parasitized erythrocytes [37], might favor the accumulation of mature P. falciparum IEs in the placenta. Changes in immunologic responses during pregnancy that could reduce parasite clearance have also been described, such as impaired placental T cell responses to infection [38, 39], changes in specific antimalarial antibodies [40, 41], and systemic immunologic impairment as reflected by raised cortisol levels during pregnancy [42]. Published studies examining the histopathology of infected placentas report that very few IEs are directly adherent to the syncytiotrophoblast surface lining the maternal blood spaces [8, 43].
The marked difference between patterns of CD36 binding of maternal isolates (absent or low) and clinical isolates from children (common and high) suggests that isolates selected after sequestration in the placenta may have exchanged this property for adherence to other molecules, such as CSA. Three placental isolates bound at relatively low levels to CD36, which has not been detected on syncytiotrophoblasts [19]. Parasites from the same placentas also bound to CSA, which may indicate the presence of a subset of parasites with CD36 binding activity or multiple adhesive specificities of some isolates. It is unclear why binding to CSA is not seen more commonly in nonpregnant persons, since thrombomodulin, which may carry CSA chains [21], is present on endothelial cells [44]. Conditions that support the binding of parasites to CSA in the placenta may be absent in the vasculature of other organs. In addition, testing adhesion to purified CSA in vitro may not be entirely representative of mechanisms in vivo.
It was surprising that none of the isolates from pregnant women collected at term or during the course of pregnancy bound to ICAM-1 at significant levels, whereas most child isolates tested over the same period did bind. These results suggest that adhesion to ICAM-1 is not involved in placental parasite sequestration. ICAM-1 is widespread in a range of vascular beds [45] and is present on the surface of syncytiotrophoblasts [22], which can support ICAM-1-dependent adhesion of IEs when grown in culture [19]. Unfavorable conditions for binding to occur or immune responses against ICAM-1 binding strains acquired during previous infections may explain the absence of ICAM-1 binding isolates among infected placentas.
In the present study, circulating (peripheral maternal) and sequestered (placental) parasite populations demonstrated antigenic and phenotypic differences. Such comparisons between circulating and sequestered parasites underscore the possible limitations of interpreting the role of adhesion of peripheral blood isolates in the pathogenesis of disease in a given organ. A greater proportion of peripheral maternal isolates collected in midpregnancy bound to CSA than did peripheral blood isolates collected at term, indicating that the former may be more representative of the placentally sequestered parasite population in regard to adhesive phenotype. The low proportion of peripheral maternal isolates collected at term that showed some binding to CSA (20%) is similar to that reported by Fried and Duffy [18], who tested similar isolates collected at term and not during the course of pregnancy.
Isolates collected from children were commonly agglutinated by adult sera, whereas agglutination of pregnancy isolates was rare, suggesting that the two parasite populations are antigenically different and that pregnancy selects in some way for subpopulations of P. falciparum variant types that are rare in the wider community. Selection may occur as a result of the adhesion of parasites to novel receptors (e.g., CSA in the placenta) in combination with other factors. A study of strain-specific immunity in Kenyan children suggests that persons with a repertoire of agglutinating antibodies against a wide range of isolates develop infections with less common or rare antigenic variants [46]. Similar selection pressures may be important during pregnancy. The diversity of agglutination seen with different placental isolates suggests that pregnancy isolates are not antigenically restricted.
Before pregnancy there may be little or no exposure to these antigenic types, but after several pregnancies, during which exposure occurs, antibody responses develop that may help prevent infection in subsequent pregnancies. This is reflected in the higher prevalence of agglutinating antibodies to placental isolates in the sera of multigravidae. Agglutinating or variant-specific antibodies correlate with protection from disease in African children [31]; however, Fried et al. [47] found no association between placental infection and such antibodies but did find an association between CSA adhesion inhibition antibodies and infection. A detailed understanding of adhesion and antigenic variation of P. falciparum infection in pregnancy and of the molecular mechanisms governing these features may further clarify the pathogenesis of malaria during pregnancy and identify immune responses that are protective.
Acknowledgments
We thank E. Chalaluka, R. Tembenu, and P. Bock for assistance with sample collection and processing; the staff of the Antenatal Clinic and Labour Ward of Queen Elizabeth Central Hospital, Blantyre, Malawi, for friendly cooperation; and to all individuals who participated in the study. We are grateful for the enthusiastic support of V. Lema, Department of Obstetrics and Gynaecology, College of Medicine, and of T. Taylor.
Financial support: National Health and Medical Research Council, Australia (medical postgraduate scholarship to J.G.B); Wellcome Trust, UK (career development fellowship to S.J.R.; research leave fellowship in Clinical Tropical Medicine to M.E.M.); District 9680 Rotary Against Malaria Programme, Australia (to J.G.B).
Footnotes
Presented in part: European Congress of Tropical Medicine, Liverpool, United Kingdom, September 1998; Australian Society of Parasitology Malaria Satellite Meeting, LaTrobe University, Bundoora, Victoria, Australia, 2 October 1998.
Informed consent was obtained from all study participants, and ethical clearance was obtained from the College of Medicine Research Committee, University of Malawi, and the Ethics Committee, Walter and Eliza Hall Institute of Medical Research.
References
- 1.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–16. [PMC free article] [PubMed] [Google Scholar]
- 2.McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg. 1984;33:517–25. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 3.Fleming AF. Tropical obstetrics and gynaecology. 1. Anaemia in pregnancy in tropical Africa. Trans R Soc Trop Med Hyg. 1989;83:441–8. doi: 10.1016/0035-9203(89)90241-1. [DOI] [PubMed] [Google Scholar]
- 4.Granja AC, Machungo F, Gomes A, Bergstrom S, Brabin B. Malaria-related maternal mortality in urban Mozambique. Ann Trop Med Parasitol. 1998;92:257–63. doi: 10.1080/00034989859816. [DOI] [PubMed] [Google Scholar]
- 5.McDermott JM, Wirima JJ, Steketee RW, Breman JG, Heyman DL. The effect of placental malaria infection on perinatal mortality in rural Malawi. Am J Trop Med Hyg. 1996;55:61–5. doi: 10.4269/ajtmh.1996.55.61. [DOI] [PubMed] [Google Scholar]
- 6.Aikawa M, Iseki M, Barnwell JW, Taylor D, Oo MM, Howard RJ. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990;43:30–7. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- 7.Berendt AR, Turner GDH, Newbold CI. Cerebral malaria: the sequestration hypothesis. Parasitol Today. 1994;10:412–4. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 8.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. Am J Pathol. 1982;109:330–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Watkinson M, Rushton DI. Plasmodial pigmentation of placenta and outcome of pregnancy in West African mothers. BMJ. 1983;287:251–4. doi: 10.1136/bmj.287.6387.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogerson SJ, Brown GV. Chondroitin sulfate A as an adherence receptor for Plasmodium falciparum-infected erythrocytes. Parasitol Today. 1997;13:70–5. doi: 10.1016/s0169-4758(96)10081-8. [DOI] [PubMed] [Google Scholar]
- 11.Berendt AR, Simmons DL, Tansey J, Newbold CI, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion molecule for Plasmodium falciparum. Nature. 1989;341:57–9. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 12.Barnwell JW, Asch AS, Nachman RL, Yamaya M, Aikawa M. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest. 1989;84:765–72. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ockenhouse CF, Tandon NN, Magowan C, Jamieson GA, Chulay JD. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989;243:1469–71. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- 14.Ockenhouse CF, Ho M, Tandon NN, et al. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis. 1991;164:163–9. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- 15.Reeder JC, Rogerson SJ, Al-Yaman F, et al. Diversity of agglutinating phenotype, cytoadherence, and rosette-forming characteristics of Plasmodium falciparum isolates from Papua New Guinean children. Am J Trop Med Hyg. 1994;51:45–55. doi: 10.4269/ajtmh.1994.51.45. [DOI] [PubMed] [Google Scholar]
- 16.Beeson JG, Chai W, Rogerson SJ, Lawson AM, Brown GV. Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A. Infect Immun. 1998;66:3397–402. doi: 10.1128/iai.66.7.3397-3402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmley RT, Takagi M, Denys FR. Ultrastructural localization of glycosaminoglycans in human term placenta. Anat Rec. 1984;210:477–84. doi: 10.1002/ar.1092100308. [DOI] [PubMed] [Google Scholar]
- 18.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 19.Maubert B, Guilbert LJ, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun. 1997;65:1251–7. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Pouvelle B, Meyer P, et al. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res Immunol. 1995;146:383–93. doi: 10.1016/0923-2494(96)81042-x. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Novakovic S, Cooke BM, Brown GV. Plasmodium falciparum-infected erythrocytes adhere to the proteoglycan thrombomodulin in static and flow-based systems. Exp Parasitol. 1997;86:8–18. doi: 10.1006/expr.1996.4142. [DOI] [PubMed] [Google Scholar]
- 22.Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ. ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts. Am J Pathol. 1997;150:1845–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Marsh K, Howard RJ. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–3. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 24.Leech JH, Barnwell JW, Miller LH, Howard RJ. Identification of a strainspecific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159:1567–75. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biggs BA, Goozé L, Wycherley K, et al. Antigenic variation in Plasmodium falciparum. Proc Natl Acad Sci USA. 1991;88:9171–4. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baruch DI, Pasloske BL, Singh HB, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 27.Smith JD, Chitnis CE, Craig AG, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–10. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 29.Baruch DI, Gormley JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeder JC, Cowman AF, Davern KM, et al. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc Natl Acad Sci USA. 1999;96:5198–202. doi: 10.1073/pnas.96.9.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nature Med. 1998;4:358–60. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogerson SJ, Beeson JG, Dzinjalamala FK, Mhango C, van den Broek N, Molyneux ME. Malaria in pregnancy in Blantyre; Presented at the Second European Congress on Tropical Medicine; Liverpool, UK. 1998. [Google Scholar]
- 33.Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1997;91:363–5. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 34.Cooke BM, Rogerson SJ, Brown GV, Coppel RL. Adhesion of malariainfected red blood cells to chondroitin sulfate A under flow conditions. Blood. 1996;88:4040–4. [PubMed] [Google Scholar]
- 35.Kutner S, Breuer WV, Ginsburg H, Aley SB, Cabantchik ZI. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J Cell Physiol. 1985;125:521–7. doi: 10.1002/jcp.1041250323. [DOI] [PubMed] [Google Scholar]
- 36.Ramsey EM, Donner MW. Placental vasculature and circulation. Georg Thieme; Stuttgart, Germany: 1980. [Google Scholar]
- 37.Miller LH, Chien S, Usami S. Decreased deformability of Plasmodium coatneyi-infected red cells and its possible relation to cerebral malaria. Am J Trop Med Hyg. 1972;21:133–6. doi: 10.4269/ajtmh.1972.21.133. [DOI] [PubMed] [Google Scholar]
- 38.Riley EM, Schneider G, Sambou I, Greenwood BM. Suppression of cell-mediated immune responses to malaria antigens in pregnant Gambian women. Am J Trop Med Hyg. 1989;40:141–4. doi: 10.4269/ajtmh.1989.40.141. [DOI] [PubMed] [Google Scholar]
- 39.Lea RG, Calder AA. The immunology of pregnancy. Curr Opin Infect Dis. 1997;10:171–6. [Google Scholar]
- 40.Nambei WS, Goumbala M, Spiegel A, Dieye A, Perraut R, Garraud O. Imbalanced distribution of IgM and IgG antibodies against Plasmodium falciparum antigens and merozoite surface protein-1 (MSP1) in pregnancy. Immunol Lett. 1998;61:197–9. doi: 10.1016/s0165-2478(97)00168-5. [DOI] [PubMed] [Google Scholar]
- 41.Deloron P, Steketee RW, Campbell GH, Peyron F, Kaseje DCO. Serological reactivity to the ring-infected erythrocyte surface antigen and circumsporozoite protein in gravid and nulligravid women infected with Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1989;83:58–62. doi: 10.1016/0035-9203(89)90705-0. [DOI] [PubMed] [Google Scholar]
- 42.Vleugels MPH, Brabin B, Eling WMC, de Graaf R. Cortisol and Plasmodium falciparum infection in pregnant women in Kenya. Trans R Soc Trop Med Hyg. 1989;83:173–7. doi: 10.1016/0035-9203(89)90632-9. [DOI] [PubMed] [Google Scholar]
- 43.Yamada M, Steketee R, Abramowsky C, et al. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am J Trop Med Hyg. 1989;41:161–8. doi: 10.4269/ajtmh.1989.41.161. [DOI] [PubMed] [Google Scholar]
- 44.Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75:329–36. [PubMed] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–54. [PubMed] [Google Scholar]
- 46.Bull PC, Lowe BS, Kortok M, Marsh K. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. 1999;67:733–7. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–2. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]