Abstract
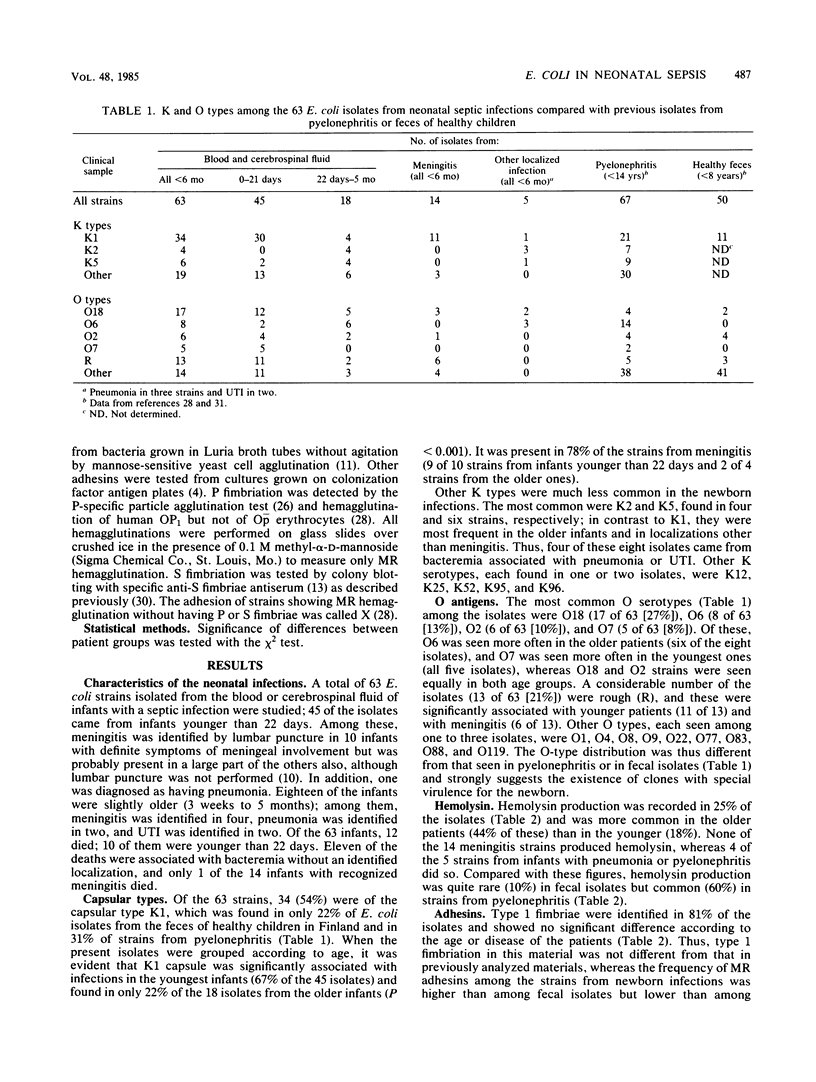
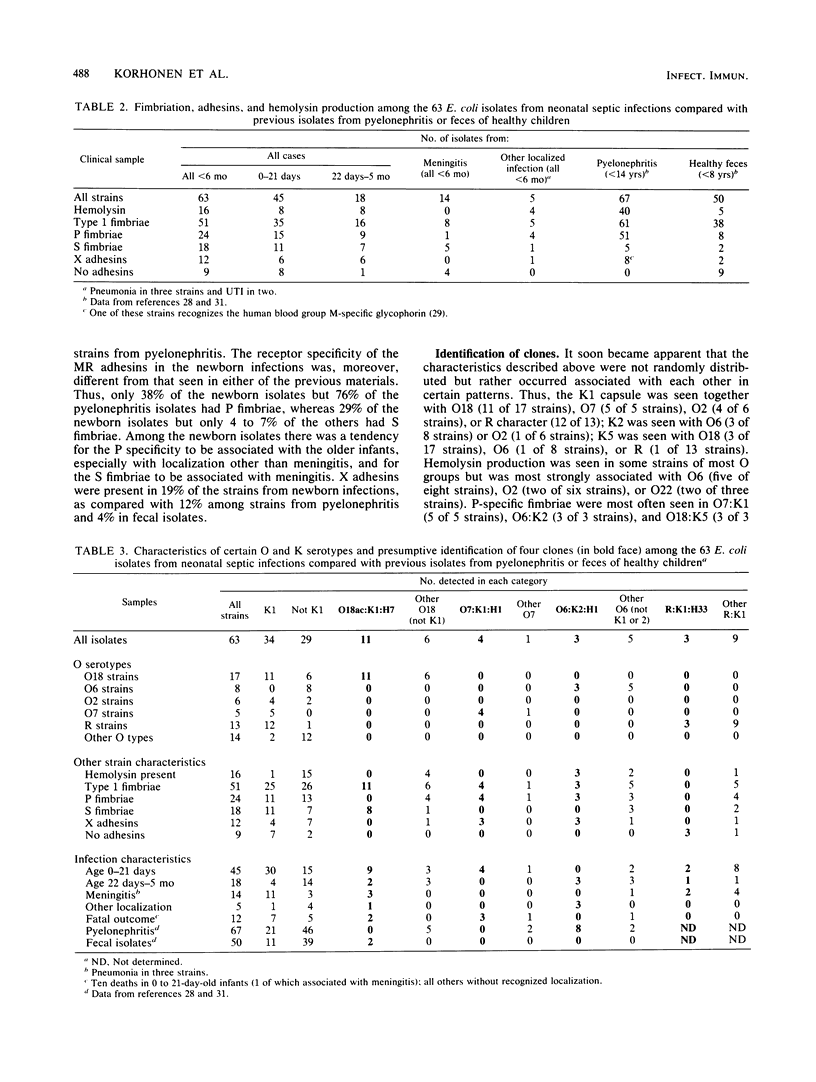
Sixty-three Escherichia coli strains isolated from neonatal sepsis or meningitis were studied and compared with previous data on fecal or urinary pyelonephritis-associated isolates from children. Characteristics significantly associated with neonatal infection were capsular type K1 (54%), O group 18 (27%), rough-type lipopolysaccharide together with K1 capsule (19%), and S fimbriae (29%). Within the neonatal infection group, the K1 capsule and rough lipopolysaccharide were most common among the youngest infants (0 to 21 days old) and in meningitis. Hemolysin production, P fimbriae, and X adhesions (adhesions not identifiable as type 1, P, or S) were significantly more common in the two materials from infections as compared with the fecal isolates. One large clone of 11 strains (O18:K1:H7, with both type 1 and S fimbriae) and three smaller ones (O7:K1:H1 and O6:K2:H1, both with type 1 and P fimbriae and X adhesions; and R:K1:H33 with no adhesions) were identified among the strains from neonatal infections. Only O6:K2:H1 strains were also common among the strains from pyelonephritis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks H. J., O'Grady F., McSherry M. A., Cattell W. R. Uropathogenic properties of Escherichia coli in recurrent urinary-tract infection. J Med Microbiol. 1980 Feb;13(1):57–68. doi: 10.1099/00222615-13-1-57. [DOI] [PubMed] [Google Scholar]
- DeBoy J. M., 2nd, Wachsmuth I. K., Davis B. R. Hemolytic activity in enterotoxigenic and non-enterotoxigenic strains of Escherichia coli. J Clin Microbiol. 1980 Aug;12(2):193–198. doi: 10.1128/jcm.12.2.193-198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J., Leinonen M., Mäkelä P. H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983 Aug 13;2(8346):355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J Biol Chem. 1982 Oct 25;257(20):11966–11970. [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Winberg J., Hultberg H. Identification of a carbohydrate receptor recognized by uropathogenic Escherichia coli. Infection. 1980;8 (Suppl 3):288–293. doi: 10.1007/BF01639597. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J Bacteriol. 1984 Nov;160(2):691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Finne J. Isolation and structural characterization of five major sialyloligosaccharides and a sialylglycopeptide from normal human urine. Eur J Biochem. 1983 Nov 2;136(2):355–361. doi: 10.1111/j.1432-1033.1983.tb07749.x. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Källenius G., Möllby R., Hultberg H., Winberg J. Rapid identification of P-fimbriated Escherichia coli by a receptor-specific particle agglutination test. Infection. 1982;10(4):209–214. doi: 10.1007/BF01666912. [DOI] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984 Nov;46(2):401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., van den Bosch J. F., MacLaren D. M., de Graaff J. Hemolysin plasmid coding for the virulence of a nephropathogenic Escherichia coli strain. Infect Immun. 1982 Jan;35(1):32–37. doi: 10.1128/iai.35.1.32-37.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


