Abstract
Saccharomyces cerevisiae linker histone Hho1p is not essential for cell viability, and very little is known about its function in vivo. We show that deletion of HHO1 (hho1Δ) suppresses the defect in transcriptional silencing caused by a mutation in the globular domain of histone H4. hho1Δ also suppresses the reduction in HML silencing by the deletion of SIR1 that is involved in the establishment of silent chromatin at HML. We further show that hho1Δ suppresses changes in silent chromatin structure caused by the histone H4 mutation and sir1Δ. These results suggest that HHO1 plays a negative role in transcriptionally silent chromatin. We also provide evidence that Hho1p hinders the de novo establishment of silent chromatin but does not affect the stability of preexistent silent chromatin. Unlike canonical linker histones in higher eukaryotes that have a single conserved globular domain, Hho1p possesses two globular domains. We show that the carboxyl-terminal globular domain of Hho1p is dispensable for its function, suggesting that the mode of Hho1p action is similar to that of canonical linker histones.
The eukaryotic genome is packed into chromatin that plays a key role in regulating DNA transactions, including transcription, replication, and recombination. The basic unit of chromatin is the nucleosome consisting of 147 bp of DNA wrapped around a protein core composed of two each of histones H2A, H2B, H3, and H4 (1). Nucleosomes in chromatin are connected by linker DNAs whose average lengths vary from organism to organism (2). Linker DNAs associate with linker histones, referred to as histone H1, that bind DNA near the entry and exit points of the nucleosome. There is evidence that linker histones facilitate chromatin condensation and regulate the 30 nm chromatin fiber structure in vitro (3). Since linker histones are involved in the formation of higher order chromatin structures and repress chromatin transcription in vitro (4–6), it was originally believed that they function as global transcription repressors in vivo. However, increasing evidence demonstrates that this is not the case. In mice, reducing the level of histone H1 to 50% of its normal level causes dramatic changes in chromatin structure, including a general decrease in nucleosome spacing and reduced chromatin compaction (7). However, expression of only a small number of genes is affected (7). Similarly, in Tetrahymena thermophilia, deletion of histone H1 reduces global chromatin compaction and affects transcription of specific genes but does not have a major effect on global transcription (8, 9). The functions of linker histones are essential in mice, and reducing the level of histone H1 by half leads to embryonic lethality (10). On the other hand, linker histones in lower eukaryotes, such as Tetrahymena and Saccharomyces cerevisiae are not essential for cell survival (8, 11).
Linker histones can be divided into two major families based on their structural characteristics (12). Members of one family have a tripartite structure consisting of a conserved globular domain flanked by a short NH2-terminal tail and a long COOH-terminal tail that are both lysine-rich, highly charged, and relatively unstructured. Linker histones in the other family lack the conserved globular domain and contain only the equivalent of the COOH-terminal domain of the tripartite family. Tripartite linker histones are generally found in multicellular eukaryotes, whereas the single domain ones are found in certain protists, such as Tetrahymena.
A search of yeast genome sequence for homologs of the conserved globular domain of histone H1 identified the HHO1 gene (13, 14). Interestingly, the sequence of HHO1 predicts a protein that resembles the tripartite linker histone but contains a second globular domain fused to its COOH terminus (12). The two globular domains of Hho1p (GI and GII) can form similar secondary and tertiary structures in vitro, but GI is significantly more stable than GII under physiological salt conditions (15–17). Initial biochemical analyses of recombinant Hho1p suggest that it has properties similar to those of canonical tripartite linker histones (18). Hho1p forms a stable 1:1 tertiary complex with reconstituted dinucleosomes, but its concentration in vivo is significantly less than that of the nucleosome cores (18–20).
There is only limited information about the in vivo function of Hho1p. Deletion of HHO1 has no noticeable effect on cell growth (14, 18, 21) and causes a reduction in the transcripts of only 27 of about 6000 genes by a factor of 2 or more, indicating that Hho1p positively regulates the expression of only a subset of genes (22). There is evidence suggesting that Hho1p is inhibitory to homologous recombination (20, 23).
One interesting question regarding Hho1p is whether it plays any role in transcriptional silencing. Silencing occurs at the HML and HMR loci, regions near the telomeres, as well as the rDNA array in yeast, which is mediated by a special silent chromatin (24). The establishment of silent chromatin is achieved via an initiation process that recruits the Sir complex consisting of Sir2p, Sir3p, and Sir4p to specific nucleation sequences, including the silencers flanking the HM loci and telomeric repeats. A Sir complex recruited to silencers or telomeric repeats is believed to deacetylate histones in adjacent nucleosomes through the deacetylase activity of Sir2p (25). The deacetylated nucleosomes then bind additional Sir complexes. This is because the Sir complex self-interacts and preferentially binds hypoacetylated histones. Through repeated cycles of histone deacetylation and Sir complex recruitment, Sir complexes propagate along the chromatin. Each silencer at the HM loci consists of binding sites for origin recognition complex (ORC),3 Rap1p, and/or Abf1p. The silencer-binding factors recruit the Sir complex through a direct interaction between ORC and Sir1p that binds to Sir4p and the binding of Rap1p to Sir3p or Sir4p. Sir1p acts only in the establishment of silent chromatin, and its deletion reduces but does not eliminate silencing (26, 27).
As a nucleosome-interacting protein, Hho1p has the potential to influence the formation and maintenance of silent chromatin. However, deletion of HHO1 seems to have no effect on silencing at HM loci, telomeres, and rDNA (14, 18, 21, 23). On the other hand, we found that overexpression of HHO1 has an inhibitory effect on silencing (28). We show in this work that Hho1p functionally interacts with core histone H4 and Sir1p and negatively regulates the de novo establishment of silent chromatin. We also present evidence suggesting that Hho1p functionally resembles canonical tripartite linker histones.
EXPERIMENTAL PROCEDURES
Plasmids and Strains—The DNA sequence from coordinates 308227 to 310203 of chromosome XVI encompassing the HHO1 ORF plus 600-bp upstream and 600-bp downstream sequences was PCR-amplified. XhoI and SpeI sites were added to the ends of this fragment during PCR to yield the XhoI-HHO1-SpeI fragment that was inserted into pRS414 (CEN-TRP1) to make pQY288. A similar BglII-HHO1-BglII fragment was inserted into pYEP13 (2 μm-LEU2) to yield pYEP13-HHO1. pQY290 was made by precisely deleting the sequence corresponding to the COOH-terminal 87 amino acids of Hho1p from pQY288. pQY292 and -293 were similarly constructed by deleting from pQY288 HHO1 sequences corresponding to amino acids 1–118 and 1–40, respectively. The HHO1 allele in each plasmid was confirmed by sequencing. pQY318 and -319 were made by replacing the HHO1 ORF in pQY288 with the ORFs of Xenopus H1° and human H1.1 genes, respectively. pMS337, -364, -366, and -367 are CEN-LEU2 plasmids encoding HHT1-HHF1, HHT1-hhf1-Y88G, HHT1-hhf1-Y98H, and HHT1-hhf1-Y98W, respectively, and pMS329 is CEN-URA3-HHT1-HHF1 (29). pUC-SK was made by inserting the sir3-8 allele and KanMX into pUC19.
Yeast strains are listed in Table 1. Strains 1m, 3m, 5m, and 7m were derived from Y1838 (MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ pMS329, from J. R. Broach) by replacing its pMS329 with pMS337, -364, -366, and -367, respectively. Strains 2m, 4m, 6m, and 8m were made by replacing the HHO1 ORF in 1m, 3m, 5m, and 7m with KanMX, respectively. Strains 1–8 were made by transforming 1m–8m, respectively, to Ura+ with EcoRI-SalI-digested pT1 (30). Strains 9–12 were made by transforming 1m–4m to Ura+ with HindIII-digested p114 (31). The HMR-E silencer in these strains was inverted to enhance silencing on the left of HMR (31). Strains 13–16 were made by transforming 1m–4m to Ura+ with BamHI-digested p102 (31). The HML-I silencer in these strains was inverted to enhance silencing on the right of HML (31). Strains 17 and 18 were made by transforming pYEP13 and pYEP13-HHO1 into 9, respectively. Strains 19 and 20 were made by transforming pYEP13 and pYEP13-HHO1 into 13, respectively. Strains 21 and 22 were made by replacing the CEN-URA3-HHF1 plasmid in YXB102 (32) with pRS414-HHF1 and -hhf1-Y88G, respectively. HHO1 in strains 21 and 22 was replaced by URA3 to yield 23 and 24, respectively. Strain 25 was derived from 21 by replacing its SIR3 with URA3. Strains 26 and 27 were made by transforming 1 and 3, respectively, to Geneticin-resistant with a PCR-produced fragment encoding 9-Myc linked to KanMX embedded in a sequence spanning the 3′ region of HHO1 ORF. Strains 28–30 are from Open Biosystems (Huntsville, AL). Strain 31 was made by replacing HHO1 in 30 with LEU2. Strains 32, 34, and 36 have been described (33, 34). Strain 37 was made by transforming 36 to Geneticin-resistant with Tth111I-digested pUC-SK. Strains 33, 35, and 38 were made by replacing HHO1 in 32, 34, and 37, respectively, with NatMX. Strains 39–45 were made by transforming plasmids pRS414 and pQY288, -290, -293, -292, -318, and -319, respectively, into strain 4. The relevant genotypes of all of the strains constructed in this work were confirmed by PCR and/or Southern blotting.
TABLE 1.
Yeast strains
| Number | Name | Genotype |
|---|---|---|
| 1m | YQY385m | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ pMS337 (CEN-LEU2-HHT1-HHF1) |
| 1 | YQY385 | YQY385m, Tel VII-L-URA3 |
| 2m | YQY386m | YQY385m, hho1Δ::KanMX |
| 2 | YQY386 | YQY386m, Tel VII-L-URA3 |
| 3m | YQY355m | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ pMS364 (CEN-LEU2-HHT1-hhf1-Y88G) |
| 3 | YQY355 | YQY355m, Tel VII-L-URA3 |
| 4m | YQY356m | YQY355m, hho1Δ::KanMX |
| 4 | YQY356 | YQY356m, Tel VII-L-URA3 |
| 5m | YQY452m | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ pMS366 (CEN-LEU2-HHT1-hhf1-Y98H) |
| 5 | YQY452 | YQY452m, Tel VII-L-URA3 |
| 6m | YQY500m | YQY452m, hho1Δ::KanMX |
| 6 | YQY500 | YQY500m, Tel VII-L-URA3 |
| 7m | YQY450m | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ pMS367 (CEN-LEU2-HHT1-hhf1-Y98W) |
| 7 | YQY450 | YQY450m, Tel VII-L-URA3 |
| 8m | YQY451m | YQY450m, hho1Δ::KanMX |
| 8 | YQY451 | YQY451m, Tel VII-L-URA3 |
| 9 | YQY379 | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ URA3-HMR-E(inverted)-HMRa-HMR-I pMS337 (CEN-LEU2-HHT1-HHF1) |
| 10 | YQY380 | YQY379, hho1Δ::KanMX |
| 11 | YQY368 | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ URA3-HMR-E(inverted)-HMRa-HMR-I pMS364 (CEN-LEU2-HHT1-hhf1-Y88G) |
| 12 | YQY384 | YQY368, hho1Δ::KanMX |
| 13 | YQY377 | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ HML-E-HMLα-HML-I(inverted)-URA3 pMS337 (CEN-LEU2-HHT1-HHF1) |
| 14 | YQY378 | YQY377, hho1Δ::KanMX |
| 15 | YQY367 | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ HML-E-HMLα-HML-I(inverted)-URA3 pMS364 (CEN-LEU2-HHT1-hhf1-Y88G) |
| 16 | YQY383 | YQY367, hho1Δ::KanMX |
| 17 | YQY379 + pYEP13 (2 μm-LEU2) | |
| 18 | YQY379 + pYEP13-HHO1 | |
| 19 | YQY377 + pYEP13 | |
| 20 | YQY377 + pYEP13-HHO1 | |
| 21 | YXB803 | MATa ura3-52 ade2-101 can1-100 trp1Δ901 his3Δ200 his5-1 LEU2-GAL10-FLP1 hhf1Δ::HIS3 hhf2Δ::LEU2 E-FRT-hml::β-FRT-I pRS414-HHF1(CEN-TRP1-HHF1) [ciro] |
| 22 | YXB815 | MATa ura3-52 ade2-101 can1-100 trp1Δ901 his3Δ200 his5-1 LEU2-GAL10-FLP1 hhf1Δ::HIS3 hhf2Δ::LEU2 E-FRT-hml::β-FRT-I pRS414-hhf1-Y88G [ciro] |
| 23 | YQY566 | YXB803, hho1Δ::URA3 |
| 24 | YQY567 | YXB815, hho1Δ::URA3 |
| 25 | YQY570 | YXB803, sir3Δ::URA3 |
| 26 | YQY478 | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ Tel VII-L-URA3 HHO1-Myc-KanMX pMS337 (CEN-LEU2-HHT1-HHF1) |
| 27 | YQY479 | MATα ura3-52 leu2-3,112 his3Δ trp1-289 (hht1-hhf1)Δ (hht2-hhf2)Δ Tel VII-L-URA3 HHO1-Myc-KanMX pMS363 (CEN-LEU2-HHT1-hhf1-Y88G) |
| 28 | BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 |
| 29 | BY4741, hho1Δ::KanMX | |
| 30 | BY4741, sir1Δ::KanMX | |
| 31 | YYZ333 | BY4741, sir1Δ::KanMX hho1Δ::LEU2 |
| 32 | YXB10 | MATa ura3-52 ade2-1 lys1-1 his5-1 can1-100 LEU2-GAL10-FLP1 FRT-E-hml::β1-I-FRT [ciro] |
| 33 | YHK13 | YXB10, hho1Δ::NatMX |
| 34 | YXB810 | YXB10, sir1::URA3 |
| 35 | YHK14 | YXB810, hho1Δ::NatMX |
| 36 | YXB10s | YXB10, sir3::URA3 |
| 37 | YXB814 | YXB10s, sir3-8-KanMX |
| 38 | YHK16 | YXB814, hho1Δ::NatMX |
| 39 | YQY512 | YQY354 + pRS414 (CEN-TRP1) |
| 40 | YQY513 | YQY354 + pQY288 (CEN-TRP1-HHO1) |
| 41 | YQY514 | YQY354 + pQY290 (CEN-TRP1-hho1-ΔGII) |
| 42 | YQY516 | YQY354 + pQY293 (CEN-TRP1-hho1-ΔN) |
| 43 | YQY517 | YQY354 + pQY292 (CEN-TRP1-hho1-ΔN-GI) |
| 44 | YQY354 + pQ318 (CEN-TRP1-XI-H1) | |
| 45 | YQY354 + pQ319 (CEN-TRP1-Hs-H1) |
RT-PCR—Total RNA was isolated from log phase cells and diluted to a concentration of 1 μg/ml. The sample was treated with DNase I and then used as template for RT-PCR with the SuperScript III one-step RT-PCR system with Platinum® TaqDNA polymerase (Invitrogen) in a 20-μl reaction. For measuring the transcript of SIR1, SIR2, SIR3, or SIR4, 1 μl of RNA was used in a 25-cycle PCR. For ACT1,1 μl of RNA was used in a 20-cycle PCR. PCR products were fractionated on 2.0% agarose gels. The proper amount of RNA used as template in RT-PCR was predetermined to be in the linear range by serial dilutions. No-RT control PCR was performed by using Platinum® TaqDNA polymerase instead of the RT/Platinum® Taq mix.
Analysis of DNA Topology—Cells grown in YPR medium (1% yeast extract, 2% bacto-peptone, and 2% raffinose) were treated with galactose (2%) for 2.5 h to induce the expression of PGAL10-FLP1. Nucleic acids were isolated using the glass bead method and fractionated on agarose gels supplemented with 13 μg/ml chloroquine. DNA circles were detected by Southern blotting. The profiles of topoisomers were determined by using the NIH Image software.
Chromatin Immunoprecipitation (ChIP)—ChIP was carried out as described (35). Briefly, the culture (50 ml) was grown to log phase and then fixed for 25 min at room temperature in 1% formaldehyde. Cells were harvested and washed twice with distilled H2O and FA-Lysis 140 buffer (50 mm HEPES-KOH, pH 7.5, 140 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% deoxycholic acid (catalog number P-8215; Sigma) at 15 μl/ml). Cell pellets were resuspended in 400 μl of FA-Lysis 140 and lysed using the glass bead method. The extract was then sonicated with a Branson Sonifier 450, and the lysate was clarified by centrifugation. According to the A260, 120 units of whole-cell extract was added to each IP for a final volume of 240 μl in FA-Lysis 140. Myc antibody was added at 1.5 μl/IP. Incubation of IP reactions was done at 4 °C overnight. Bound chromatin was precipitated with Protein A-Sepharose beads for 2 h at 4 °C. The beads were washed, and the immune complexes were eluted twice with 200 μl of 1% SDS, 0.1 m NaHCO3 at room temperature. The cross-links were then reversed at 65 °C for 5 h in the presence of NaCl and ethanol-precipitated overnight at –20 °C. The recovered material was RNase A- and Proteinase K-treated and phenol/chloroform-extracted. Purified DNA was resuspended in 150 μl of 1× Tris-EDTA. Six μl of each sample was used in 25-μl PCRs for 28 cycles. In PCRs, the proper amount of input and immunoprecipitated chromatin DNA used was predetermined to be in the linear range by serial dilutions. Input chromatin was added to PCRs as a 1:10 dilution. PCR products were separated on a 1.2% agarose gel. ChIP data were analyzed as described in “Results”. The sequences of the PCR primers used are available upon request.
RESULTS
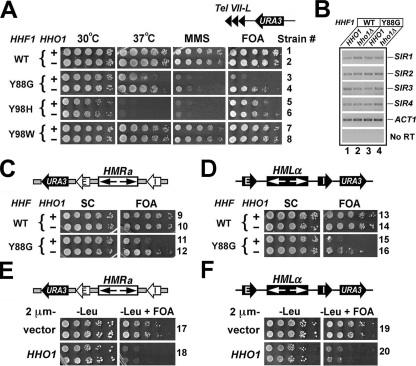
Deletion of HHO1 Suppresses the Defect in Transcriptional Silencing Caused by the Y88G Mutation in Histone H4—In an attempt to systematically investigate the potential role of HHO1 in transcriptional silencing, we monitored silencing of the URA3 reporter placed near Tel VII-L (the left telomere of chromosome VII) and the HML and HMR loci in HHO1 wild type and null strains (Fig. 1, A, C, and D, top). URA3 silencing was measured as cell growth on medium containing 5-fluoroorotic acid (FOA), since cells expressing URA3 are sensitive to killing by FOA (36). As predicted, URA3 was strongly silenced at Tel VII-L and the HM loci in wild type cells (Fig. 1, robust growth of strains 1, 9, and 13 on FOA medium). Deletion of HHO1 had no effect on URA3 silencing (Fig. 1, compare 2 with 1, 10 with 9, and 14 with 13 on FOA). This is consistent with previous studies that revealed no role of HHO1 in transcriptional silencing (14, 18, 21). However, it is formally possible that Hho1p plays a role in silencing that is redundant with or antagonistic to functions of other factors, such as histones, involved in the formation and/or maintenance of silent chromatin. To test this idea, we set out to look for synthetic effects of hho1Δ and histone mutations on transcriptional silencing.
FIGURE 1.
HHO1 functionally interacts with histone H4 and negatively regulates transcriptional silencing. A, phenotypes of the Y88G mutant and their suppression by hho1Δ. Shown are growth phenotypes of strains 1–8 under different conditions. Odd numbered strains are wild type (WT) for HHO1 (+), whereas even numbered ones are hho1Δ (–). Cells of each strain were grown to late log phase, and serial 10-fold dilutions were spotted on four synthetic complete (SC) plates and allowed to grow for 3 days under different conditions as indicated. FOA, SC supplemented with 1 mg/ml 5-fluoroorotic acid. MMS, SC with 0.02% methyl methanesulfonate. The histone H4 (HHF1) alleles are indicated on the left, and strain numbers are on the right. The silencing reporter construct is illustrated above the FOA panel. B, levels of the transcripts of genes SIR1 through SIR4 in H4-Y88G and hho1Δ single and double mutants as measured by RT-PCR. RNAs were isolated from strains 1–4 grown to log phase and used as template for RT-PCR with five pairs of primers for SIR1 through SIR4 and ACT1, respectively. The PCR products were analyzed by agarose gel electrophoresis. C, HHO1 deletion suppresses the effect of H4-Y88G on HMR silencing. Shown are growth phenotypes of strains 9–12 on SC and FOA media. Each strain bears a modified HMR locus with the URA3 reporter gene inserted to the left of an inverted HMR-E silencer, as shown at the top. D, HHO1 deletion suppresses the effect of H4-Y88G on HML silencing. Shown are growth phenotypes of strains 13–16 on SC and FOA media. The silencing reporter construct is illustrated at the top. E and F, HHO1 is a high copy inhibitor of HM silencing. Shown are growth phenotypes of strains 17–20 on –Leu (SC minus leucine) and –Leu+FOA media.
A salient feature of silent chromatin is the hypoacetylation of lysines in the NH2-terminal tails of core histones (24). We examined if hho1Δ had synthetic interactions with a series of lysine to arginine or glutamine mutations in the NH2-terminal tails of H3 and H4 but found that hho1Δ neither suppressed nor exacerbated the phenotypes of the histone mutants with regard to telomeric silencing, cell growth, and resistance to the DNA-damaging agent methyl methanesulfonate (MMS) (data not shown). Therefore, we found no evidence for functional interactions between Hho1p and the NH2-terminal tails of histones H3 and H4.
We next examined if Hho1p functionally interacts with the globular domains of core histones that interact to form the nucleosome core. Three tyrosine residues, Tyr-72, -88, and -98, in the globular domain of histone H4 play a key role in the interaction between the H3/H4 tetramer and the H2A/H2B dimers (1, 29). Santisteban et al. (29) demonstrated the importance of these tyrosine residues by showing that H4-Y72G, -Y88G, and -Y98H mutants are sensitive to high temperatures (Ts–) (Fig. 1A, compare 3 and 5 with 1 at 37 °C). Here we showed that the H4-Y88G and -Y98H mutants were also sensitive to MMS (Fig. 1A, compare 3 and 5 with 1 on MMS). Moreover, the Y88G mutation abolished URA3 silencing near Tel VII-L or the HM loci (Fig. 1, compare 3 with 1, 11 with 9, and 15 with 13 on FOA). On the other hand, cells with H4 Tyr-98 changed to tryptophan, a substitution that conserved the side chain aromatic ring, had none of the phenotypes of Y88G or Y98H (Fig. 1A, compare 7 with 1). These results support the notion that hydrophobic interactions between H3/H4 and H2A/H2B mediated by H4 Tyr-88 and Tyr-98 are important for various cellular functions.
HHO1 deletion partially suppressed the defect in telomeric silencing of the H4-Y88G mutant (Fig. 1A, compare 4 with 3 on FOA). It also partially suppressed the silencing defect at the HM loci (Fig. 1, C and D, compare 12 with 11 and 16 with 15 on FOA). In addition, hho1Δ suppressed the Ts– phenotype and, to a lesser extent, MMS sensitivity of H4-Y88G (Fig. 1A, 37 °C and MMS panels, compare 4 with 3). HHO1 deletion had no effect on the phenotypes of the H4-Y98H and -Y98W mutants (Fig. 1A, compare 6 with 5 and 8 with 7). These results demonstrate that Hho1p functionally interacts with histone H4 in an allele-specific manner and plays a negative role in various cellular functions, including transcriptional silencing. Consistently, increasing the dosage of the HHO1 gene reduced transcriptional silencing in histone H4 wild type cells (Fig. 1, E and F, compare 18 with 17 and 20 with 19).
It is formally possible that restoration of silencing in the H4-Y88G mutant by hho1Δ is due to increased expression of the SIR1 through SIR4 genes. However, the levels of the SIR genes as measured by RT-PCR in the H4-Y88G hho1Δ double mutant were not significantly different from those in the H4-Y88G and hho1Δ single mutants (Fig. 1B). Therefore, hho1Δ does not suppress the silencing defect of H4-Y88G by increasing the expression of SIR genes.
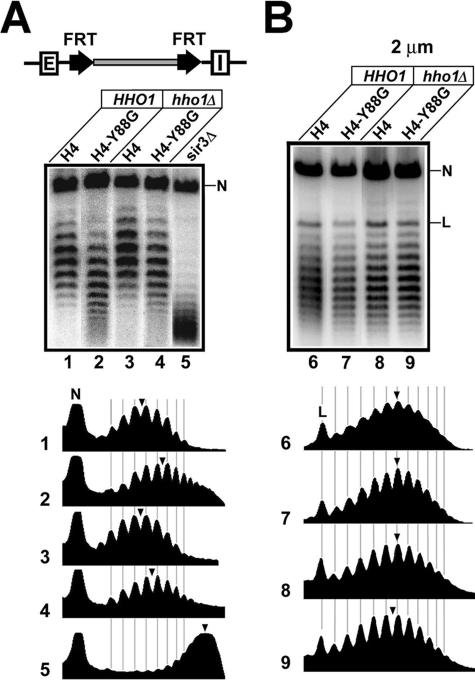
Hho1p Plays a Negative Role in Silent Chromatin—We investigated whether the negative role of HHO1 in silencing revealed above reflected changes in the structure of silent chromatin using a DNA topology-based assay. The topology of eukaryotic DNA is determined by multiple factors, including the density of nucleosomes along the DNA and the configurations of nucleosomes. The major contribution to the negative supercoiling of DNA is its wrapping into nucleosomes with an average linking number change (ΔLk) of about –1 per nucleosome formed (37, 38). DNA at HML and HMR is more negatively supercoiled when these loci are silenced than when they are derepressed, and the degree of negative supercoiling is an indicator of the state of silent chromatin (39, 40). We asked if the histone H4-Y88G mutation and hho1Δ affected the structure of silent chromatin by comparing the supercoiling of HML DNA in H4-Y88G and hho1Δ single and double mutants as well as wild type cells. This was achieved by using site-specific recombination to excise HML as a minichromosome circle whose topology could be readily examined (39).
In the strains used for measuring the supercoiling of HML DNA, the modified HML locus excluding the E and I silencers was bracketed by two copies of Flp1p recombination target (FRT) for the site-specific recombinase Flp1p (Fig. 2A, top), and an inducible PGAL-FLP1 gene was resident elsewhere in the genome. Activation of PGAL-FLP1 by galactose would lead to the expression of Flp1p and recombination between the FRT sites, resulting in the excision of HML as a closed circle (39). After deproteinization, the topoisomers of the circle can be resolved by gel electrophoresis in the presence of the DNA intercalator chloroquine (Fig. 2A, lane 1). Deletion of SIR3 reduced the negative supercoiling of the HML circle by a ΔLk of ∼7 (Fig. 2A, compare the centers of topoisomer distributions in lanes 1 and 5; note that more negatively supercoiled topoisomers migrate more slowly under the conditions used). The H4-Y88G mutation reduced the negative supercoiling of HML DNA by a ΔLk of 2 (Fig. 2A, compare 2 with 1). This effect was specific to HML, since H4-Y88G did not reduce the negative supercoiling of the 2 μm plasmid (Fig. 2B, compare 7 with 6). It was clear that the level of negative supercoiling of HML DNA in the H4-Y88G mutant was between those in the wild type and sir3Δ strains (Fig. 2A, compare 2 with 1 and 5). This suggests that HML chromatin in the H4-Y88G mutant is in an intermediate state between fully silenced and derepressed structures. In other words, H4-Y88G alters, but does not dismantle, silent chromatin.
FIGURE 2.
HHO1 suppresses the alteration in silent chromatin structure induced by the H4-Y88G mutation. A, effects of H4-Y88G and hho1Δ single and double mutations on the supercoiling of HML DNA. The modified HML locus in strains 21–25 is illustrated at the top. Two FRTs bracket the HML locus, excluding the HML-E and -I silencers. Cells grown in YPR medium (1% yeast extract, 2% bacto-peptone, and 2% raffinose) were treated with galactose (2%) for 2.5 h to induce the expression of PGAL10-FLP1. Nucleic acids were isolated and fractionated on agarose gels supplemented with 13 μg/ml chloroquine. Under this condition, more negatively supercoiled circles migrate more slowly (39). After Southern blotting, the topoisomers of the HML circle were detected by an HML-specific probe. The nicked form of the HML circle is indicated by N. The profiles of topoisomers in lanes 1–5 were determined by using the NIH Image software and are shown at the bottom. The center of distribution of topoisomers in each lane is marked by an arrowhead. B, effects of H4-Y88G and hho1Δ single and double mutations on the supercoiling of the 2 μm plasmid. DNA from strains 1–4 grown in YPD (1% yeast extract, 2% bacto-peptone, and 2% glucose) was analyzed by agarose gel electrophoresis in the presence of chloroquine. The nicked and linear forms of the plasmid are indicated by N and L, respectively.
Linker histones in higher organisms modulate global chromatin structure, including the spacing of adjacent nucleosomes in chromosomes (7, 41). However, Hhop1 does not seem to possess this activity in wild type yeast cells (18). Consistently, we found that hho1Δ had no effect on the topology of the 2 μm plasmid (Fig. 2B, compare 8 with 6). hho1Δ also did not affect the supercoiling of HML DNA (Fig. 2A, compare 3 with 1), which is in line with the fact that hho1Δ per se does not affect transcriptional silencing (Fig. 1).
Since hho1Δ partially suppresses the silencing defect of histone H4 mutations (Fig. 1), we asked whether it could also suppress the reduction in HML supercoiling caused by H4-Y88G. We found that the level of negative supercoiling of HML in the H4-Y88G hho1Δ double mutant was higher (by a ΔLk of –1) than that in the H4-Y88G mutant but lower (by a ΔLk of 1) than that in the H4 wild type strain (Fig. 2B, compare 4 with 1 and 2). Therefore, hho1Δ partially suppresses the reduction in negative supercoiling caused by H4-Y88G, suggesting that HHO1 negatively regulates silent chromatin in the H4-Y88G mutant.
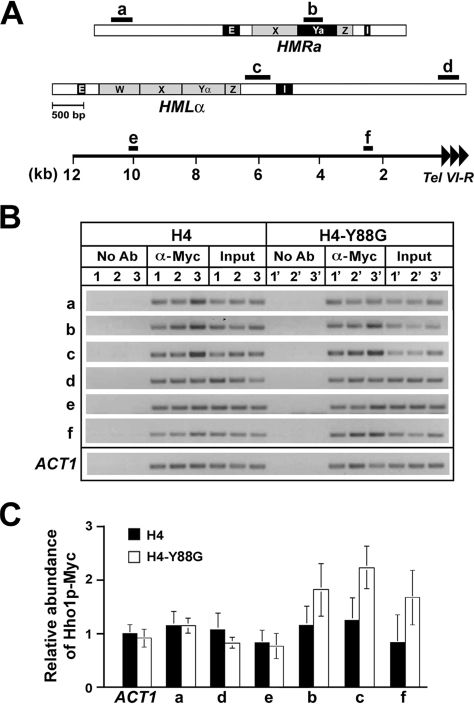
Hho1p Is Associated with both Silent Chromatin and Active Chromatin—Our finding that Hho1p plays an inhibitory role in silent chromatin led us to ask whether it is preferentially associated with silent chromatin. Hho1p has been shown to bind nucleosomes in vitro (18). Previous ChIP experiments showed that Hho1p is widely associated with the yeast genome (19, 20). However, the association of Hho1p with silent chromatin has not been systematically examined.
To measure the binding of Hho1p to silent loci using ChIP, we COOH-terminally tagged Hho1p with the Myc epitope. Cells bearing Myc-tagged Hho1p exhibit the same phenotypes as their untagged counterparts, and Hho1p-Myc is expressed as determined by Western blotting (data not shown). We performed ChIP to examine the association of Hho1p-Myc with sequences in silent and active loci using the anti-Myc antibody. Sequences b and c are within silent HML and HMR flanked by silencers (Fig. 3A). Sequence a is located 1.8 kb to the left of HMR, whereas d is 2.6 kb to the right of HML (Fig. 3A). Both a and d are outside of the silent HML and HMR domains (31, 42). Sequences e and f are 10 and 2.5 kb, respectively, from the right telomere of chromosome VI (Tel VI-R) (Fig. 3A). Silent chromatin is limited to a ∼3-kb domain immediately adjacent to Tel VI-R,so f but not e is within silent chromatin (43–45). The abundance of sequences a–f as well as a control sequence at the ACT1 locus in the immunoprecipitated chromatin fragments was measured by PCR. Three independent ChIP experiments were performed, and a gel picture of the PCR products was presented in Fig. 3B. The intensity of each band was quantified and normalized against input control. The relative abundance of Hho1p-Myc associated with a sequence was estimated as the ratio of the intensity of the corresponding band to that of the ACT1 control (taken as 1.0). The mean of data from all three experiments (together with the S.D.) was graphed in Fig. 3C. We found that the abundance of Hho1p-Myc in euchromatin regions a, d, and e as well as in the silent chromatin regions b, c, and f were all comparable with that at ACT1 (Fig. 3C, compare filled bars a–f with ACT1). Therefore, Hho1p is associated with both silent and active loci and is not specifically enriched in or excluded from silent chromatin.
FIGURE 3.
Association of Hho1p with silent and active chromatin regions. A, schematics of the HMR and HML loci and flanking sequences as well as the subtelomeric region of the right arm of chromosome VI. The HMR-E and -I silencers, HML-E and -I silencers, and the W, X, Ya, Yα, and Z elements at the HM loci are indicated. The locations of DNA segments a to f tested in ChIP are indicated as black bars. Tandem arrowheads, telomeric repeats. B, ChIP analysis of the abundance of Hho1p-Myc around HMR, HML, and Tel VI-R. The abundance of sequences a–f as well as a control sequence at the ACT1 locus in the immunoprecipitated chromatin fragments was measured by PCR before (Input) and after (α-Myc) chromatin IP. No Ab, samples from mock IP without antibody. Three independent ChIP experiments (designated 1–3 for the H4 strain 26, and 1′–3′ for the H4-Y88G strain 27) were performed. C, quantification of the ChIP data shown in A. See “Results” for a description.
Given the roles of H4 Tyr-88 in the interaction between the H3/H4 tetramer and H2A/H2B dimers, it is possible that the H4-Y88G mutation alters the conformation of the nucleosome and affects the interaction between nucleosomes and Hho1p. We examined the association of Hho1p with chromatin in the H4-Y88G mutant. As shown in Fig. 3, B and C, H4-Y88G did not significantly affect the association of Hho1p with active chromatin (compare open and filled bars at ACT1; a, d, and e). H4-Y88G seemed to increased Hho1p abundance at silent chromatin by up to 2-fold (compare open and filled bars at b, c, and f). The difference in Hho1p abundance at sequence c between wild type H4 and H4-Y88G was statistically significant, since the p value (calculated using Student's t test) was <0.04. On the other hand, the p values for data at b and f were <0.15 and <0.06, respectively. Therefore, the difference between H4 and H4-Y88G in Hho1p abundance detected at b and f might not be statistically significant. We conclude that H4-Y88G does not enhance the association of Hho1p with active chromatin but increases Hho1p abundance at least in silent chromatin at HML, which is correlated with reduced transcriptional silencing at HML in the H4-Y88G mutant, as shown in Fig. 1.
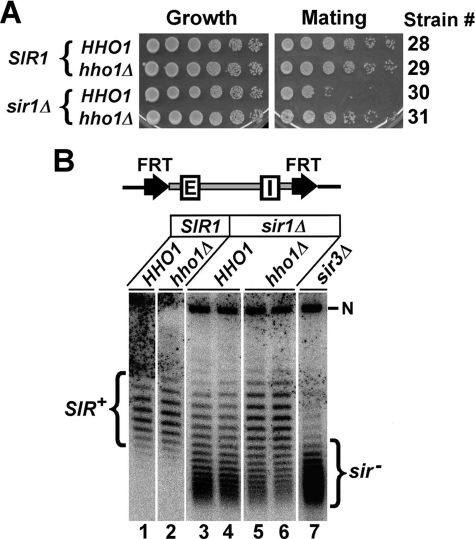
HHO1 Deletion Suppresses the Silencing Defect of sir1Δ—The above results suggest that Hho1p is inhibitory to transcriptional silencing, which becomes manifest when silent chromatin is compromised by mutations in histone H4. We wanted to identify other conditions that could also reveal the negative role of HHO1 in silencing. To this end, we tested if hho1Δ suppresses the effect of sir1Δ on HML silencing. SIR1 deletion reduces HML silencing, as evidenced by the decrease in the mating efficiency of MATa sir1Δ cells (Fig. 4A, compare 30 with 28; note that the mating efficiency of a MATa strain is inversely proportional to the expression state of HML). We showed that hho1Δ partially reversed the decrease in HML silencing caused by sir1Δ (Fig. 4A, compare 31 with 30).
FIGURE 4.
HHO1 deletion suppresses the silencing defect caused by sir1Δ. A, shown are growth phenotypes of MATa strains 28–31 on SC medium (Growth) and synthetic minimum medium lacking amino acids coated with cells of the MATα tester strain DC17 (MATα his1)(Mating). Only diploid cells resulting from the mating of the MATa and MATα cells can growth on the Mating plate. The relevant genotype of each strain is indicated on the left, and strain number is on the right. B, effects of sir1Δ and hho1Δ single and double mutations on the supercoiling of HML DNA. The modified HML locus in strains 32–36 is illustrated at the top. Two FRTs bracket the HML locus, including the HML-E and -I silencers. DNA samples isolated after the induction of the excision of the HML circle were analyzed by agarose gel electrophoresis in the presence of 13 μg/ml chloroquine. The nicked form of the HML circle is indicated by N. The topoisomers of HML from wild type and sir3Δ strains are designated SIR+ and sir–, respectively.
Unlike Sir2p, Sir3p, and Sir4p, which form the Sir complex as an integral part of silent chromatin, Sir1p is only involved in the initiation stage of the formation of silent chromatin. Whereas HML is completely derepressed in all cells in a sir2Δ, sir3Δ, or sir4Δ culture, a sir1Δ culture consists of two populations of mitotically stable cells with HML being silenced in one population but derepressed in the other (26, 27). Consistently, we found that the topoisomers of the HML circle from a sir1Δ culture consisted of a mixture of topoisomers resembling those from SIR+ cells where HML was silenced and those from sir– cells where HML was derepressed (Fig. 4B, compare lanes 3 and 4 with lanes 1 and 7). Deletion of HHO1 significantly increased the proportion of sir1Δ cells harboring silenced HML (Fig. 4B, compare 5 and 6 with 3 and 4). These results suggest that hho1Δ partially suppresses the defect in the formation of silent chromatin caused by sir1Δ.
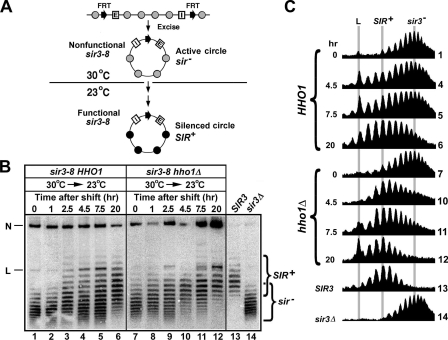
HHO1 Negatively Regulates the de Novo Establishment of Silent Chromatin—Based on the functional interaction between HHO1 and SIR1 described above and the role of SIR1 in the initiation of silencing, we hypothesize that HHO1 regulates the de novo establishment of silent chromatin. We tested this model by monitoring the silencing state of a silencer-bearing HML circle (that is initially derepressed) following the activation of the silencing apparatus in a strain bearing sir3-8, a temperature-sensitive allele of SIR3 (Fig. 5A). sir3-8 is nonfunctional at 30 °C but functional at 23 °C (46). HML circles excised from sir3-8 cells growing at 30 °C have active chromatin and reduced negative supercoiling as found in a sir3– strain (32). Subsequent incubation of the cells at 23 °C leads to the gradual acquisition of circles with silent chromatin and higher negative supercoiling, as found in SIR+ cells (32). This is because the silencers in the HML circle promote de novo establishment of silent chromatin (32).
FIGURE 5.
HHO1 negatively regulates the de novo establishment of transcriptionally silent chromatin. A, strategy for examining the de novo establishment of silent chromatin on the HML circle. Shaded and filled circles denote nucleosomes in active chromatin and silent chromatin, respectively. See“Results” for a description. B, examination of the kinetics of establishment of silent chromatin on the HML circle in strains 37 (sir3-8 HHO1) and 38 (sir3-8 hho1Δ). Cells of each strain grown at 30 °C in YPR medium were shifted to galactose medium and incubated for 2.5 h to induce excision of the HML circle. Cells were then shifted to YPD medium and incubated at 23 °C for up to 20 h. Aliquots of the culture were harvested at time points 0, 1, 2.5, 4.5, 7.5, and 20 h. DNA was isolated from all samples and fractionated by agarose gel electrophoresis in the presence of 13 μg/ml chloroquine. N and L, nicked and linear forms of the HML circle, respectively. The topoisomers in the SIR3 wild type strain 32 and sir3Δ strain 36 are designated SIR+ and sir–, respectively. C, the profiles of topoisomers in lanes 1, 4–6, 7, and 10–14 were determined by NIH Image. The center of distribution of topoisomers in lanes 13 (SIR+) and 14 (sir–) as well as the position of the linear form of the circle (L) are marked by shaded lines.
A pair of isogenic sir3-8 HHO1 and sir3-8 hho1Δ strains were constructed in which HML, including its silencers, was flanked by FRTs (Fig. 5A). Each strain was first grown to late log phase in noninducing YPR medium at 30 °C. Galactose was then added to the culture, which was further incubated for 2.5 h to induce PGAL-FLP1 and the excision of the HML circle. Cells were then shifted to YPD and incubated at 23 °C. The short half-life of Flp1p and the stringent repression by glucose of PGAL-FLP1 ensured that circles were excised during, and only during, the 2.5 h of galactose induction (39, 47, 48). Aliquots of the culture were taken at various time points for DNA isolation. For the sir3-8 HHO1 strain, the HML circle isolated before the shift from 30 to 23 °C assumed a topology characteristic of HML circles from a sir– strain (Fig. 5, B and C, compare 1 and 14). As the incubation time at 23 °C increased, the proportion of topoisomers of the HML circle resembling those in SIR+ cells increased (Fig. 5, B and C, compare 2–6 with 13), indicating that silent chromatin was established on more and more HML circles in the culture. Deletion of HHO1 increased the rate of the accumulation of SIR+ topoisomers and the concomitant decrease of the proportion of sir– topoisomers (Fig. 5, B and C, compare 8–12 with 2–6). For example, after 7.5 h of incubation at 23 °C, the population of SIR+ topoisomers was markedly larger than that of sir– ones in the hho1Δ strain, whereas the proportion of SIR+ topoisomers in the HHO1 strain was equal to or less than that of sir– topoisomers (Fig. 5, B and C, compare lanes 11 and 5). Therefore, we conclude that HHO1 negatively regulates de novo establishment of silent chromatin.
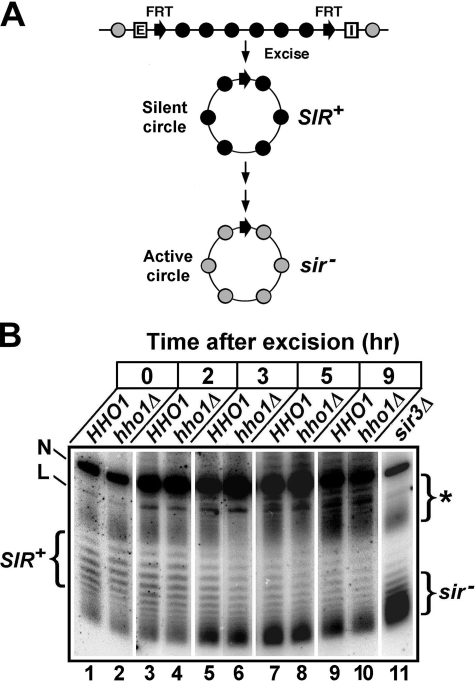
HHO1 Does Not Affect the Stability of Preexistent Silent Chromatin—We also investigated if HHO1 regulates the stability of silent chromatin. We have previously shown that an HML circle excised without silencers would gradually lose its silent state as the host cell progresses through the cell cycle (Fig. 6A) (39). This is probably the result of cell cycle-dependent events that disrupt silent chromatin. We examined if hho1Δ had any effect on the kinetics of this process. Strain 21 with its HML, excluding its silencers, bracketed by FRTs and its hho1Δ derivative (strain 23) was first grown to log phase and then treated with galactose for 2.5 h to induce the excision of the HML circle. During this 2.5-h incubation, in which cells continued to grow, a small portion of the circles had already lost their silenced state, as evidenced by the appearance of some topoisomers that resembled those in the sir3Δ strain (Fig. 6B, compare lanes 1 and 2 with lane 11). The cells were then shifted to YPD to repress PGAL-FLP1, and the fate of the HML circles was followed during subsequent cell growth. In HHO1 cells, as expected, the proportion of topoisomers with high negative supercoiling (SIR+) characteristic of silent chromatin gradually decreased, whereas the proportion of topoisomers with lower negative supercoiling (sir–) characteristic of active chromatin increased (Fig. 6B, lanes 1, 3, 5, 7, and 9). This conversion of the HML circles form the SIR+ to the sir– state was not significantly affected by hho1Δ (Fig. 6B, compare lanes 2, 4, 6, 8, and 10 with 1, 3, 5, 7, and 9, respectively). Therefore, HHO1 does not affect the stability of preexistent silent chromatin.
FIGURE 6.
HHO1 does not affect the stability of preexistent silent chromatin. A, strategy for examining the stability of silent chromatin on silencer-free HML circles. Shaded and filled circles denote nucleosomes in active chromatin and silent chromatin, respectively. See “Results” for a description. B, examination of the kinetics of the conversion of HML silent chromatin to active chromatin after its excision from the genome in strains 21 (HHO1) and 23 (hho1Δ). Cells of each strain grown to log phase in YPR were treated with galactose for 2.5 h to induce excision of the HML circle. Cells were then shifted and diluted into YPD medium and incubated for 9 h. Aliquots of the culture were harvested at time points 0, 2, 3, 5, and 9 h. DNA was isolated from the samples and fractionated by agarose gel electrophoresis in the presence of chloroquine. N and L, nicked and linear forms of the HML circle, respectively. Topoisomers of the HML circle in the sir3Δ strain 31 are designated sir–. The asterisk indicates cross-hybridizing DNA.
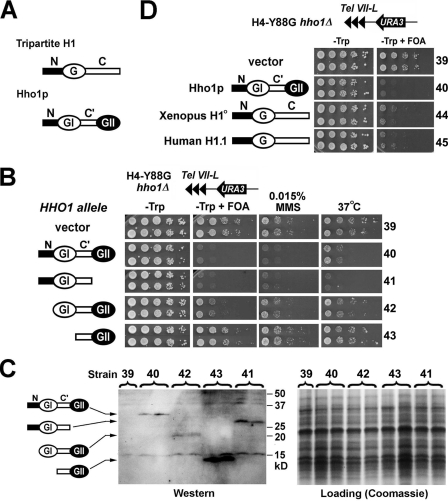
Hho1p Functionally Resembles Tripartite Linker Histones—Unlike the tripartite linker histones in higher organisms, Hho1p contains two globular domains (GI and GII) separated by a sequence (designated C′) that is a shorter version of the COOH-terminal domain of a tripartite linker histone (Fig. 7A). We examined which domain(s) of Hho1p is important for its function. We made CEN-ARS plasmids carrying full-length and truncated alleles of HHO1 flanked by its endogenous upstream and downstream regulatory sequences (Fig. 7B, left). These plasmids were transformed into the H4-Y88G hho1Δ strain 4, in which URA3 silencing near Tel VII-L is subject to inhibition by HHO1 (Fig. 1). Western blotting analysis confirmed that the Hho1p variants encoded by the plasmids were expressed (Fig. 7C).
FIGURE 7.
Hho1p functionally resembles tripartite linker histones in metazoans. A, schematics of the structures of tripartite linker histone H1 and yeast Hho1p. N, G, and C, the NH2-terminal, globular, and COOH-terminal domains, respectively. C′, the sequence between GI and GII domains of Hho1p. B, GII of Hho1p is dispensable for Hho1p function. Shown are growth phenotypes of strains 39–43 under different conditions. The schematic of the HHO1 allele in each strain is shown on the left. C, Western blotting analysis of full-length and truncated Hho1p proteins. Protein extracts from strains 45–49 were run on SDS-PAGE and strained with Coomassie Blue (right) or probed with an anti-Hho1p antiserum (left). The antiserum was raised against both a peptide from the N domain of Hho1p and a peptide from GII and is therefore able to detect both NH2- and COOH-terminally truncated Hho1p proteins (28). D, Xenopus linker histone H1° and human H1.1 ectopically expressed in yeast have effects on silencing similar to those of Hho1p. Shown are growth phenotypes of strains 39, 40, 44, and 45 on –Trp and –Trp+FOA media. The silencing reporter construct in these strains is illustrated at the top.
Consistent with data shown in Fig. 1, URA3 silencing in the H4-Y88G hho1Δ strain bearing the empty vector was robust (Fig. 7B, growth of strain 39 on –Trp+FOA). Full-length HHO1 abolished URA3 silencing (Fig. 7B, lack of growth of strain 40 on –Trp+FOA), confirming that Hho1p inhibits silencing in the H4-Y88G mutant (Fig. 1). Deletion of GII had no effect on the ability of Hho1p to inhibit silencing (Fig. 7B, compare 41 with 40). Deleting the NH2-terminal tail of Hho1p (N) moderately affected its negative role in silencing (Fig. 7B, compare 42 with 40). Deleting the NH2-terminal tail plus GI abolished the effect of Hho1p on silencing (Fig. 7B, compare 43 with 39 and 40). These results suggest that GII is dispensable for the role of Hho1p in inhibiting silencing in the H4-Y88G mutant. Moreover, we showed that GII was also dispensable for the roles of Hho1p in conferring MMS sensitivity and Ts– to the H4-Y88G mutant (Fig. 7B, MMS and 37 °C panels). Therefore, GII is not required for the Hho1p functions examined here.
Since Hho1p lacking GII structurally resembles tripartite linker histones and retains the functions of intact Hho1p, it is possible that the mode of Hho1p function is similar to that of tripartite linker histones. To test this hypothesis, we examined if canonical tripartite linker histones ectopically expressed in yeast could complement hho1Δ. We made CEN-ARS plasmids carrying Xenopus H1° and human H1.1 ORFs flanked by endogenous upstream and downstream sequences of HHO1 (Fig. 7D, left) and transformed them into the H4-Y88G hho1Δ strain 4. It was clear that, like HHO1, Xenopus H1° and human H1.1 (controlled by the HHO1 promoter) were also able to inhibit transcriptional silencing in the H4-88G mutant (Fig. 7D, compare 44 and 45 with 39 and 40).
DISCUSSION
Linker histones are abundant nucleosome-binding proteins that are believed to help stabilize higher order chromatin structures and inhibit DNA-dependent activities, but the mechanism of their functions has not been well defined. S. cerevisiae linker histone Hho1p is not required for normal cell growth, and it affects the expression of only a small subset of genes (22). To gain insights into Hho1p function, we examined possible genetic interactions between HHO1 and the core histone genes. We found that hho1Δ suppresses the phenotypes of mutations in the globular domain of histone H4. Tyr-88 of histone H4 is involved in hydrophobic interactions between the H3/H4 tetramer and H2A/H2B dimers (1, 29). The H4-Y88G mutation renders cells temperature-sensitive and sensitive to DNA damage and disrupts transcriptional silencing (Fig. 1). All of these phenotypes are partially suppressed by hho1Δ (Fig. 1), suggesting that Hho1p negatively regulates cellular functions, which is normally counteracted by intact histone H4. The genetic interaction between HHO1 and histone H4 supports the notion that Hho1p functions by regulating chromatin structure.
HHO1 does not affect transcriptional silencing under normal conditions (14, 18, 21, 23). However, increasing HHO1 dosage inhibits silencing (Fig. 1) (28), suggesting that Hho1p has the ability to negatively regulate silencing, which is possibly offset by intact silent chromatin. In line with this notion, we showed that the inhibitory role of Hho1p in silencing became manifest when silent chromatin was compromised by the histone H4-Y88G mutation or sir1Δ (Figs. 1 and 4). It is unlikely that Hho1p regulates silencing indirectly by affecting the expression of genes encoding silencing factors, since hho1Δ has no effect on the expression of these genes (Fig. 1B) (22). We think Hho1p as a chromatin-binding protein is likely to affect silent chromatin directly. This is consistent with the fact that hho1Δ partially reverses the change in silent chromatin induced by the H4-Y88G mutation (Fig. 2), suggesting that HHO1 plays an inhibitory role in the formation and/or maintenance of silent chromatin.
Like linker histones in higher eukaryotes, Hho1p is abundant and widely associated with the yeast genome (19, 20). We found that Hho1p is not specifically enriched in or excluded from silent chromatin under normal conditions, which correlates with its lack of a role in silencing (Fig. 1 and 3). On the other hand, in the H4-Y88G mutant, Hho1p abundance is specifically increased at least within silent chromatin at HML, and silencing is reduced (Figs. 1 and 3). It is possible that disruption of H3/H4 tetramer-H2A/H2B dimer interaction by H4-Y88G changes the conformation of the nucleosome, thereby increasing its affinity for Hho1p across the genome. This notion seems contrary to the fact that the level of Hho1p associated with euchromatin is not affected by H4-Y88G (Fig. 3). However, we think it is the combination of H4-Y88G-induced changes in nucleosome conformation and the unique features of silent chromatin (e.g. histone hypoacetylation and hypomethylation) that specifically enhances the association of Hho1p with silent chromatin. A high Hho1p abundance might alter the compaction of silent chromatin and/or affect the proper function of the Sir complex, thereby abrogating transcriptional silencing.
Sir1p functions in the initiation of the formation of silent chromatin at HM loci by bridging the silencer-binding ORC complex and the Sir complex (49). A sir1Δ culture consists of a mixture of two discrete populations of cells, one with derepressed and the other with silenced HML (26, 27). We found that hho1Δ increases the proportion of sir1Δ cells with silenced HML (Fig. 4). This suggests that Hho1p has an inhibitory effect on the establishment of silent chromatin. In support of this notion, we showed that hho1Δ accelerated the de novo establishment of silent chromatin on a silencer-containing HML circle (Fig. 5). It is possible that an Hho1p-bound nucleosome is a less favorable substrate for the Sir2p histone deacetylase and/or has reduced affinity for the Sir complex, thereby hindering the propagation of silent chromatin. Alternatively, or in addition, Hho1p may act to inhibit the silencer from recruiting the Sir complex through Rap1p and/or Abf1p, the other silencer-binding proteins. Along this line, hho1Δ may suppress the silencing defect of a sir1Δ strain by allowing Rap1p and/or Abf1p to work more efficiently.
Given that Hho1p negatively regulates the de novo establishment of silent chromatin, we think the reason why Hho1p does not affect silencing under normal conditions is that the maintenance of silent chromatin structure usually does not require its de novo establishment. This is based on the proposed robustness/resilience of silenced chromatin (24). One could imagine that an accidental loss of Sir complexes from one or a few nucleosomes in silent chromatin can be readily remedied by Sir complexes still bound to neighboring nucleosomes because they can recruit more Sir complexes. This has been used to explain the epigenetic inheritance of silent chromatin during DNA replication, in which the “old” nucleosomes in silent chromatin on the parental stand are distributed to the daughter stands and modify and help recruit Sir complexes to newly deposited nucleosomes (24). We think the robustness/resilience of silent chromatin maintained by the intact silencing machinery normally offsets the inhibitory effect of Hho1p. In accord with this, we showed that HHO1 does not affect the stability of preexistent silent chromatin. However, when the silencing machinery is compromised (e.g. by histone mutations or sir1Δ), stochastic damages to silent chromatin may lead to large scale disruptions that can only be repaired by de novo establishment of silent chromatin, which is subject to inhibition by HHO1.
Hho1p has a unique structure with two conserved globular domains GI and GII. We showed that GII is dispensable for the functions of Hho1p (Fig. 7). Since Hho1p deleted for GII is similar to a tripartite linker histone, this suggests that the mode of function of Hho1p is similar to that of tripartite linker histones. In fact, under physiological salt conditions, only GI is stably folded in vitro (15–17), indicating that Hhop1 may assume a tripartite structure in vivo with GII serving as part of the COOH-terminal unstructured domain. We further showed that both Xenopus histone H1° and human H1.1 introduced into yeast can complement hho1Δ (Fig. 7), suggesting that Hho1p and metazoan linker histones are functionally conserved.
Acknowledgments
We thank Qiong Liu for assistance and Drs. M. Mitchell Smith, Maria Santisteban, James Broach, and Jeff Hayes for plasmids and strains.
This work was supported, in whole or in part, by National Institutes of Health Grant GM62484 (to X. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ORC, origin recognition complex; ORF, open reading frame; ChIP, chromatin immunoprecipitation; MMS, methyl methanesulfonate; FRT, Flp1p recombination target; RT, reverse transcription; IP, immunoprecipitation; FOA, 5-fluoroorotic acid; SC, synthetic complete.
References
- 1.Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F., and Richmond, T. J. (1997) Nature 389 251–260 [DOI] [PubMed] [Google Scholar]
- 2.Woodcock, C. L., Skoultchi, A. I., and Fan, Y. (2006) Chromosome Res. 14 17–25 [DOI] [PubMed] [Google Scholar]
- 3.Robinson, P. J., and Rhodes, D. (2006) Curr. Opin. Struct. Biol. 16 336–343 [DOI] [PubMed] [Google Scholar]
- 4.Hannon, R., Bateman, E., Allan, J., Harborne, N., and Gould, H. (1984) J. Mol. Biol. 180 131–149 [DOI] [PubMed] [Google Scholar]
- 5.Shimamura, A., Sapp, M., Rodriguez-Campos, A., and Worcel, A. (1989) Mol. Cell Biol. 9 5573–5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laybourn, P. J., and Kadonaga, J. T. (1991) Science 254 238–245 [DOI] [PubMed] [Google Scholar]
- 7.Fan, Y., Nikitina, T., Zhao, J., Fleury, T. J., Bhattacharyya, R., Bouhassira, E. E., Stein, C. L., Woodcock, C. L., and Skoultchi, A. I. (2005) Cell 123 1199–1212 [DOI] [PubMed] [Google Scholar]
- 8.Shen, X., Yu, L., Weir, J. W., and Gorovsky, M. A. (1995) Cell 82 47–56 [DOI] [PubMed] [Google Scholar]
- 9.Shen, X., and Gorovsky, M. A. (1996) Cell 86 475–483 [DOI] [PubMed] [Google Scholar]
- 10.Fan, Y., Nikitina, T., Morin-Kensicki, E. M., Zhao, J., Magnuson, T. R., Woodcock, C. L., and Skoultchi, A. I. (2003) Mol. Cell Biol. 23 4559–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey, A. C., and Downs, J. A. (2004) Mol. Microbiol. 53 771–775 [DOI] [PubMed] [Google Scholar]
- 12.Kasinsky, H. E., Lewis, J. D., Dacks J. B., and Ausio, J. (2001) FASEB J. 15 34–42 [DOI] [PubMed] [Google Scholar]
- 13.Landsman, D. (1996) Trends Biochem. Sci. 21 287–288 [PubMed] [Google Scholar]
- 14.Ushinsky, S. C., Bussey, H., Ahmed, A. A., Wang, Y., Friesen, J., Williams, B. A., and Storms, R. K. (1997) Yeast 13 151–161 [DOI] [PubMed] [Google Scholar]
- 15.Ono, K., Kusano, O., Shimotakahara, S., Shimizu, M., Yamazaki, T., and Shindo, H. (2003) Nucleic Acids Res. 31 7199–7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali, T., Coles, P., Stevens, T. J., Stott, K., and Thomas, J. O. (2004) J. Mol. Biol. 338 139–148 [DOI] [PubMed] [Google Scholar]
- 17.Ali, T., and Thomas, J. O. (2004) J. Mol. Biol. 337 1123–1135 [DOI] [PubMed] [Google Scholar]
- 18.Patterton, H. G., Landel, C. C., Landsman, D., Peterson, C. L., and Simpson, R. T. (1998) J. Biol. Chem. 273 7268–7276 [DOI] [PubMed] [Google Scholar]
- 19.Freidkin, I., and Katcoff, D. J. (2001) Nucleic Acids Res. 29 4043–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downs, J. A., Kosmidou, E., Morgan, A., and Jackson, S. P. (2003) Mol. Cell 11 1685–1692 [DOI] [PubMed] [Google Scholar]
- 21.Escher, D., and Schaffner, W. (1997) Mol. Gen. Genet. 256 456–461 [DOI] [PubMed] [Google Scholar]
- 22.Hellauer, K., and Turcotte, B. (2001) J. Biol. Chem. 276 13587–13592 [DOI] [PubMed] [Google Scholar]
- 23.Li, C., Mueller, J. E., Elfline, M., and Bryk, M. (2008) Mol. Microbiol. 67 906–919 [DOI] [PubMed] [Google Scholar]
- 24.Rusche, L. N., Kirchmaier, A. L., and Rine, J. (2003) Annu. Rev. Biochem. 72 481–516 [DOI] [PubMed] [Google Scholar]
- 25.Moazed, D. (2001) Curr. Opin. Cell Biol. 13 232–238 [DOI] [PubMed] [Google Scholar]
- 26.Pillus, L., and Rine, J. (1989) Cell 59 637–647 [DOI] [PubMed] [Google Scholar]
- 27.Xu, E. Y., Zawadzki, K. A., and Broach, J. R. (2006) Mol. Cell 23 219–229 [DOI] [PubMed] [Google Scholar]
- 28.Veron, M., Zou, Y., Yu, Q., Bi, X., Selmi, A., Gilson, E., and Defossez, P. A. (2006) Genetics 173 579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santisteban, M. S., Arents, G., Moudrianakis, E. N., and Smith, M. M. (1997) EMBO J. 16 2493–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bi, X., Yu, Q., Sandmeier, J. J., and Zou, Y. (2004) Mol. Cell Biol. 24 2118–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou, Y., Yu, Q., Chiu, Y.-H., and Bi, X. (2006) Genetics 174 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu, E. Y., Bi, X., Holland, M. J., Gottschling, D. E., and Broach, J. R. (2005) Mol. Cell Biol. 25 1846–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi, X., Yu, Q., Sandmeier, J. J., and Elizondo, S. (2004) J. Mol. Biol. 344 893–905 [DOI] [PubMed] [Google Scholar]
- 34.Zou, Y., and Bi, X. (2008) Nucleic Acids Res. 36 5189–5200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu, Y. H., Yu, Q., Sandmeier, J. J., and Bi, X. (2003) Genetics 165 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Leeuwen, F., and Gottschling, D. E. (2002) Methods Enzymol. 350 165–186 [DOI] [PubMed] [Google Scholar]
- 37.Germond, J. E., Hirt, B., Oudet, P., Gross-Belard, M., and Chambon, P. (1975) Proc. Natl. Acad. Sci. U. S. A. 72 1842–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson, R. T., Thoma, F., and Brubaker, J. M. (1985) Cell 42 799–808 [DOI] [PubMed] [Google Scholar]
- 39.Bi, X., and Broach, J. R. (1997) Mol. Cell Biol. 17 7077–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng, T. H., Li, Y. C., and Gartenberg, M. R. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 5521–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Campos, A., Shimamura, A., and Worcel, A. (1989) J. Mol. Biol. 209 135–250 [DOI] [PubMed] [Google Scholar]
- 42.Donze, D., Adams, C. R., Rine, J., and Kamakaka, R. T. (1999) Genes Dev. 13 698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hecht, A., Strahl-Bolsinger, S., and Grunstein, M. (1996) Nature 383 92–96 [DOI] [PubMed] [Google Scholar]
- 44.Lieb, J. D., Liu, X., Botstein, D., and Brown, P. O. (2001) Nat. Genet. 28 327–334 [DOI] [PubMed] [Google Scholar]
- 45.Xu, F., Zhang, Q., Zhang, K., Xie, W., and Grunstein, M. (2007) Mol. Cell 27 890–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller, A., and Nasymth, K. (1984) Nature 312 247–251 [DOI] [PubMed] [Google Scholar]
- 47.Volkert, F. C., and Broach, J. R. (1986) Cell 46 541–550 [DOI] [PubMed] [Google Scholar]
- 48.Holmes, S. G., and Broach, J. R. (1996) Genes Dev. 10 1021–1032 [DOI] [PubMed] [Google Scholar]
- 49.Triolo, T., and Sternglanz, R. (1996) Nature 381 251–253 [DOI] [PubMed] [Google Scholar]