Abstract
Multidrug and toxic compound extrusion (MATE) transporters represent a large family in plants, but their functions are poorly understood. Here, we report the function of a rice (Oryza sativa) MATE gene (Os03g0216700, OsFRDL1), the closest homolog of barley (Hordeum vulgare) HvAACT1 (aluminum [Al]-activated citrate transporter 1), in terms of metal stress (iron [Fe] deficiency and Al toxicity). This gene was mainly expressed in the roots and the expression level was not affected by either Fe deficiency or Al toxicity. Knockout of this gene resulted in leaf chlorosis, lower leaf Fe concentration, higher accumulation of zinc and manganese concentration in the leaves, and precipitation of Fe in the root's stele. The concentration of citrate and ferric iron in the xylem sap was lower in the knockout line compared to the wild-type rice. Heterologous expression of OsFRDL1 in Xenopus oocytes showed transport activity for citrate. Immunostaining showed that OsFRDL1 was localized at the pericycle cells of the roots. On the other hand, there was no difference in the Al-induced secretion of citrate from the roots between the knockout line and the wild-type rice. Taken together, our results indicate that OsFRDL1 is a citrate transporter localized at the pericycle cells, which is necessary for efficient translocation of Fe to the shoot as a Fe-citrate complex.
Multidrug and toxic compound extrusion (MATE) proteins are widely present in bacteria, fungi, plants, and mammals (Omote et al., 2007). These proteins are characterized by having 400 to 700 amino acids with 12 transmembrane helices. Although no apparent consensus sequence is conserved in all MATE proteins, they share approximately 40% sequence similarity. MATE protein was first identified as a multidrug transporter for norfloxacin and ethidium bromide in Vibrio parahaemolyticus and Escherichia coli (Morita et al., 1998), but subsequent studies have shown that the MATE proteins transport various organic substrates, such as alkaloids, berberine, and acriflavine (Omote et al., 2007). Therefore, MATE-type transporters are proposed to function as fundamental molecular machinery for the excretion of metabolic and xenobiotic organic compounds from the body.
Plants have a higher diversity of MATE-type transporters than bacteria and animals (Omote et al., 2007). For example, in Arabidopsis (Arabidopsis thaliana), there are 58 MATE orthologs (Yazaki, 2005). In the rice (Oryza sativa) genome, there are at least 40 MATE genes based on our search from the database (http://cdna01.dna.affrc.go.jp/cDNA). However, only a few of them have been functionally characterized. Previous studies have shown that MATE proteins in plants have various functions. For example, an Arabidopsis MATE protein TT12, which is expressed specifically in ovules and developing seeds, is suggested to be involved in the vacuolar sequestration of flavonoids in the seed coat endothelium (Debeaujon et al., 2001). Furthermore, TT12 was reported to act as a vacuolar flavonoid/H+-antiporter (Marinova et al., 2007). ALF5, another member of the Arabidopsis MATE gene family, is expressed strongly in the root epidermis and is required for protection of the roots from inhibitory compounds, including polyvinylpyrrolidone or pyrrolidone (Diener et al., 2001). ALF5 has been proposed to play a direct role in either the vacuolar sequestration or the cellular efflux of these toxins. AtDTX1, which was identified from the Arabidopsis cDNA library using a bacterial mutant defective in multidrug resistance, is localized on the plasma membrane of the roots (Li et al., 2002). It serves as an efflux carrier for plant-derived alkaloids, antibiotics, and other toxic compounds. It is also capable of detoxifying cadmium (Li et al., 2002). The involvement of a MATE protein in vacuolar transport of the endogenous alkaloid berberine has also been reported in Coptis japonica (Otani et al., 2005).
Recently, three studies have reported that some MATE proteins are involved in the transport of citrate, which is required for iron (Fe) translocation or aluminum (Al) detoxification. FRD3 from Arabidopsis has been demonstrated to be a citrate transporter, which is required for Fe translocation from the roots to the shoots (Durrett et al., 2007). It is localized at the pericycle and cells internal to the pericycle cells in roots (Green and Rogers, 2004). Defects in this transporter resulted in the precipitation of Fe in the root vasculature (Green and Rogers, 2004; Durrett et al., 2007). On the other hand, two studies have shown that Al-induced secretion of citrate in barley (Hordeum vulgare) and sorghum (Sorghum bicolor) is mediated through MATE transporters (Furukawa et al., 2007; Magalhaes et al., 2007). Secretion of citrate from the roots is a mechanism of Al resistance in a number of plant species (Ma et al., 2001; Kochian et al., 2005; Delhaize et al., 2007). Furukawa et al. (2007) identified a gene, HvAACT1, in barley and found that it is responsible for the Al-induced secretion of citrate. The HvAACT1 is localized on the plasma membrane of epidermal cells of the roots and citrate efflux through this transporter is activated by Al. In sorghum, a similar gene SbMATE was identified (Magalhaes et al., 2007). Functional analysis of SbMATE showed that it is also a citrate transporter, which is required for Al resistance. In the rice genome, there are six close homolog genes of AtFRD3, HvAACT1, and SbMATE (Fig. 1); however, the function of all these genes is still unknown. In this study, we performed a functional analysis of OsFRDL1, the closest homolog of HvAATC1 in rice. However, our results demonstrated that OsFRDL1 is not involved in the Al-induced secretion of citrate like HvAACT1, but in the efficient translocation of Fe in the xylem.
Figure 1.
Phylogenetic relationship of OsFRDL1-like proteins in different plant species. The amino acid sequences were aligned by ClustalW.
RESULTS
Phenotype of OsFRDL1 Knockout Line
OsFRDL1 (Os03g0216700) was initially isolated as a homolog of AtFRD3 (Fig. 1; Inoue et al., 2004). However, its function and role in rice has not been known. In this study, to examine the function and role of OsFRDL1 in rice, we obtained two independent Tos-17 insertion lines; ND8025 (ND) and NC2637 (NC) for this gene. Tos-17 was inserted in the twelfth exon of OsFRDL1 in ND, but in the tenth intron in NC (Fig. 2A). The full-length transcript of OsFRDL1 was not detected in the homozygous ND line, but a small amount was found in the homozygous NC line (Fig. 2B), indicating that OsFRDL1 was knocked out in the ND line, but knocked down in the NC line.
Figure 2.
Tos-17 insertion lines of OsFRDL1. A, Scheme of the Tos-17 integration sites in ND8025 and NC2637. The location of the Tos-17 insertions is indicated by triangles. B, Expression of OsFRDL1 mRNA in the roots of wild-type rice (WT), ND8025, and NC2637. The expression was examined by RT-PCR.
Because AtFRD3 has been implicated in the Fe translocation in Arabidopsis (Green and Rogers, 2004), we first investigated the involvement of OsFRDL1 in Fe nutrition by growing the plants at low and high Fe concentrations. When the wild-type rice and two Tos-17 insertion lines were grown at 10 μm Fe (as FeSO4), no visible difference was observed among the three lines (Fig. 3A). However, at 0.2 μm Fe, chlorosis was observed in the newly expanded leaves of two Tos-17 lines, but not in the wild type (Fig. 3B).
Figure 3.
Phenotype of Tos-17 insertion lines of OsFRDL1. Three lines, including wild-type rice (WT) and two Tos-17 insertion lines (ND and NC), were grown in a nutrient solution containing 10 μm (A) or 0.2 μm (B) FeSO4. Ferric accumulation in the roots was examined with Perls blue staining in the roots of WT (C and F), ND (D and G), and NC (E and H) grown in 10 μm FeSO4. Red arrow shows ferric precipitation in the stele of a lateral root. Bars = 100 μm.
The Fe3+ precipitation in the roots was investigated at 10 μm Fe with Perls blue staining (Green and Rogers, 2004). Fe precipitation was observed in the epidermal cells of the roots of all three lines (Fig. 3, C–E), which was not observed in Arabidopsis roots (Green and Rogers, 2004). This distinct precipitation in rice is attributed to secreted oxygen from rice roots, which oxidizes ferrous iron (Fe2+) into insoluble ferric iron (Fe3+) on the root surface (Horiguchi, 1995). However, there was no difference in the epidermal staining among three lines (Fig. 3, C–E). In contrast, heavy staining was observed in the central vascular part of the knockout line, ND (Fig. 3, D and G), but not in the wild-type line (Fig. 3, C and F). In the knockdown line NC, staining was also observed in the central vascular part (Fig. 3, E and H), although the intensity was not as strong as that of ND.
We further investigated the link between Fe accumulation detected with Perls blue staining in the root stele and Tos-17 insertion using heterozygous progeny of ND. Genotyping analysis with specific primers in 50 seedlings showed that wild-type, heterozygotes, and homozygotes segregated at 10:27:13, respectively. All 13 mutant homozygote seedlings showed heavy staining in the central vascular part. These results demonstrate that the phenotypes observed in ND are caused by loss of function of OsFRDL1. We then used ND, which shows clearer phenotypes, for further investigations.
Knockout of OsFRDL1 Results in Decreased Fe Concentration in the Shoots
We compared the Fe concentration in the roots and shoots between the wild-type rice and the knockout line (ND). The concentration of Fe in the shoots was significantly lower in the knockout line than in the wild-type rice at either Fe concentration (Fig. 4A). The shoot Fe concentration of ND was 77% and 53.5% of the wild-type rice, respectively, at 0.2 and 10 μm. The shoot Fe concentration of ND at 10 μm Fe was higher than that of wild type at 0.2 μm. This is in agreement with the phenotype of ND at 10 μm Fe (no chlorosis; Fig. 3A). By contrast, the concentration of Fe in the roots was 2 times higher in the knockout line than in the wild-type line at 0.2 μm Fe (Fig. 4B). At 10 μm Fe, the root Fe increased to an extremely high concentration, probably due to precipitation of Fe on the root epidermal layer (Fig. 4C). The root Fe concentration is also slightly higher in ND, but there was no significant difference between ND and the wild-type rice. These results indicate that knockout of OsFRDL1 causes accumulation of Fe in the roots and decreased Fe concentration in the shoots.
Figure 4.
Fe concentration in the shoots (A) and roots (B and C). Wild-type rice (WT) and a knockout line (ND) were cultivated in nutrient solution containing 0.2 μm (B) or 10 μm (C) FeSO4. The concentration of Fe was determined by atomic absorption spectrophotometer. Data as means ± sd (n = 3).
Analysis of other metals showed that the shoot concentration of zinc (Zn) and manganese (Mn) was 15% higher in ND than in wild type at 0.2 μm Fe, but there was no difference between the two lines at 10 μm Fe (data not shown). There was no difference in the shoot copper (Cu) concentration between ND and wild type at either Fe concentrations. Because Fe deficiency is more pronounced in the ND knockout line, higher concentrations of Zn and Mn in the ND shoots at lower Fe concentration probably resulted from Fe deficiency-enhanced expression of IRT1, a Fe2+ transporter (Eide et al., 1996). IRT1 has been shown to transport other divalent metals, such as Zn and Mn, in addition to Fe (Eide et al., 1996; Korshunova et al., 1999). In fact, the expression of OsIRT1 was markedly enhanced in ND roots at 0.2 μm Fe (data not shown). Overall, the shoot concentration of Zn, Mn, and Cu was higher at 0.2 μm Fe than that at 10 μm Fe. This is probably due to lower expression of IRT1 at a higher Fe concentration.
Fe3+ and Citrate Were Decreased in the Xylem Sap of the Knockout Line of OsFRDL1
Accumulation of Fe in the roots and decreased Fe concentration in the shoots of the knockout line suggest that OsFRDL1 is involved in the translocation of Fe from the roots to the shoots. Fe was reported to be translocated in the form of a Fe-citrate complex in xylem sap (Tiffin, 1970; Durrett et al., 2007). Therefore, we compared the concentration of Fe and citrate in the xylem sap between ND and wild type at different Fe concentrations in the external solution. The concentration of citrate in the xylem sap of ND was less than one-half that of wild type at either external Fe concentration (Fig. 5A). However, there was no significant difference in malate concentration in the xylem sap between ND and wild type (Fig. 5B). The total Fe concentration in the xylem sap was significantly lower in ND than in wild type (Fig. 5C). In a separate experiment with 10 μm Fe, speciation analysis showed that there was no difference in Fe2+ concentration in the xylem sap between ND and wild type, but the concentration of Fe3+ in ND xylem sap was less than one-half that in wild type (Fig. 5D).
Figure 5.
Organic acids and Fe in the xylem sap. Wild-type rice (WT) and a knockout line (ND) were cultivated in nutrient solution containing 0.2 μm or 10 μm FeSO4 for 3 weeks. Xylem sap was collected from the cut ends for 1 h. Citrate (A) and malate (B) were determined with HPLC and total Fe (C) was by atomic absorption spectrophotometry. Fe3+ and Fe2+ (D) in the xylem sap were also analyzed. Data as means ± sd (n = 3).
Expression Pattern of OsFRDL1 and Localization of OsFRDL1
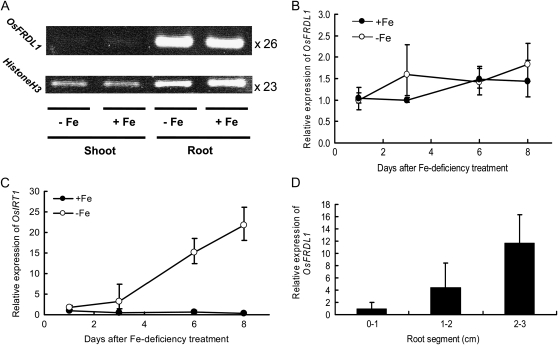
The expression of OsFRDL1 was examined with quantitative reverse transcription (RT)-PCR. OsFRDL1 was mainly expressed in the roots, but not in the shoots (Fig. 6A). A time course experiment showed that the expression in the roots was not affected by Fe deficiency (Fig. 6, A and B), in contrast to IRT1, which expression was greatly increased with development of Fe deficiency (Fig. 6C). Interestingly, the expression of OsFRDL1 was higher in the mature root zone than in the root tip (Fig. 6D). This result is in agreement with Fe precipitation and development of xylem.
Figure 6.
Expression pattern of OsFRDL1. A, Expression of OsFRDL1 mRNA in the roots and shoots of rice grown in a nutrient solution with or without Fe. The expression was examined by RT-PCR. Label on right side shows cycle number. B, Time-dependent expression of OsFRDL1 mRNA in the roots of rice subjected to Fe deficiency for 1, 3, 6, and 8 d. C, Time-dependent expression of OsIRT1 mRNA in the roots of rice subjected to Fe deficiency for different days. D, Expression of OsFRDL1 mRNA at different root regions. The expression level was quantified by real-time RT-PCR. Histone H3 mRNA level was used as an internal control. Data as means ± sd (n = 3).
The localization of OsFRDL1 was examined with an anti-OsFRDL1 antibody. Immunostaining showed that OsFRDL1 was localized at pericycle cells (Fig. 7, A and B). This result is consistent with OsFRDL1 promoter GUS staining (Inoue et al., 2004). No signal was observed in the knockout line (Fig. 7C), indicating the high specificity of the anti-OsFRDL1 antibody. The subcellular localization of OsFRDL1 was found to be localized on the plasma membrane by using heterologous expression in the onion (Allium cepa) epidermal cells (data not shown), which was the same as that reported previously (Inoue et al., 2004).
Figure 7.
Tissue-specific localization of OsFRDL1 in rice root. A and B, Immunostaining with anti-OsFRDL1 antibody in the roots (20 mm from the tip) of wild-type rice grown in a nutrient solution containing 10 μm FeSO4. C, Immunostaining in the roots of knockout line (ND). Scale bars = 100 μm.
OsFRDL1 Was Not Involved in the Al-Induced Secretion of Citrate
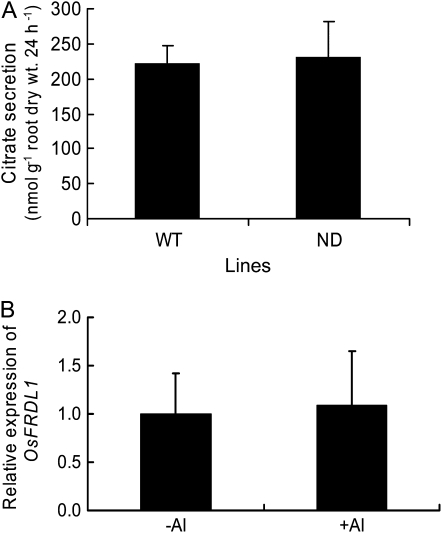
Rice secretes citrate from the roots in response to Al, although the amount secreted is small (Ma et al., 2002). To investigate whether OsFRDL1 is also involved in the Al-induced secretion of citrate, we compared citrate secretion between ND with wild type in the presence of Al. However, there was no significant difference in the Al-induced secretion of citrate between the two lines (Fig. 8A). The expression of OsFRDL1 was not affected by the exposure to Al (Fig. 8B). These results indicate that OsFRDL1 is not responsible for Al-induced secretion of citrate from the rice roots.
Figure 8.
Role of OsFRDL1 in Al stress. A, Al-induced citrate secretion. Seedlings of wild-type rice (WT) and a knockout line (ND) were exposed to 0.5 mm CaCl2 (pH4.5) solution containing 50 μm AlCl3. Root exudates were collected for 24 h after Al treatment. Citrate was determined by an enzymatic method. B, Effect of Al on the expression of OsFRDL1 in rice root. The roots were exposed to 50 μm AlCl3 for 3 h. Data as means ± sd (n = 3).
OsFRDL1 Is Able to Transport Citrate in a Heterologous Expression System
The above results suggest that OsFRDL1 encodes a transporter for citrate at the plasma membrane of pericycle cells, which is required for efficient translocation of Fe3+ as a citrate complex from the roots to the shoots. To confirm whether OsFRDL1 has activity to transport citrate, we expressed OsFRDL1 in Xenopus oocytes. The efflux activity for citrate was significantly higher in the oocytes injected with OsFRDL1 cRNA than in oocytes injected with water (Fig. 9). This result indicates that OsFRDL1 is able to transport citrate out of the cells.
Figure 9.
Efflux transport activity for citrate in Xenopus oocytes. OsFRDL1 cRNA or water was injected into Xenopus oocytes. After 1-d cultivation, the oocytes were injected with 14C-labeled citrate. The release of 14C-labeled citrate from the oocytes was determined 2 h later. Data as means ± sd (n = 3).
DISCUSSION
OsFRDL1 has 87% sequence homology with HvAACT1 and shares 57% sequence identity with AtFRD3 at the amino acid level (Fig. 1). However, our results indicate that OsFRDL1 is not involved in the Al-induced secretion of citrate like HvAACT1, but in Fe translocation like AtFRD3. OsFRDL1, HvAACT1, and AtFRD3 are from different plant species, but all show efflux activity for citrate when they were expressed in heterologous systems (Fig. 9; Durrett et al., 2007; Furukawa et al., 2007), indicating that they are all efflux transporters of citrate. Since the expression of OsFRDL1 was not responsive to Al (Fig. 8B), their different roles may be attributed to different tissue-specific localization. HvAACT1 is localized on the plasma membrane of the root epidermal cells, while AtFRD3 and OsFRDL1 are on the pericycle cells (Fig. 7; Green and Rogers, 2004). It is likely that activation of HvAACT1 by Al is required to transport citrate out of the cells (Furukawa et al., 2007), but Al usually stops at the epidermal cells and outer cortex cells (Ma et al., 2004). Therefore, Al may not reach the pericycle cells to activate OsFRDL1. This is supported by the finding that citrate secretion occurred when AtFRD3 was overexpressed in Arabidopsis under the control of the 35S promoter even in the absence of Al (Durrett et al., 2007).
Paddy rice is usually cultivated under reduced soil conditions, where excess Fe2+ is present. Therefore, paddy rice has developed a strategy to prevent excess Fe uptake by oxidation of Fe2+ to insoluble Fe3+ on root surfaces (Fig. 3; Horiguchi, 1995). In our experiment, we found that a Fe2+ supplement of only 0.2 μm is sufficient for healthy growth of wild-type rice (Fig. 3). When the plants were supplied with 50 times more Fe2+ (10 μm), most Fe was retained in the roots (Fig. 4C), supporting the role of roots in preventing excess Fe uptake. Rice roots are able to take up both Fe2+ and Fe3+ phytosiderophore complexes (Ishimaru et al., 2006). However, under submerged conditions, the contribution of uptake from the Fe3+ phytosiderophore complex is likely negligible because of the limited amount of phytosiderophore secretion and diffusion in water. Studies have shown that the transport of Fe2+ from the external solution to the root cells is mediated by OsIRT1, a Fe2+ transporter (Bughio et al., 2002; Ishimaru et al., 2006). However, it is unknown how Fe is released into the xylem from the root cells. Our speciation analysis of xylem sap showed that Fe is present in the form of both Fe2+ and Fe3+ (Fig. 5D), suggesting that part of Fe2+ taken up into the root cells is oxidized to Fe3+ before loading into the xylem. When OsFRDL1 at the pericycle cells was knocked out, the concentration of Fe3+, but not Fe2+, in the xylem sap was reduced (Fig. 5D) and Fe3+ was precipitated in the central vascular part (Fig. 3, D and G). Because the pH of the xylem sap is high (around 6.0), our results suggest that Fe3+ is released into the xylem by an unidentified transporter and its subsequent translocation to the shoots requires citrate to prevent Fe3+ precipitation by forming a complex.
The concentration of citrate in the xylem sap was much higher than that of Fe, even in the knockout line (Fig. 5A). The question, therefore, arises why Fe deficiency-induced chlorosis in the knockout line occurred at low external Fe concentrations (Fig. 3A). This is probably due to the complexation of citrate with other cations. In the xylem sap, other cations, such as calcium (Ca), magnesium (Mg) are present at higher concentrations than Fe; therefore, most citrates form complexes with these cations. In fact, speciation simulation with GeoChem software showed that only a small percentage of total citrate in the xylem sap chelates Fe.
In the OsFRDL1 knockout line (ND), the citrate concentration in the xylem sap did not decrease to zero (Fig. 5A). This suggests that there are other transporters either for citrate or for citrate-metal complexes. In the rice genome, there are five more homologs of OsFRDL1 (Fig. 1); therefore, the functions of these homologs should be examined in the future in terms of release of citrate to the xylem.
Overall, the function of OsFRDL1 is similar to AtFRD3 in terms of localization, transport, substrate, expression pattern, and phenotype of knockout lines. In Arabidopsis, knockout of AtFRD3 resulted in overaccumulation of Fe in the shoots (Rogers and Guerinot, 2002), but in rice, a decreased concentration of Fe in the shoots was found (Fig. 4A). This difference may be attributed to the Fe concentrations used in the experiment. We used relatively low Fe concentrations (up to 10 μm), but in the experiment with Arabidopsis, 100 μm ferrous sulfate was used (Rogers and Guerinot, 2002). In fact, when the Arabidopsis knockout line was grown on potting soil with less Fe availability, the leaf Fe concentration was 10% lower than in the wild-type line (Lahner et al., 2003), which is similar to the rice knockout line. The other possible reason is that, unlike Arabidopsis, rice roots have oxidation capacity, which prevents excess accumulation of ferrous iron.
In conclusion, our results clearly demonstrate that OsFRDL1 is a transporter of citrate, which is required for efficient translocation of Fe under limited Fe conditions. It is constitutively expressed and localized at the pericycle cells of rice roots.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Two rice (Oryza sativa) Tos-17 insertion lines, ND8025 and NC2637 for OsFRDL1, were obtained from the rice genome resource center. The homozygous lines were screened by PCR using OsFRDL1-specific primers (5′-GGTCTCACTGTTGTGCTTGGG-3′ and 5′-GCTATGGTCCTCAGGCTCATG-3′) and a left-border Tos-17 primer (5′-ATTGTTAGGTTGCAAGTTAGTTAAGA-3′). Progeny for genetic analysis was derived from a heterozygous line of ND8025 selected by PCR as described above. Seeds including wild-type rice (cv Nipponbare), two Tos-17 insertion homozygous lines, and the heterozygous progeny of ND8025 were soaked in water overnight at 25°C in the dark and then transferred to a net floating on 0.5 mm CaCl2 solution. On day 7, seedlings were transferred to a 3.5-L plastic pot containing one-half-strength Kimura B solution and grown in a greenhouse at 22°C to 25°C. After 10 d, the seedlings (five plants per pot) were transferred to a 1.2-L pot containing freshly prepared nutrient solution. The nutrient solution contained the macronutrients (mm): (NH4)2SO4 (0.18), MgSO4·7H2O (0.27), KNO3 (0.09), Ca(NO3)2·4H2O (0.18), and KH2PO4 (0.09), and the micronutrients (μm): MnCl2·4H2O (0.5), H3BO3 (3), (NH4)6Mo7O24·4H2O (1), ZnSO4·7H2O (0.4) and CuSO4·5H2O (0.2), supplied with either 0.2 or 10 μm FeSO4. The pH of this solution was adjusted to 5.5 and the nutrient solution was renewed every 2 d. Fe2+ was not oxidized during 2 d. After 10 to 15 d more growth, the seedlings were used for following experiments. Each experiment was repeated at least three times with three replicates each.
Metal Analysis in Roots and Shoots
Plants cultivated with different Fe concentrations for 15 d as described above were harvested and separated into the roots and shoots. After drying at 70°C for 2 d, the samples were ground to fine powder and digested with 5 mL of 11 m HNO3 for 5 h at 150°C. The metal concentration was then determined by atomic absorption spectrometry (Z-2000; Hitachi).
Analysis of the Xylem Sap
Before collection of xylem sap, the nutrient solution was renewed. After 6 h, xylem sap was collected from the cut end for 1 h with a micropipette after decapitating the plant 3 cm above the roots. The concentration of Fe and other cations was determined using flameless atomic absorption spectrometry (Z-2000; Hitachi). Malate concentrations in the sap were analyzed with HPLC using an ion-exclusion column (Shimpack SCR-102H; 8.0 mm × 30 cm; Shimadzu) with a guard column (6.0 mm × 5 cm). Citrate concentrations in the xylem sap were analyzed with HPLC using a reverse-phase column (Cosmossil Packed Column 5C18-PAQ 4.6 i.d. × 250 mm; Nakalai tesque). The mobile phase was a dilute perchloric acid solution (pH 2.1) run at 40°C, and peaks were detected by postcolumn bromothymol blue method at the wavelength of 425 nm (Ma et al., 1997).
Fe Speciation Analysis
Xylem sap collected as described above were immediately used for determination of Fe3+ and Fe2+ by QuantiChrom iron assay kit (Bioassay System) according to the manufacturer's instructions.
Perls Blue Staining
Perls blue staining was performed with the roots of wild-type rice, two Tos-17 lines, and a ND heterozygous progeny. Briefly, equal amounts of solutions of 4% (v/v) HCl and 4% (w/v) potassium ferrocyanide were mixed immediately prior to use. Seedlings (1-week-old) were exposed to the staining solution and vacuum infiltrated for 15 min. The seedlings were then rinsed with water and approximately 200-μm cross-sections were prepared by free hand. The staining was observed under optical microscope.
Quantitative Real-Time RT-PCR
Total RNA was extracted from the roots and leaves of rice plants subjected to Fe deficiency for 1, 3, 6, and 8 d. The relative transcript levels of OsFRDL1, OsIRT1, and histone H3 (internal control) were determined by quantitative real-time RT-PCR as described previously (Yamaji and Ma, 2007). Primer sequences used were OsFRDL1, 5′-TCACCAATGCTAAGGCCTGC-3′ (forward) and 5′-AACCACGGAAAACACCCTG-3′ (reverse); OsIRT1, 5′-GTCGTCAAGGCGTTCGCGTC-3′ (forward) and 5′-CGAACGGGAACTCCGACCAC-3′ (reverse); histone H3, 5′-AGTTTGGTCGCTCTCGATTTCG-3′ (forward) and 5′-TCAACAAGTTGACCACGTCACG-3′ (reverse). Expression data were normalized with the expression level of histone H3 and the data for the root of Fe deficiency were compared with those of Fe sufficiency by the ΔΔCt method. The expression of OsFRDL1 was also quantified in the roots exposed to 0 or 50 μm Al for 3 h.
Immunostaining of OsFRDL1 Protein
The synthetic peptide C-EEKTAAAAAAPEDLPA (positions 102–107 of OsFRDL1) was used to immunize rabbits to obtain antibodies against OsFRDL1. The roots of both wild-type and Tos-17 line (ND) grown at 10 μm FeSO4 were used for immunostaining as described previously (Yamaji and Ma, 2007). Fluorescence from the secondary antibody (Alexa Fluor 555 goat anti-rabbit IgG; Molecular Probes) was observed with a fluorescence microscope (Axio Imager with Apotome; Carl Zeiss).
Al-Induced Citrate Secretion
To compare organic acids secreted from rice roots in response to Al, root exudates from both Nipponbare and the knockout line (ND) were collected. Seedlings were exposed to a 0.5 mm CaCl2 (pH 4.5) solution overnight and then to a 0.5 mm CaCl2 (pH 4.5) solution containing 50 μm AlCl3 for 24 h. Root exudates collected were then passed through a cation-exchange resin column (16 × 14 mm) filled with 5 g of Amberlite IR-120B resin (H+ form), followed by an anion-exchange resin column (16 × 14 mm) filled with 2 g of AG 1 × 8 resin (100–200 mesh; formate form). Organic acids retained on an anion-exchange resin were eluted with 2 n HCl and the eluate was concentrated to dryness with a rotary evaporator (40°C). After evaporation, the residue was dissolved in 1 mL of milli-Q water; the concentration of organic acids was analyzed by enzymatic method according to Delhaize et al. (1993).
Transport Activity Assay in Xenopus Oocytes
Oocytes were isolated from adult female Xenopus laevis frogs as described before (Ma et al., 2006). Selected oocytes were incubated for 1 d in modified Barth's saline at 18°C until the injection of cRNA. The open reading frame of OsFRDL1 was amplified and cloned as described in Ma et al. (2006) and the cRNA with cap analog was synthesized with mMASSAGGE mMACHINE high-yield capped RNA transcription kit (Ambion) according to the manufacturer's instructions. Fifty nanoliters cRNA (1 ng nL−1) were injected into the selected oocytes using a Nanoject II automatic injector (Drummond Scientific). As a negative control, 50 nL of RNase-free water were injected. After 1-d culture, oocytes with or without OsFRDL1 expression were injected with 50 nL of 2.4 mm 14C-labeled citrate (2.3 nCi/oocytes; Amersham; 4–5 oocytes/replicates). The oocytes were washed for 5 min in modified Barth's saline buffer (pH 5.0) and then transferred into a new 500-μL buffer at 18°C. At the end of the experiments, the oocytes were homogenized with 0.1 n HNO3. Radioactivity of the buffer and homogenized oocytes was measured with a liquid scintillation counter (Aloka liquid scintillation system).
Acknowledgments
We thank the Rice Genome Resource Center for providing Tos-17 seeds.
This work was supported by the Program of Promotion of Basic Research Activities for Innovative Biosciences, by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 18380052 to J.F.M.), by a Sunbor grant, and by the Ohara Foundation for Agricultural Science.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jian Feng Ma (maj@rib.okayama-u.ac.jp).
Open Access articles can be viewed online without a subscription.
References
- Bughio N, Yamaguchi H, Nishizawa NK, Nakanishi H, Mori S (2002) Cloning an iron-regulated metal transporter from rice. J Exp Bot 53 1677–1682 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJM, Leon-Kloosterziel KM, Koorneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13 853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Ryan PR (2007) The roles of organic anion permeases in aluminum resistance and mineral nutrition. FEBS Lett 581 2255–2262 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener AC, Gaxiola RA, Fink GR (2001) Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13 1625–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrett TP, Gassmann W, Rogers EE (2007) The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 144 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48 1081–1091 [DOI] [PubMed] [Google Scholar]
- Green L, Rogers EE (2004) FRD3 controls iron localization in Arabidopsis. Plant Physiol 136 2523–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi T (1995) Rhizosphere and root functions. In T Matsuo, K Kumazawa, R Ishii, K Ishihara, H Hirata, eds, Science of the Rice Plant, Vol 2. Nobunkyo, Tokyo, pp 221–248
- Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) A rice FRD3-like (OsFRDL1) gene is expressed in the cells involved in long-distance transport. Soil Sci Plant Nutr 50 1133–1140 [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, et al (2006) Rice plants take up as an Fe3+-phytosiderophore and Fe2+. Plant J 45 335–346 [DOI] [PubMed] [Google Scholar]
- Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274 175–195 [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40 37–44 [DOI] [PubMed] [Google Scholar]
- Lahner B, Gong J, Mahmoudian M, Smith E, Abid K, Rogers E, Guerinot M, Harper J, Ward J, McIntyre L, et al (2003) Ionomics the genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotechnol 21 1215–1221 [DOI] [PubMed] [Google Scholar]
- Li L, He Z, Pandey GK, Tsuchiya T, Luan S (2002) Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem 277 5360–5368 [DOI] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E (2001) Aluminum resistance in plants and the complexing role of organic acids. Trends Plant Sci 6 273–278 [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen R, Nagao S, Tanimoto E (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol 45 583–589 [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen RF, Zhao ZQ, Wissuwa M, Takeuchi Y, Ebitani T, Yano M (2002) Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant Cell Physiol 43 652–659 [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H (1997) Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant Cell Physiol 38 1019–1025 [Google Scholar]
- Magalhaes JV, Liu J, Guimarães CT, Lana UGP, Alves VMC, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, et al (2007) A member of the multidrug and toxic compound extrusion ‘MATE’ family is a major gene that confers aluminum tolerance in sorghum. Nat Genet 39 1156–1161 [DOI] [PubMed] [Google Scholar]
- Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul J, Debeaujon I, Klein M (2007) The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19 2023–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T (1998) NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother 42 1778–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y (2007) The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci 11 587–593 [DOI] [PubMed] [Google Scholar]
- Otani M, Shitan N, Sakai K, Martinoia E, Sato F, Yazaki K (2005) Characterization of vacuolar transport of the endogenous alkaloid berberine in Coptis japonica. Plant Physiol 138 1939–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Guerinot ML (2002) FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14 1787–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin LO (1970) Translocation of iron citrate and phosphorus in xylem exudate of soybean. Plant Physiol 45 280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF (2007) Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol 143 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8 301–307 [DOI] [PubMed] [Google Scholar]