Abstract
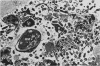
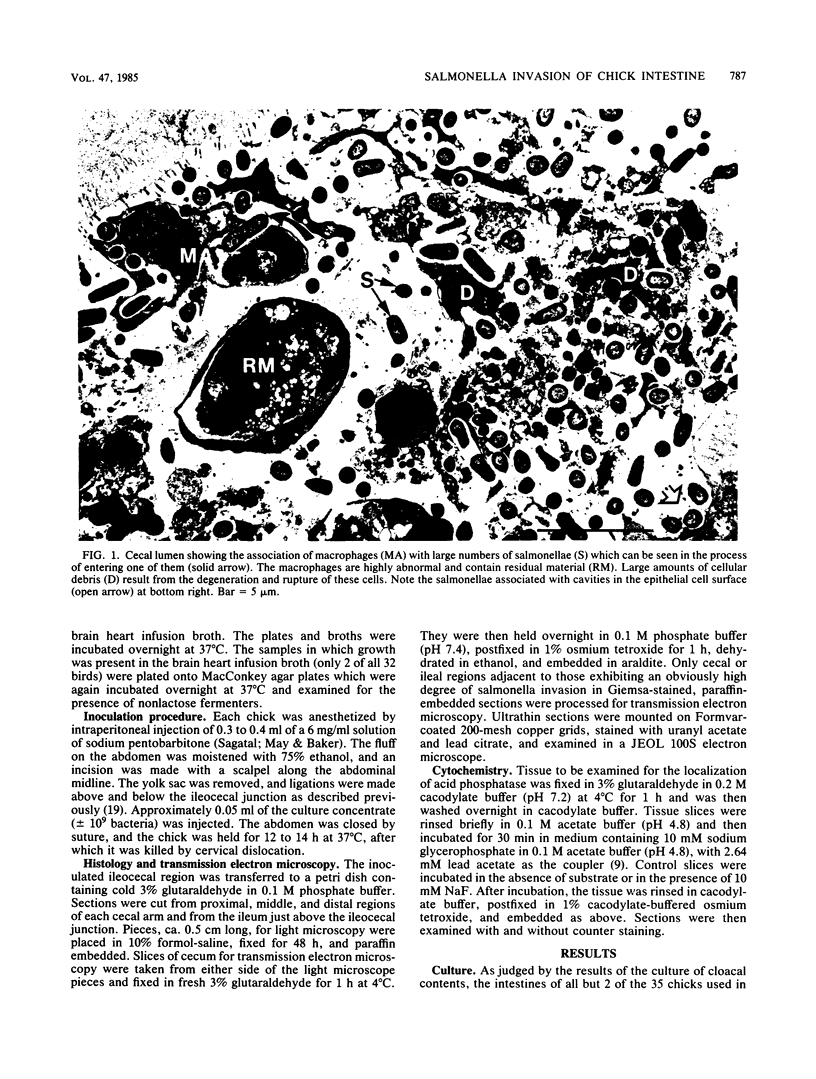
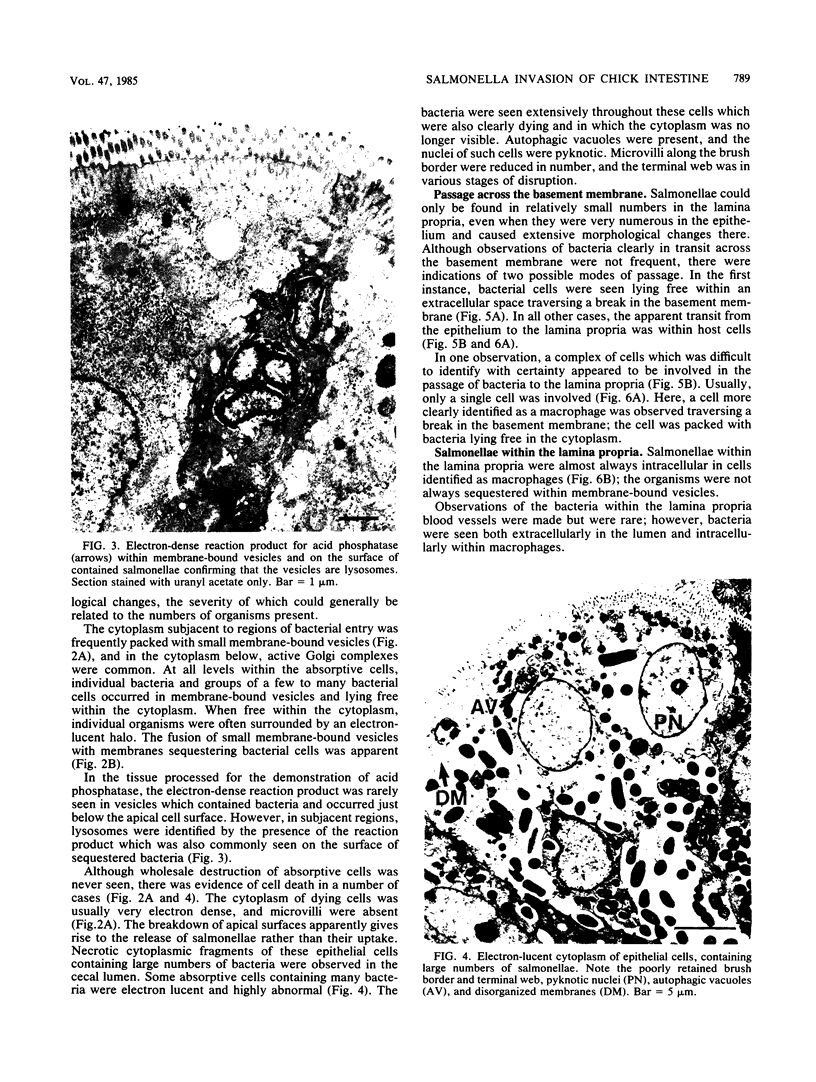
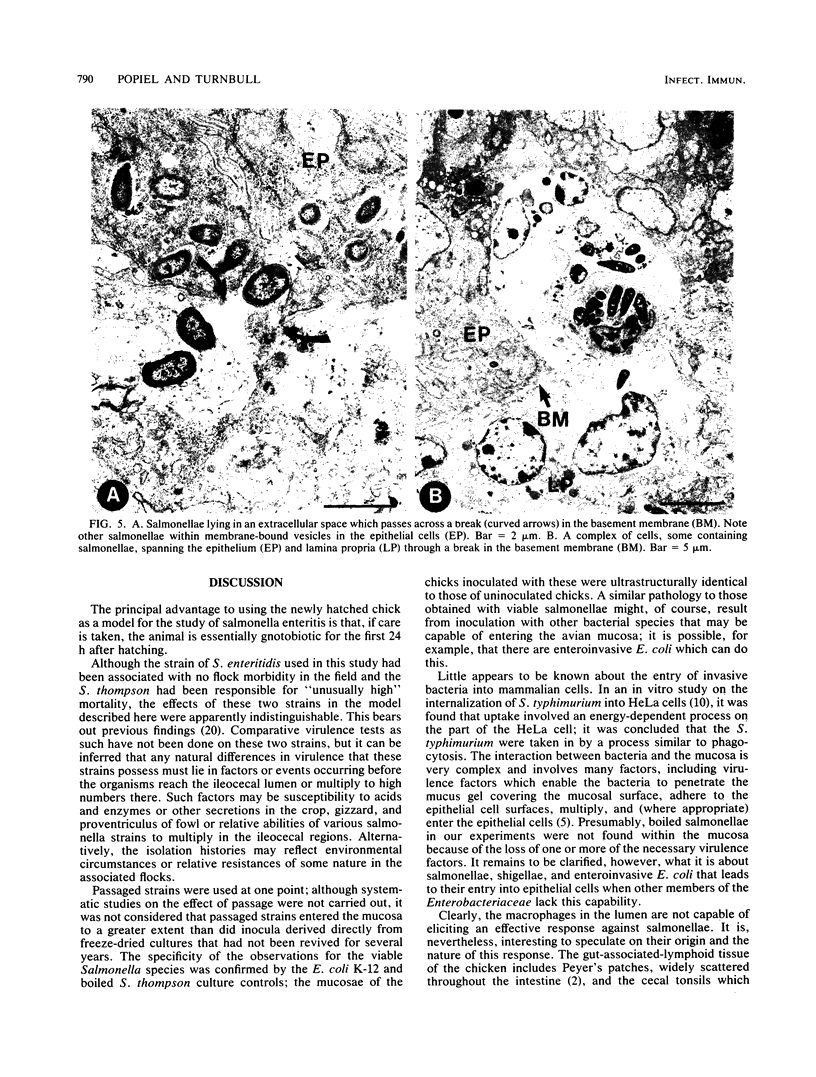
The passage of Salmonella enteritidis and S. thompson across the cecal mucosa has been visualized in an electron microscope study with the freshly hatched chick as a model. The uptake of salmonellae by macrophages took place in the cecal lumen; the macrophages became abnormal and often ruptured to release organisms back into the lumen. The entry of bacteria into the epithelial cells was associated with a series of pathological changes, beginning with the appearance of active Golgi apparatus and the production of a variety of lysosomal vesicles. Salmonellae became sequestered within lysosomes but were unaffected by the presence of hydrolytic enzyme. Epithelial cell death was related to particularly large numbers of bacteria. Fragments of invaded epithelial cells, especially those undergoing cell death, contributed to the cytoplasmic debris and released further salmonellae into the lumen. Bacteria were never observed in large numbers below the basement membrane, and there was no significant pathology in the lamina propria tissue. Wandering cells, identified as macrophages and containing the bacteria, were observed spanning the epithelial and lamina propria regions through breaks in the basement membrane. It is suggested that the passage of bacteria from the epithelium to the lamina propria is primarily the result of capture and transport within host macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS G. D., BAUER H., SPRINZ H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963 Mar;12:355–364. [PubMed] [Google Scholar]
- Befus A. D., Johnston N., Leslie G. A., Bienenstock J. Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer's patches. J Immunol. 1980 Dec;125(6):2626–2632. [PubMed] [Google Scholar]
- Bienenstock J., Gauldie J., Perey D. Y. Synthesis of IgG, IgA, IgM by chicken tissues: immunofluorescent and 14C amino acid incorporation studies. J Immunol. 1973 Oct;111(4):1112–1118. [PubMed] [Google Scholar]
- Day D. W., Mandal B. K., Morson B. C. The rectal biopsy appearances in Salmonella colitis. Histopathology. 1978 Mar;2(2):117–131. doi: 10.1111/j.1365-2559.1978.tb01700.x. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B., Holbrook K. A., Olah I., Perkins W. D., Stinson R. An electron and light microscope study on the caecal tonsil: the basic unit of the caecal tonsil. Dev Comp Immunol. 1981 Winter;5(1):95–104. doi: 10.1016/s0145-305x(81)80011-0. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Bowen I. D. The fine structural localization of acid phosphatase in pore cells of embryonic and newly hatched Deroceras reticulatum (Pulmonata: Stylommatophora). Cell Tissue Res. 1979 Dec;204(2):253–265. doi: 10.1007/BF00234637. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Nilsson L. Endocytosis of Salmonella typhimurium 395 MS and MR10 by HeLa cells. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):322–328. doi: 10.1111/j.1699-0463.1977.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Madge D. S. Intestinal absorption in experimental Salmonella enterocolitis in mice. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):445–463. doi: 10.1016/0300-9629(72)90124-7. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Allen C. L., Stevens D. P. Phagocytosis of Giardia muris by macrophages in Peyer's patch epithelium in mice. Infect Immun. 1981 Aug;33(2):591–601. doi: 10.1128/iai.33.2.591-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout W. R., Formal S. B., Dammin G. J., Giannella R. A. Pathophysiology of Salmonella diarrhea in the Rhesus monkey: Intestinal transport, morphological and bacteriological studies. Gastroenterology. 1974 Jul;67(1):59–70. [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H. Electron-Microscope Studies of Experimental Salmonella Infection in the Preconditioned Guinea Pig: II. Response of the Intestinal Mucosa to the Invasion by Salmonella typhimurium. Am J Pathol. 1967 Jul;51(1):137–161. [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C. Food poisoning with special reference to Salmonella -- its epidemiology, pathogenesis and control. Clin Gastroenterol. 1979 Sep;8(3):663–714. [PubMed] [Google Scholar]
- Turnbull P. C., Richmond J. E. A model of salmonella enteritis: the behaviour of Salmonella enteritidis in chick intestine studies by light and electron microscopy. Br J Exp Pathol. 1978 Feb;59(1):64–75. [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C., Snoeyenbos G. H. Experimental salmonellosis in the chicken. 1. Fate and host response in alimentary canal, liver, and spleen. Avian Dis. 1974 Apr-Jun;18(2):153–177. [PubMed] [Google Scholar]