Abstract
Visceral hypersensitivity is an important clinical feature associated with irritable bowel syndrome which in some patients has been linked to prior infection. Here we employ an animal model in which transient infection leads to persistent gut dysfunction to investigate the role of altered 5-HT metabolism upon afferent mechanosensensitivity in the post-infected gut. Jejunal segments isolated from Trichinella spiralis-infected mice were used to assess 5-HT metabolism whilst afferent activity in T. spiralis-infected mice was studied by extracellular recordings from jejunal mesenteric afferent bundles and patch clamp recordings of isolated nodose ganglion neurons (NGNs). During acute infection, intestinal 5-HT content and release increased, 5-HT turnover decreased and afferent discharge in response to mechanical stimulation was attenuated. By day 28 post infection (PI), 5-HT turnover had normalized, but 5-HT content and release were still elevated. This was associated with afferent mechano-hypersensitivity, which persisted for 8 weeks PI and was susceptible to 5-HT3 receptor blockade. NGNs from post-infected animals were more excitable than controls but their current densities in response to 2-methyl-5-HT were lower. T. spiralis infection increased mucosal 5-HT bioavailability and affected the spontaneous activity and mechanosensitivity of gastrointestinal sensory nerves. This involved an initial hyposensitivity occurring during acute infection followed by long-term hypersensitivity in the post-infectious period that was in part mediated by 5-HT acting via 5-HT3 receptors. Functional down-regulation of 5-HT3 receptors also occurs in the post-infected animals, which may represent an adaptive response to increased mucosal 5-HT bioavailability.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by chronic abdominal pain and altered bowel habits (Drossman, 1999; Hungin et al. 2003; Dunlop et al. 2003a). However, the mechanisms contributing to the pathophysiology of IBS are poorly understood partly due to the heterogeneity in the patient population. Recent evidence though, suggests an important role for serotonin (5-HT) in the development of IBS symptoms in one well-defined group of patients who develop their symptoms post infection, typically after a bout of acute bacterial gastroenteritis. Studies have reported biochemical abnormalities in the gut mucosa of these post-infected IBS (PI-IBS) patients including hyperplasia of the 5-HT-containing enterochromaffin (EC) cells and depressed 5-hydroxyindole acetic acid (5-HIAA)/5-HT ratios, which suggests an impaired serotonin transporter (SERT) function (Dunlop et al. 2003a,b, 2005). Genetic profiles of biopsies from IBS patients have shown changes indicative of altered 5-HT bioavailability which were predictive of the patient population and used as a diagnostic indicator of IBS (Camilleri, 2007).
Animal models of IBS include stress, neonatal insult, chemical injury and nematode infection (Mayer & Collins, 2002). In some of these models, hypersensitivity to distension is reported while in others mechanosensitivity may be normal but chemosensitivity exaggerated (Bercik et al. 2004; Aerssens et al. 2007). This suggests that variability in the severity of the inflammatory response, and its timing relative to resolution may play a role in determining the sensory endpoint. Clinically, this may be important in patients with inflammatory bowel disease (IBD) that have normal bowel sensitivity during periods of active inflammation, but exhibit symptoms similar to those seen in patients with IBS when in remission (Minderhoud et al. 2004; Bercik et al. 2005).
Most of the studies using animal models have focused on the post-infectious period when inflammation has resolved but long-term changes persist both in the bowel wall and in the neural mechanisms that process nociceptive information (Bercik et al. 2004). In this study we employed the Trichinella spiralis-infected mouse, a well-characterized model of gut dysfunction which has been proposed as an animal model of PI-IBS (Mayer & Collins, 2002; Bercik et al. 2004). Infection with T. spiralis results in a transient enteric inflammatory response which resolves to leave persistent gut dysfunction including hypercontractility, altered peristalsis and exaggerated visceromotor responses to colorectal distension (Barbara et al. 1997; Vallance et al. 1997; Bercik et al. 2004; Akiho et al. 2005).
Persistent changes have also been described in the mucosa following T. spiralis infection, including EC cell hyperplasia and decreased immunostaining for SERT (Wheatcroft et al. 2005), which reflects an altered 5-HT bioavailability similar to that seen in PI-IBS patients (Dunlop et al. 2003a,b, 2005). Depressed SERT activity and EC cell hyperplasia appears to be a feature of inflammatory processes in the gut (Linden et al. 2003, 2005; O'Hara et al. 2004; Coates et al. 2004).
Since it is well established that 5-HT is involved in sensory signalling within the bowel wall (Gershon, 2003), we hypothesized that changes in 5-HT metabolism might be involved in the initiation or maintenance of hypersensitivity in the afferents supplying the post-infectious bowel. We tested this by examining the time course of changes in 5-HT metabolism and afferent sensitivity following infection with T. spiralis and the extent to which hypersensitivity may be influenced by pharmacological manipulation of the 5-HT3 receptor.
Methods
Animals and Trichinella infection
Experimental treatment
All experimental procedures were performed in accordance with the UK Animals Scientific Procedures Act (1986). Adult male mice (Harlan, UK) weighing approximately 24 g were used. Animals were kept in filtered cages under controlled ambient temperature and light–dark cycle (12 h : 12 h). The T. spiralis colony was maintained through serial infection in adult NIH Swiss mice as previously described (Kalia et al. 2007).
Recording of jejunal mesenteric afferent nerve activity
Experimental treatment
Afferent recordings were performed following infection of NIH Swiss mice with T. spiralis larvae (260–300 larvae per mouse) administered by oral gavage (0.2 ml in 0.9% saline). Control mice included naive and sham-infected (0.9% saline) animals at different time points and since there was no significant different between these, the data have been pooled. Animals were examined at three different time points post infection (PI): the first between days 14 and 16 (d14–16)PI represented the late inflammatory phase (Bercik et al. 2004), whilst the second and third time points covering days 28–36 (d28–36)PI and days 48–56 (d48–56)PI represented the early and late post-infectious phases, respectively.
Tissue preparation
Animals were killed by cervical dislocation, and their abdominal cavity opened and bathed in cold Krebs solution (in mm: NaCl 120; KCl 5.9; NaH2PO4 1.2; MgSO4 1.2; NaHCO3 15.4; CaCl2 2.5; glucose 11.5) that was gassed with carbogen (95% O2, 5% CO2). Segments of jejunum, 3 cm long, were harvested 10–20 cm proximal to the ileocaecal junction, such that a non-bifurcating mesenteric bundle emanated centrally from each segment. Individual segments were placed into a Sylgard-lined organ chamber (8 ml) which was continually superfused with gassed Krebs solution at a flow rate of 8 ml min−1 and maintained at 33–34°C. The segments were securely attached at either end to an input and outlet port. The input port was connected to a Perfusor VI syringe pump, which allowed continuous intraluminal perfusion of Krebs solution through the segments (0.2 ml min−1) when the outlet port was open, but allowed periodic distension when closed. Intraluminal pressure was recorded via a pressure amplifier (NL 108, Digitimer, UK) connected in series with the input port. The mesenteric bundle was pinned out to the base of the chamber and a mesenteric nerve was dissected out from the bundle and drawn into a suction electrode.
The preparation was stabilized for 60 min before any experimental procedures were started. To test the mechanosensitivity of jejunal afferents, the preparation was distended by closing a tap attached to the outlet port. This allowed the intestinal segment to be distended to an intraluminal pressure of 60 mmHg. The tap was then opened, returning the pressure to baseline. This procedure was repeated at 1000 s intervals throughout the course of the experiment to test the reproducibility of the nerve response to ramp distensions and the response during test conditions. Drugs were added to the Krebs solution perfusing the organ bath. However, to achieve rapid steady-state conditions following application of 2-Me-5-HT, perfusion was switched off prior to application directly into the organ bath and perfusion resumed 60 s later.
Mesenteric afferent nerve recordings
Nerve activity was recorded with a Neurolog headstage (NL100, Digitimer) and electrical signals amplified (NL104), filtered (NL125, band pass 200–3000 Hz) and acquired (20 kHz sampling rate) to a personal computer through a Micro 1401 MKII interface running Spike2 software (Cambridge Electronic Design, UK). Intraluminal pressure was sampled at 100 Hz.
High performance liquid chromatography (HPLC) determination of jejunal 5-HT metabolism
Experimental procedure
Studies were performed on infected NIH mice at d14, d28 or d56PI, or on uninfected controls. Animals were killed by cervical dislocation, and the abdominal cavity opened and bathed in an ice-cold solution of carbogen-gassed Krebs containing 10 μm pargyline and 1 mmN-acetylcysteine. Segments of jejunum, 1 cm long, were removed, cut into strips, weighed and then either incubated for 10 min at 37°C in 1 ml of the same buffer to determine basal extracellular 5-HT levels (defined as released 5-HT minus reuptake) or snap frozen in liquid nitrogen to determine 5-HT content.
Samples of the incubation solution were analysed using HPLC in order to determine extracellular 5-HT levels. For content analysis, tissues were placed in 1 ml of ice-cold 0.1 m perchloric acid and 0.02% (w/v) sodium metabisulphite and homogenized using an ultrasonic homogenizer (MSE ultrasonic disintegrator, MSE Scientific Instruments, Crawley, UK). Samples were then centrifuged at 15 000 g for 15 min, the supernatant filtered (0.45 μm filter) and subjected to HPLC analysis. The protein content of the tissue was measured using the Bradford protein assay method and 5-HT levels expressed per milligram of tissue protein.
Samples were injected onto a TARGA C18 analytical HPLC column (25 cm × 2.1 mm) (Higgins Analytical, CA, USA). An L-7110 solvent delivery pump (Merck Hitachi, Poole, UK) was used to circulate the mobile phase (flow rate, 0.15 ml min−1) which consisted of (in mm: sodium dihydrogen orthophosphate 150; EDTA 1; 1-octane sulphonic acid sodium salt 1; methanol 14%, adjusted to pH 4.7 filtered and degassed). 5-HT oxidation was detected via an electrochemical detector (Antec Leyden, the Netherlands) with the glass carbon fibre working electrode potential set at +0.70 V with reference to a saturated KCl-filled Ag/AgCl reference electrode. Oxidation currents were acquired to a personal computer through an Analog Interface Module and System Gold software (Beckman, High Wycombe, UK).
Patch clamping of isolated nodose ganglia neurons
Tissue preparation
Sham-treated or T. spiralis (d28–56PI)-infected NIH Swiss mice were injected with Fast Blue (4%, 10 μl) i.p. Animals were killed 3–7 days later by pentobarbitone overdose followed by exsanguination. The head was removed and the nodose ganglia were located by following the cervical vagi to the base of the skull. The ganglia were dissected free of adherent connective tissue, removed, and placed in cold physiological saline. The tissue was incubated in collagenase (1 mg ml−1) and dispase (4 mg ml−1) for 10 min, titurated with a fire-polished Pasteur pipette, and incubated again for an additional 5 min. The dissociated neurons were plated onto poly l-lysine-coated coverslips and stored in a humidified incubator at 37°C, under 95% air–5% CO2, and in DMEM with 1% penicillin–streptomycin, 2 mm glutamine and 0.2% glucose. Neurons were retrieved 18–48 h later for electrophysiological recordings.
Patch clamp recordings
Coverslips containing adherent nodose neurons were placed in a custom-designed recording chamber (volume 0.75 ml) and continuously perfused with extracellular solution (in mm: NaCl 140; KCl 5; CaCl2 2; MgCl2 1; Hepes 10; glucose 13) at a flow rate of 1–2 ml min−1 and maintained at room temperature throughout the experiments. Using a Nikon TE200 inverted microscope with fluorescence attachments, fluorescent neurons were identified for recording. Conventional whole-cell recordings were performed using thin walled borosilicate glass microelectrodes, manufactured with a Narishige PP830 vertical pipette puller, and fire polished using a Narishige MF 830 microforge. Electrodes were filled with internal solution containing (in mm: KCl 140, EGTA 10, glucose 10, Hepes 10). Recordings were performed using a Multiclamp 700A voltage clamp amplifier (Axon Instruments, Sunnydale, CA, USA), and the pCLAMP 8.2 software package. Current clamp recordings were performed, and rheobase (current threshold) was determined by a series of 30 ms current steps. The number of spikes at twice rheobase was determined using a 500 ms current pulse. To examine 5-HT-induced currents, cells were voltage clamped at −60 mV and 2-Me-5-HT (100 μm) was applied to the cell using a fast flow solution switching system (RSC 160, Bio-Logic, France), and the resultant change in holding current measured.
Data analysis
The mean firing frequency (impulses (imp) s−1) was measured with a time constant of 10 s. Firing rates were measured using a bin width of 1 s. The stimulus–response curves (mean afferent discharge plotted against intraluminal pressure) for whole nerve or single unit activities were plotted using a customized script program (CED, Cambridge, UK). Spontaneous nerve activity (imp s−1) was measured over 300 s intervals using the X–Y plot function of the Spike 2 program (version 5).
Single unit analysis was performed off-line using Spike 2 software as previously described in order to identify single units within multiunit afferent nerve preparations (Hillsley & Grundy, 1998). Single units assigned to an individual template were then examined using principal component analysis to identify and eliminate ambiguous waveforms.
Mechanosensitive single units were classified into high threshold (HT), wide dynamic range (WDR) and low threshold (LT) on the basis of their response profile during ramp distensions as previously described (Booth et al. 2008). Briefly, the magnitude of the afferent response at 20 mmHg reflecting low threshold (LT) activation was expressed as a percentage of the maximum response at 60 mmHg. This value is referred to as %LT and for a linear stimulus–response function would be 33%. Values higher than this reflect disproportionate low threshold sensitivity, while values below this have disproportionate high threshold sensitivity. In classifying afferent fibres, values for %LT > 55 are defined as LT. HT units are those responding with a %LT of < 15% whereas afferents with a more linear increase in discharge over the range of distension used (> 15%, < 55%) were termed WDR units. A χ2 test was performed to determine significant differences in the prevalence of each afferent fibre type in control and infected animals.
For the physiological experiments, naive and sham-infected data showed no difference and were pooled for subsequent analysis. For the 5-HT metabolism studies, data are derived from the mean of three samples per animal. All data are expressed as mean ± s.e.m. Significant differences between data points were determined using paired or unpaired t test and by 1- or 2-way ANOVA with additional post hoc tests as appropriate. A probability of P < 0.05 was considered statistically significant. Statistical analysis was performed using Graphpad Prism version 4.00 (San Deigo, CA, USA).
Chemicals
Granisetron HCl 1 mg ml−1 in 0.9% saline was obtained from the Royal Hallamshire Hospital, Sheffield. 2-Me-5-HT was purchased from Tocris Cookson, UK. Chemicals were purchased from BDH.
Abbreviations
EC, enterochromaffin; 5-HIAA, 5-hydroxyindole acetic acid; HPLC, high performance liquid chromatography; 5-HT, serotonin; HT, high threshold unit; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; LT, low threshold unit; NGNs, nodose ganglion neurons; PI-IBS, post-infectious irritable bowel syndrome; SERT, serotonin transporter; WDR, wide dynamic range unit.
Results
T. spiralis infection alters the bioavailability and metabolism of 5-HT in jejunal segments
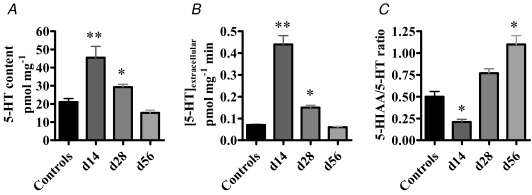
The effect of infection upon 5-HT bioavailability and metabolism in mouse jejunal segments is summarized in Fig. 1. 5-HT content and extracellular 5-HT levels were significantly increased above control values in d14 and d28PI preparations. However, by d56PI, both extracellular 5-HT and total 5-HT content had normalized to control values. In contrast, the 5-HIAA/5-HT ratio, a marker of 5-HT turnover, had decreased by d14PI but was significantly elevated by d56PI, suggesting that in the late post-infected gut, 5-HT turnover is augmented.
Figure 1. 5-HT metabolism is altered in the jejunum of T. spiralis-infected animals.
5-HT content (A) and extracellular 5-HT levels (B) were significantly augmented at d14 (n = 12) and d28 (n = 12) PI, but had normalized by d56 (n = 12) PI. C, 5-HIAA/5-HT ratio was significantly reduced at d14PI but was significantly elevated at d56PI. ** P < 0.001; * P < 0.01 versus controls (n = 18).
Spontaneous activity of the mesenteric afferent nerve is altered in T. spiralis-infected mice
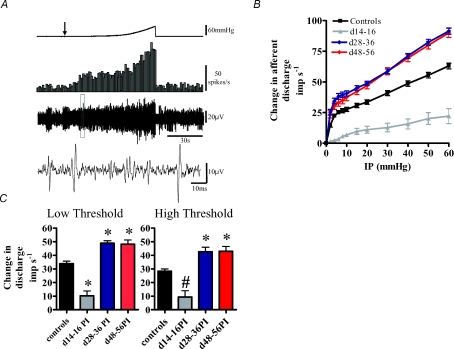
The jejunal mesenteric nerve preparations of both control and infected animals exhibited an irregular spontaneous activity which occurred in the absence of any changes in intraluminal pressure (Fig. 2A). Whole-nerve spontaneous activity in control preparations was significantly higher than that observed in d14–16PI preparations but was significantly lower that that observed in both d28–36PI and d48–56PI preparations (Table 1A). Within each mesenteric afferent nerve recording, it was possible to identify single units which contributed to the whole nerve activity profiles (Fig. 2A). Single unit activity followed a similar pattern to the whole nerve activity profiles, whereby control preparation single unit activity was higher than that observed in d14–16PI preparations although this was not significant. However, single unit activity in control preparations was found to be significantly lower than in both d28–36PI and d48–56PI preparations (Table 1B).
Figure 2. Mesenteric afferent neurons are differentially sensitized to mechanical stimulation following T. spiralis infection.
A, a representative trace showing the mesenteric afferent response to jejunal ramp distension in control animals. Top trace: ramp distension profile. Arrow denotes start of ramp distension. Second trace from top: nerve discharge rate. Third trace from top: raw recording of whole nerve activity. Bottom trace: expanded view of the raw nerve activity (within the boxed region) to illustrate individual units. B, pressure–response profiles of multiunit activity from 43 control and 54 post-infected preparations. The pressure–response profile was significantly attenuated at d14–16PI compared to controls (P <0.0001, n = 8), but was significantly augmented at d28–36PI (P < 0.0001, n = 24) and at d48–56PI (P < 0.0001, n = 22). Significant differences were detected above 2 mmHg for all three time points (P ≤ 0.05 Bonferroni post hoc analysis). C, comparison of the afferent discharge responses at low threshold (0–20 mmHg) and high threshold (20–60 mmHg) distension pressures. At both low and high thresholds, afferent discharge is significantly attenuated at d14–16PI, but is augmented at both d28–56PI and d48–56PI. *P < 0.05; #P < 0.01; *P < 0.001, one-way ANOVA.
Table 1.
Baseline activity values for control and post-infected preparations for group nerve activity (A) and single unit activity (B)
| Baseline activity | ||
|---|---|---|
| Sample group (n) | (imp s−1) | P |
| A. Group nerve activity | ||
| Controls (43) | 21.2 ± 1.5 | n/a |
| d14–16PI (8) | 8.5 ± 4.0 | P <0.05 |
| d28–36PI (24) | 31.1 ± 1.9 | P <0.01 |
| d48–56PI (22) | 29.1 ± 2.7 | P <0.05 |
| B. Single unit activity | ||
| Controls (42) | 0.78 ± 0.09 | n/a |
| d14–16PI (23) | 0.39 ± 0.1 | P > 0.05 |
| d28–36PI (47) | 1.48 ± 0.28 | P <0.05 |
| d48–56PI (21) | 1.86 ± 0.29 | P <0.05 |
Values are mean ±s.e.m., statistical analysis performed by one-way ANOVA.
Mesenteric afferent nerve mechanosensitivity is altered following T. spiralis infection
We investigated the effect of transient inflammation upon jejunal afferent nerve mechanosensitivity, using a repeated ramp distension protocol. In control preparations, this procedure induced a biphasic increase in afferent discharge (Fig. 2A). The initial phase of this response involved a rapid increase in afferent discharge which occurred during the first 5 mmHg change in intraluminal pressure (Fig. 2B). This was then followed by a slower increase in afferent discharge which occurred between 5 and 20 mmHg. The final phase of the distension response profile involved an increased rate of afferent discharge between 20 and 60 mmHg.
In the T. spiralis-infected animals, ramp distension of jejunal segments evoked profoundly different nerve response profiles compared to control animals (Fig. 2B). In preparations from d14–16PI animals, afferent discharge was significantly attenuated compared to control responses. In d28–36PI and d48–56PI preparations, afferent discharge was significantly augmented compared to controls. We summarized these results in a series of bar graphs whereby the low threshold component of the pressure–response profile was taken as the change in afferent discharge occurring between a 0–20 mmHg change in intraluminal pressure and the high threshold response was taken between 20 and 60 mmHg. Analysis of these data showed that afferent discharge was attenuated in d14–16PI preparations compared to controls at both low and high threshold distension pressures, whereas the afferent discharge in d28–36PI and d48–56PI preparations was augmented compared to controls at both low and high threshold distension levels (Fig. 2C). Since this study aimed to investigate the mechanisms underlying the development of afferent mechano-hypersensitivity, the d14–16PI preparations were not studied any further.
We were concerned that the compliance changes observed in these studies (see online Supplemental material) could have contributed to the altered afferent mechanosensitivity because the degree of stretch would have been greater in the more compliant post-infected bowel. Consequently we re-analysed the stimulus–response function using distending volume rather than pressure. We noted that the changes in afferent discharge were also seen when the discharge rate was plotted against filling volume (see Supplemental material). Furthermore, the compliance changes appeared to be most pronounced at the low threshold (0–20 mmHg) phase of distension at d28–36PI and were unchanged during the high threshold (20–60 mmHg) distension phase.
Single unit responses to jejunal distension in control preparations
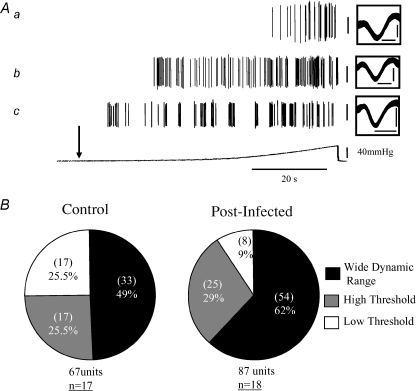
Single unit analysis of the whole nerve pressure–response profiles revealed three functional types of mechano-sensitive afferent fibre with distinct activation profiles in control preparations (Fig. 3A). The low threshold (LT) units were disproportionately activated by low-pressure distensions, typically reaching a plateau of elevated firing at intraluminal pressures of approximately 20 mmHg. LT units gave rise to a %LT value of 67.7 ± 3.6% (95% confidence interval (CI): 60.03–75.5%n = 17). The wide dynamic range (WDR) units also had a low threshold for activation but responded in a linear fashion, with afferent discharge increasing throughout the rise in intraluminal pressure suggesting that they can be activated at both physiological and nociceptive distension pressures. These unit subtypes comprised the most abundant afferent fibre types in the mesenteric nerve bundles (Fig. 3B) and possessed a %LT of 33.8 ± 2.1% (95% CI: 29.5–38.1%, n = 33). The third fibre subtype identified responded at higher thresholds only and were termed high threshold (HT) units. These had a %LT of 9.2 ± 1.2% (95% CI: 6.5–11.9%, n = 17).
Figure 3. Jejunal afferent fibre types recorded from the mesenteric nerves of control and post-infected preparations.
A, waveform analysis of the mesenteric afferent response to jejunal ramp distension revealed three mechanosensitive afferent fibre subtypes with characteristic activation profiles. Representative traces illustrate (a) a high threshold (HT) unit, (b) a wide dynamic range (WDR) unit, and (c) a low threshold (LT) unit. Vertical calibration bars, 50 μV. Downward arrow denotes start of distension profile. See Results for detailed description of units. Boxes show an expanded view of overlaid individual units. Horizontal calibration bars, 1 ms. Vertical calibration bars, 50 μV. B, percentages of unit subtypes in control and post-infected animals. In post-infected animals, afferent subunit composition is significantly altered compared to controls (χ2 = 6.4, P = 0.04). Numbers in parentheses represent the number of units identified as LT, WDR or HT (out of 67 units in control animals and 87 units in post-infected animals). Numbers underlined represent the number of experiments subjected to single unit analysis.
Single unit responses to distension in post-infectious preparations
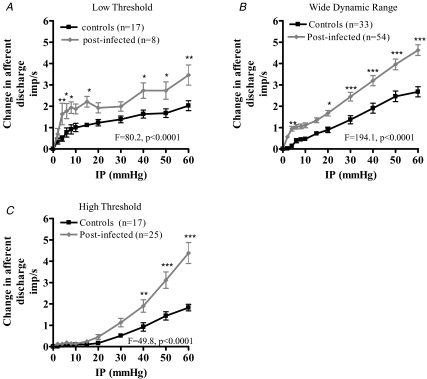
We compared the responses to distension of single units in post-infected preparations (d28–56PI) to determine what contribution these units have to the generation of afferent hypersensitivity in the post-infected animals. Single unit composition of the mesenteric nerve bundle was similar as in control preparations with LT, WDR and HT units being present. The LT subtypes possessed a %LT of 76.5 ± 8.2% (95% CI: 57.2–96.0%, n = 8), whilst the %LT values of the WDR and HT units were 37.9 ± 1.4% (95% CI: 35.1–40.6%, n = 54) and 9.5 ± 0.7% (95% CI: 7.8–11.1%, n = 25), respectively. The relative proportion of the afferent units significantly differed between the post-infected (days 28–56) and control preparations (P = 0.04, χ2 = 6.4) with fewer LT and increased numbers of WDR and HT fibres being prevalent in the post-infected preparations (Fig. 3B). All three afferent fibre subtypes demonstrated a significant sensitization in their response to distension compared to the control responses (Fig. 4A–C).
Figure 4. Summary of low threshold (A), wide dynamic range (B) and high threshold (C) jejunal mesenteric afferent fibre responses to mechanical stimulation in control and post-infected animals.
The curves for the 3 fibre types from the post-infected animals are significantly different from the controls (Bonferroni post hoc analysis, * P < 0.05; ** P < 0.01; *** P < 0.001).
The role of 5-HT in generating afferent hypersensitivity
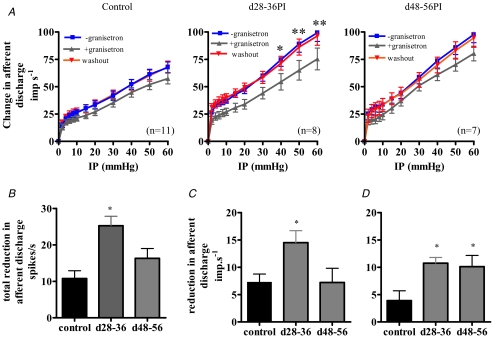
The effect of pharmacological inhibition on the afferent response to distension was investigated in control and post-infected animals using the 5-HT3 antagonist granisetron. Bath application of granisetron (30 μm, 10 min) significantly decreased the spontaneous activity in control and post-infected preparations (Table 2). However, granisetron had a significantly greater inhibitory effect upon the spontaneous activity in d28–36PI preparations compared to controls. Granisetron decreased spontaneous activity in control preparations by 4.5 ± 0.9 spikes s−1 (n = 11) whilst in d28–36PI and d48–56PI preparations, spontaneous activity was decreased by 7.5 ± 0.75 spikes s−1 (P <0.05 one-way ANOVA versus controls, n = 8) and 4.58 ± 0.8 spikes s−1 (P > 0.05 one way ANOVA versus controls, n = 7), respectively. Furthermore, granisetron (30 μm, 10 min) caused a significant decrease in the afferent response to distension in control and post-infected preparations (Fig. 5A). We compared the effect of granisetron on the control and post-infective distension responses and found that granisetron caused a significantly greater reduction in the total afferent discharge in only the d28–36PI preparations (Fig. 5B). However, analysing the distension response profiles between 0 and 20 mmHg (Fig. 5C) and between 20 and 60 mmHg (Fig. 5D) showed that, compared to the control responses, the low threshold response is attenuated at d28–36PI only, whilst the high threshold response is attenuated at both d28–36PI and d48–56PI.
Table 2.
Baseline activity for control and post-infected preparations in the absence and presence of granisetron
| Baseline activity (imp s−1) | |||
|---|---|---|---|
| Group (n) | − granisetron | + granisetron | P |
| Controls (11) | 19.3 ± 2.4 | 14.8 ± 2.0 | P = 0.0008 |
| d28–36PI (8) | 28.6 ± 3.2 | 21.1 ± 2.5 | P = 0.0001 |
| d48–56PI (7) | 21.6 ± 4.1 | 17.1 ± 3.1 | P = 0.0013 |
Values are mean ± s.e.m. Statistical analysis is by paired t test.
Figure 5. Pharmacological modulation of the jejunal afferent fibre response to mechanical stimulation.
A, bath application of granisetron (30 μm) resulted in a significant decrease in the mechanosensitivity of controls (P = 0.0001, n = 11), d28–36PI (P < 0.0001, n = 8) and d48–56PI (P < 0.0001, n = 7) preparations (*P < 0.05; ** P < 0.01, Bonferroni post hoc analysis). Mechanosensitive responses recovered upon removal of granisetron. B, the decrease in afferent firing with 30 μm granisetron during ramp distension (0–60 mmHg) is greater in the d28–36PI preparations compared to both controls and d48–56PI preparations (* P < 0.001, one-way ANOVA with Tukey's post hoc test). C, 30 μm granisetron blocks the low threshold response (0–20 mmHg) in the d28–36PI preparations compared to both controls and d48–56PI preparations (* P < 0.05, one-way ANOVA with Tukey's post hoc test). D, 30 μm granisetron blocks the high threshold response (20–60 mmHg) in both the d28–36PI preparations and d48–56PI preparations compared to controls (* P < 0.05, one-way ANOVA with Tukey's post hoc test).
The functional properties of nodose ganglion neurons (NGNs) in control and post-infected animals
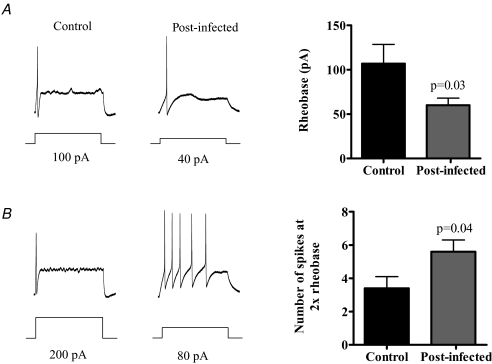
In order to determine whether there were changes in the baseline excitability of afferent nerves, we performed patch clamp studies on isolated NGNs from control and post-infected animals. We found that approximately 25–30% of NGNs in post-infected and control animals were labelled by i.p. injection of Fast Blue. Afferent firing during current injection revealed a significantly reduced rheobase in the post-infected neurons compared to controls (Fig. 6A) and was accompanied by an increase in repetitive firing, with significantly more spikes evoked by a 2 × rheobase current pulse (Fig. 6B).
Figure 6. Effect of infection on excitability of nodose ganglion neurones.
A, NGNs from post-infected animals (n = 21) were more excitable than controls (n = 16), with a lower rheobase. B, an increased number of spikes generated at 2 × rheobase.
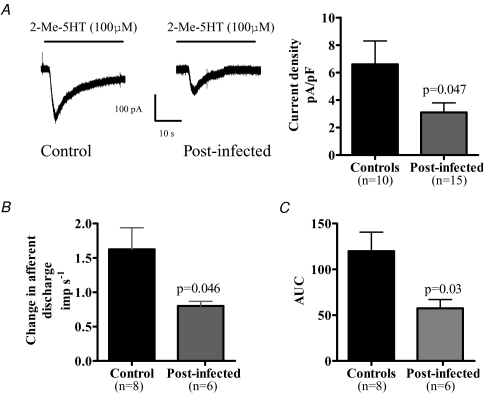
We investigated whether infection caused an increase in the number of 5-HT3 receptors by performing voltage clamp experiments on NGNs from control and post-infected animals. We employed the specific 5-HT3 agonist 2-Me-5-HT for these studies to investigate whether application of this agonist increased currents in isolated NGNs. Application of 2-Me-5-HT evoked a rapid inward current from these cells (Fig. 7A) and whilst the proportion of neurons responding to 2-Me-5-HT was similar in post-infected animals compared to controls (48% compared to 65%, P = 0.38, Fisher exact test), the post-infected neurons showed a significant reduction in the size of the currents evoked by 2-Me-5-HT (Fig. 7A).
Figure 7. 5-HT3 receptor activity is decreased in post-infected animals.
A, 5-HT3-mediated currents evoked in NGNs are reduced in post-infected animals. B, bath application of 2-Me-5-HT (100 μm) to jejunal segments resulted in an increased afferent discharge. Peak single unit activity in response to 2-Me-5-HT was reduced in the post-infected preparations compared to controls. C, area under the curve (AUC) analysis of afferent response to 2-Me-5-HT in jejunal segments. The AUC was reduced in post-infected animals compared to controls.
The response of mesenteric afferents to bath application of the 5-HT3 receptor agonist 2-Me-5-HT
We investigated whether infection changed the 5-HT3-mediated response in mesenteric afferents using 2-Me-5-HT. Bath application of 2-Me-5-HT (100 μm final concentration) induced a rapid increase in afferent discharge in both control and post-infected preparations which reversed rapidly upon washout. The peak single unit response to 2-Me-5-HT and the area under the curve (AUC) were significantly attenuated in the post-infected preparations compared to controls (Fig. 7B and C).
Discussion
This study set out to investigate the effect of transient inflammation upon 5-HT availability and visceral afferent signalling processes in T. spiralis-infected mice. Enteric infection altered mesenteric afferent responses to mechanical stimulation, with an initial hyposensitivity occurring during the inflammatory period being followed by a persistent post-infectious hypersensitivity. This study is, to our knowledge, the first to report such biphasic changes in the mechanosensory signalling pathways in the gut. Furthermore, since these events occurred in parallel with altered mucosal 5-HT metabolism, we hypothesized that an increased availability of 5-HT could contribute towards the afferent mechano-hypersensitivity observed in the post-infectious phase of this model.
Persistent changes in the mucosa of T. spiralis-infected animals have been reported including hyperplasia of the 5-HT-containing EC cells (Wheatcroft et al. 2005) which could account for the increased 5-HT bioavailability we observed at d14PI and d28PI. 5-HT has been established as a powerful signalling molecule in the gut, activating vagal (Blackshaw & Grundy, 1993; Hillsley & Grundy, 1998; Hillsley et al. 1998) and high threshold colonic afferents (Hicks et al. 2002; Coldwell et al. 2007). Consequently, the increased 5-HT availability could have a profound effect upon these 5-HT-mediated signalling processes.
Turnover of 5-HT is also a critical determinant of the efficacy of 5-HT-mediated signalling processes and is reliant upon the activity of the high affinity serotonin transporter protein (SERT). We measured 5-HT turnover as a ratio of 5-HIAA/5-HT (Blakely et al. 1991) since upon its release from EC cells, 5-HT is taken up by SERT-expressing enterocytes and oxidized to 5-HIAA by monoamine oxidase (Dunlop et al. 2005). The altered 5-HT turnover associated with this model, whereby long-term EC cell hyperplasia is matched by an elevated 5-HT turnover, suggests that adaptive processes occur in the gut in response to infection in an attempt to normalize 5-HT levels. This adaptive process would serve to protect the 5-HT receptors from desensitization, and the catastrophic ramifications in terms of signalling and motility processes which would otherwise occur in the gut of these animals following such an event.
Sensory signalling from the gastrointestinal tract can be initiated by a variety of luminal factors, including distension. Circumferential and longitudinal stretch of the gut wall activates extrinsic pathways which can signal pain in humans (Ritchie, 1973) and produce pseudoaffective responses in animals (Larsson et al. 2003). In this study, mesenteric afferents responded to ramp distensions as previously observed (Booth et al. 2001, 2008; Rong et al. 2004) with contributions from distinct populations of LT, WDR and HT mechanosensitive fibres. The LT afferents were activated at low distension pressures and saturated within physiological limits of distension. Conversely, the HT units were activated by high distension pressures and did not saturate fully during ramp distensions to 60 mmHg. However, the magnitude of their response was similar to the LT fibres. WDR afferent fibres were activated at low levels of distension but did not saturate at physiological distension pressures and responded throughout the distension range. These fibre subtypes appear to be analogous to the muscular afferents present in the mouse colon and bladder which are also capable of being sensitized in response to inflammation (Jones et al. 2005; Xu & Gebhart, 2008).
We were not able to discriminate between vagal or spinal pathways in this study, but work in vagotomized animals (Booth et al. 2008) has suggested that the LT units are predominantly vagal, whilst the WDR and HT units are predominantly spinal, although a small percentage of vagal units can respond into the nociceptive range (Booth et al. 2008). Furthermore, we could not rule out the possibility that our recordings included contributions from mechanosensitive intestinofugal fibres (Szurszewski & Weems, 1976), although studies have shown that their contribution to jejunal mechanosensitivity is small (Booth et al. 2008). These results show that the jejunum contains a varied population of mechanosensitive afferent fibres capable of responding to both physiological and potentially noxious levels of distension.
Our findings that afferent hyposensitivity to mechanical stimulation occurred in the late inflammatory phase of infection were surprising. It is possible that this phenomenon occurs as a result of muscular and neuronal damage caused by the presence of the parasite in the intestine. However, it is also becoming apparent that many endogenous mediators have the capacity to decrease, as well as increase, afferent sensitivity within the gut. Studies have shown that somatostatin inhibits mesenteric afferent responses to mechanical and chemical stimulation via SST2 receptor activation (Booth et al. 2001). Furthermore in an animal model of colitis, visceral perception in periods of chronic inflammation was normalized through the up-regulation of opioid modulators whereas periods of acute inflammation were associated with hyperalgesia (Verma-Gandhu et al. 2007). Thus afferent firing may reflect a balance between mediators that increase excitability and those with an inhibitory action. In the early phase of T. spiralis infection, the actions of inhibitory mediators may be dominant and this is currently under investigation. Furthermore these findings correlate with the results of a study in humans whereby active Crohn's disease is associated with increased thresholds for discomfort in response to rectal distension whereas individuals with IBS exhibit heightened sensitivity (Bernstein et al. 1996).
In the post-infectious preparations, however, we found that basal activity was increased and the sensitivity of jejunal afferents responding at both low and high distending pressures was augmented, suggesting that afferent hypersensitivity had developed. Analysis of the single unit responses to distension showed that their response magnitudes to both low and high threshold stimuli had increased. The composition of afferent subunits responding to distension also changed in the post-infected preparations, with 90% of the units corresponding to a WDR or HT phenotype compared to 75% in control preparations. This altered fibre composition may reflect an ability of the LT fibres to develop a WDR response profile in the post-infected gut which would have the effect of extending their response profile into the nociceptive range. The functional significance of these altered signalling paradigms is clear. The augmented response to physiological levels of distension means that afferent fibres discharge at a greater rate to low levels of stimulation. Secondly, most of the afferent fibres contributing to the distension response can encode both physiological stimuli and into the noxious range. Sensitization of these units would give rise to a lowered pain threshold and also contribute to the generation of afferent hypersensitivity in the post-infected gut.
In order to investigate the mechanisms underlying these changes, we studied the properties of vagal afferent neurons at the cellular level. Previous studies in T. spiralis-infected mice have demonstrated that dorsal root ganglion neurons are hyper-excitable post infection (Bercik et al. 2004), a phenomenon which was also observed in N. brasiliensis-infected mice (Hillsley et al. 2006). In this study we performed complimentary experiments on NGN neurons, and found that these too were more excitable in the post-infectious period. We believe that the increased excitability of these neurons could account for basal and mechano-hypersensitivity, through a reduction in threshold for activation, and an increased discharge at lower levels of stimulation.
We also implicated a role for 5-HT in mediating components of the afferent hypersensitivity associated with the post-infectious phase. Endogenous 5-HT release has been shown to contribute to the basal activity of jejunal afferents via 5-HT3 receptor activation, since this activity was susceptible to partial blockade by granisetron (Hillsley et al. 1998). We hypothesized that the augmented basal activity observed at d28–36PI could be due in part to the increased 5-HT availability associated with this time point, since more 5-HT would be available to activate 5-HT-sensitive afferent fibres, or increase the receptive field of activation by diffusing further within the gut wall. Our findings that granisetron had a greater inhibitory effect on basal activity at d28–36PI compared with either controls or d48–56PI preparations supported this hypothesis but since basal activity was still augmented at d48–56PI, and the fact that granisetron did not completely block basal activity in either control or post-infectious preparations, suggests that other mechanisms are also contributing to this phenomenon.
Our findings also indicated that 5-HT contributed to the generation of mechanosensitivity at d28–36PI, a time when mucosal 5-HT bioavailability is increased. Granisetron significantly attenuated the total distension response at d28–36PI compared to controls, whilst having no effect on the total distension response at d48–56PI. However, these data are controversial with respect to previous studies in vivo (Hillsley & Grundy, 1998; Hillsley et al. 1998) and in vitro (Hicks et al. 2002; Coldwell et al. 2007) which did not find a 5-HT3-mediated mechanosensitive component in rat jejunal or colonic preparations, although comparisons of our data with these studies are compromised by species and experimental differences that exist between these studies. For example, the thinner bowel wall in the mouse may allow mucosally released 5-HT better access to mechanosensitive endings than in the rat.
It is likely that other mechanisms are contributing to the afferent hypersensitivity observed during this study. However, 5-HT3 receptor up-regulation is unlikely to be contributing to the effects seen here since our results from the experiments with 2-Me-5-HT on isolated NGNs and at the nerve terminal suggest that 5-HT3 receptor activity is decreased in post-infected animals. Interestingly, enteric 5-HT3B subunit expression was attenuated in SERT-deficient mice (Liu et al. 2002) as an adaptive response to the increased 5-HT bioavailability associated with this animal. We believe that a similar adaptive response is probably also occurring in the T. spiralis-infected animals and that the net sensitivity reflects a balance between increased 5-HT bioavailability and down-regulation of the 5-HT3 receptors. However, the high concentration of granisetron needed in the current study (30 μm) may reflect non-specific actions although it should be noted that high concentrations would also be necessary for penetration of the bath-applied drug into the deeper layers where the sensory endings are located.
Recent reports showed neither mechano-hypersensitivity nor increased baseline activity following transient inflammation in either rat colonic afferents, in response to dextran sulphate sodium (DSS) treatment (Larsson et al. 2006; Coldwell et al. 2007), or in mice infected with N. brasileinsis (Aerssens et al. 2007). The inflammatory response associated with these models primarily affects the mucosa, and thus may not impact on mechanosensitivity generated from deeper layers in the gut wall. T. spiralis infection causes extensive transmural damage within the small intestine which perhaps allows a greater degree of afferent nerve sensitization affecting afferents throughout the gut wall.
In conclusion, we have provided evidence that T. spiralis infection causes an increase in the excitability of gastrointestinal sensory nerves and a sensitization of mechano-sensitive afferent fibres that is in part dependent upon the release of 5-HT. Furthermore, there appears to be a functional down-regulation of 5-HT3 receptors at the cell membrane in response to T. spiralis infection, suggesting that the increased role of 5-HT is not due to increased function of the receptor but is probably a combination of increased baseline excitability and 5-HT bioavailability.
Acknowledgments
This study was supported by the BBSRC (C.K.). M.B. was supported by a fellowship from the Canadian Association of Gastroenterology. We thank Ms Danielle Estoppy and Mrs Elizabeth Martin for technical support.
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.156984/DC1
References
- Aerssens J, Hillsley K, Peeters PJ, de Hoogt R, Stanisz A, Lin JH, Van den Wyngaert I, Göhlmann HW, Grundy D, Stead RH, Coulie B. Alterations in the brain–gut axis underlying visceral chemosensitivity in Nippostrongylus brasiliensis-infected mice. Gastroenterology. 2007;132:1375–1387. doi: 10.1053/j.gastro.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology. 2005;129:131–141. doi: 10.1053/j.gastro.2005.03.049. [DOI] [PubMed] [Google Scholar]
- Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224–1232. doi: 10.1053/gast.1997.v113.pm9322517. [DOI] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Collins SM. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin North Am. 2005;34:235–245. vi–vii. doi: 10.1016/j.gtc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Bercik P, Wang L, Verdu EF, Mao YK, Blennerhassett P, Khan WI, Kean I, Tougas G, Collins SM. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology. 2004;127:179–187. doi: 10.1053/j.gastro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Niazi N, Robert M, Mertz H, Kodner A, Munakata J, Naliboff B, Mayer EA. Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain. 1996;66:151–161. doi: 10.1016/0304-3959(96)03062-x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Booth CE, Kirkup AJ, Hicks GA, Humphrey PP, Grundy D. Somatostatin sst2 receptor-mediated inhibition of mesenteric afferent nerves of the jejunum in the anesthetized rat. Gastroenterology. 2001;121:358–369. doi: 10.1053/gast.2001.26335. [DOI] [PubMed] [Google Scholar]
- Booth CE, Shaw J, Hicks GA, Kirkup AJ, Winchester W, Grundy D. Influence of the pattern of jejunal distension on mesenteric afferent sensitivity in the anaesthetized rat. Neurogastroenterol Motil. 2008;20:149–158. doi: 10.1111/j.1365-2982.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Pharmacogenomics and serotonergic agents: research observations and potential clinical practice implications. Neurogastroenterol Motil. 2007;19(Suppl. 2):40–45. doi: 10.1111/j.1365-2982.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Coldwell JR, Phillis BD, Sutherland K, Howarth GS, Blackshaw LA. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J Physiol. 2007;579:203–213. doi: 10.1113/jphysiol.2006.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA. The Rome criteria process: diagnosis and legitimization of irritable bowel syndrome. Am J Gastroenterol. 1999;94:2803–2807. doi: 10.1111/j.1572-0241.1999.02803.x. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003a;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003b;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3:600–607. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Hicks GA, Coldwell JR, Schindler M, Ward PA, Jenkins D, Lynn PA, Humphrey PP, Blackshaw LA. Excitation of rat colonic afferent fibres by 5-HT3 receptors. J Physiol. 2002;544:861–869. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol. 1998;509:717–727. doi: 10.1111/j.1469-7793.1998.717bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 1998;506:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Lin JH, Stanisz A, Grundy D, Aerssens J, Peeters PJ, Moechars D, Coulie B, Stead RH. Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice. J Physiol. 2006;576:257–267. doi: 10.1113/jphysiol.2006.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia N, Hardcastle J, Grasa L, Keating C, Pelegrin P, Bardhan KD, Grundy D. Intestinal secretory and absorptive function in Trichinella spiralis mouse model of post-infective gut dysfunction role of bile acids. Gut. 2007;57:41–49. doi: 10.1136/gut.2006.118356. [DOI] [PubMed] [Google Scholar]
- Larsson M, Arvidsson S, Ekman C, Bayati A. A model for chronic quantitative studies of colorectal sensitivity using balloon distension in conscious mice – effects of opioid receptor agonists. Neurogastroenterol Motil. 2003;15:371–381. doi: 10.1046/j.1365-2982.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- Larsson MH, Rapp L, Lindström E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol Motil. 2006;18:144–152. doi: 10.1111/j.1365-2982.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- Liu MT, Rayport S, Jiang Y, Murphy DL, Gershon MD. Expression and function of 5-HT3 receptors in the enteric neurons of mice lacking the serotonin transporter. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1398–G1411. doi: 10.1152/ajpgi.00203.2002. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- Minderhoud IM, Oldenburg B, Wismeijer JA, Berge Henegouwen GP, Smout AJ. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49:469–474. doi: 10.1023/b:ddas.0000020506.84248.f9. [DOI] [PubMed] [Google Scholar]
- O'Hara JR, Ho W, Linden DR, Mawe GM, Sharkey KA. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G998–G1007. doi: 10.1152/ajpgi.00090.2004. [DOI] [PubMed] [Google Scholar]
- Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Grundy D. Jejunal atterent nerve sensitivity in wild-type and TRPVI Knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH, Weems WA. A study of peripheral input to and its control by post-ganglionic neurones of the inferior mesenteric ganglion. J Physiol. 1976;256:541–556. doi: 10.1113/jphysiol.1976.sp011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance BA, Blennerhassett PA, Collins SM. Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. Am J Physiol Gastrointest Liver Physiol. 1997;272:G321–G327. doi: 10.1152/ajpgi.1997.272.2.G321. [DOI] [PubMed] [Google Scholar]
- Verma-Gandhu M, Verdu EF, Bercik P, Blennerhassett PA, Al Mutawaly N, Ghia JE, Collins SM. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut. 2007;56:358–364. doi: 10.1136/gut.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.