Abstract
Acute ischaemia–reperfusion disrupts capillary fine structure and increases leukocyte adhesion in postcapillary venules. We determined whether chronic muscle ischaemia has similar consequences, and whether it is possible to ameliorate its effect on muscle performance. Following ischaemia (unilateral ligation, common iliac artery) rat hindlimb muscles were examined without other intervention or following treatment with an xanthine oxidase inhibitor (allopurinol), a Na+/H+ exchange blocker (amiloride), or an oxygen free radical scavenger (vitamin E). No significant leukocyte adhesion or rolling, nor changes in capillary fine structure were observed 3 days postsurgery, when limb use was limited. However, leukocyte rolling and adhesion almost trebled by 7 days (P < 0.001), when normal gait was largely restored. Capillary fine structure was disturbed over a similar time course, e.g. relative endothelial volume (control 46%, 7 days 61%; P < 0.05), that resolved by 5 weeks. Where activity was increased by mild electrical stimulation 3 days after ligation muscles showed enhanced capillary swelling (endothelial volume 66%versus 50%, P < 0.005), but improved fatigue index (52%versus 16%, P < 0.001) as a result of greater blood flow. Muscle fatigue after ligation was related to the extent of contraction-induced hyperaemia (R2= 0.725), but not capillary swelling. Amiloride, and to a lesser extent allopurinol but not vitamin E, significantly decreased leukocyte rolling and adhesion, as well as capillary endothelial swelling. We conclude that increased activity of ischaemic muscles on recovery is likely to accentuate acidosis accompanying changes in microcirculation and contribute to enhanced muscle fatigue, whereas formation of oxygen free radicals may be attenuated by endogenous protective mechanisms.
Peripheral vascular diseases affect about 7% of people over 55 years of age (Leng et al. 1996). The incidence increases with age and is usually linked with impairment in coronary or cerebral circulation (Rothwell et al. 2005), thus representing serious health impairment in the ageing population. So far the most effective treatment of intermittent claudication, a symptom of peripheral vascular disease, has been exercise. However, intensive activity can also damage ischaemic muscles (Hudlickáet al. 1994). One of the reasons for muscle damage may be an impaired muscle microcirculation, indicated by capillary endothelial swelling and increased leukocyte adhesion. Swollen endothelium has been demonstrated by electron microscopy in acutely ischaemic muscles (Strock & Majno, 1969), especially when followed by reperfusion (Gidlöf et al. 1988), and increased permeability (Suval et al. 1987). Leukocyte adhesion to venular endothelium has been demonstrated in skeletal muscle (Nolte et al. 1992) and other tissue as a general feature of reperfusion injury (Ley, 1992). Either or both of these processes may contribute to impaired capillary perfusion due to microvascular narrowing or blockade. Changes in microcirculation similar to those following reperfusion were observed during haemorrhagic shock (Perry & Granger, 1992; Kretchmar & Engelhardt, 1994). Capillary endothelial swelling could be decreased by amiloride blockade of Na+/H+ exchange (Mazzoni et al. 1992), which is also activated during reperfusion (Masereel et al. 2003), while leukocyte adhesion in muscles exposed to ischaemia–reperfusion was prevented by oxygen free radical scavengers (Gute et al. 1998).
There are few data on capillary fine structure and leukocyte behaviour in chronically ischaemic muscles. Hou et al. (1995) described increased leukocyte adhesion in rat cremaster muscle 3 weeks after ligation of its feed artery. We have previously shown that capillary endothelial swelling and increased leukocyte adhesion occurred in chronically ischaemic muscles 2 weeks after unilateral ligation of the common iliac artery in the rat, and that some changes were also found in contralateral muscles (Hickey et al. 1992, 1993; Egginton et al. 1993). While it is impossible to assess the contribution of these two processes to impairment of capillary perfusion, clearly they are linked (this study; Anderson et al. 2006). Leukocyte adhesion represents an obstacle to capillary perfusion in acute reperfusion, and also results in increased capillary permeability, increased interstitial volume and hence tissue pressure, and consequently a drastic decrease in the number of perfused capillaries (Jerome et al. 1994). The highest percentage of capillaries with swollen endothelium (70% compared with 27% in control muscles) was found in extensor digitorum longus (EDL) 7 days after ligation (Hughes & Hudlická, 1992). Consistent with an impaired microcirculation, the time spent stationary by red blood cells was significantly increased, and surface PO2 significantly decreased in comparison with control muscles (Dawson et al. 1990). Additionally, the number of adhering and rolling leukocytes was increased, suggesting activation of the venular endothelium (Anderson et al. 2006). In combination, these changes may explain the diminished muscle performance seen at this time (Hughes & Hudlická, 1992; Milkiewicz et al. 2006). Changes in leukocyte adhesion and the extent of capillary endothelial swelling may be related to muscle activity, since the animals start to use the ischaemic leg 3–7 days after ligation. In agreement with this, the proportion of rolling and adhering leukocytes was normal in EDL 3 days after ligation when the ischaemic leg is little used, but when the ligated muscles were subjected to a mild increase in activity by electrical stimulation for 2 out of the 3 days, the proportion of adhering leukocytes increased in comparison with muscles that were not stimulated (Anderson et al. 2006). We reasoned that similar changes would be observed in capillary fine structure.
The mechanism underlying the effect of muscle activity on capillary endothelial swelling is not clear. Ischaemic muscles have low pH (Challis et al. 1986) and muscle activity would certainly increase local acidosis. Acidosis was thought to be responsible for capillary swelling in shock (Mazzoni et al. 1992) which was diminished by amiloride, and an amiloride analogue also reduced capillary lumen narrowing resulting from systemic blood acidosis in combination with decreased blood flow (Mazzoni et al. 1994). Furthermore, it is possible that reperfusion which occurs after acute muscle ischaemia might also occur with a chronic insult: femoral artery blood pressure dropped immediately after ligation but slightly recovered 7 days later, indicating partial reperfusion (Hudlickáet al. 1994), and hence swelling as well as leukocyte adhesion could be triggered by generation of oxygen free radicals.
We hypothesised that increased muscle activity at an early stage after interruption of blood supply may accelerate metabolic changes induced by ischaemia (e.g. lowering of pH) and reperfusion that occurs gradually, and thus lead to capillary damage and microcirculatory dysfunction. Such changes may be accentuated by imposition of additional muscle activity, such as seen with therapeutic exercise. We tested this hypothesis by determining whether these responses could be attenuated by pharmacological intervention, or intensified by muscle stimulation.
Methods
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986, and had local ethical committee approval.
Surgical procedures
Experiments were performed on male Sprague–Dawley rats (Rattus norvegicus) using animals of 390–450 g for blood flow and muscle performance estimations (5–7 animals were used in each of the treatment groups, Table 1), and ∼290 g body mass for intravital microscopy observations (5 rats per group). The common iliac artery was ligated 3–5 mm below the bifurcation from the abdominal aorta under halothane anaesthesia (2% Fluothane, ICI, in oxygen at 2 l min−1) and aseptic conditions. The animals were given systemic analgesic (2.5 ml kg−1 buprenorphine, s.c., Temgesic, National Veterinary Services, Stoke-on-Trent, UK) and topical antibiotic (Duplocillin LA, National Veterinary Services, Stoke-on-Trent, UK) twice a day for the first 2 days. The animals recovered within 20–30 min and received either distilled water or 0.5 ml of the xanthine oxidase inhibitor allopurinol (50 mg kg−1; Sigma-Aldrich Co., UK), the Na+/H+ exchanger blocker amiloride (5 mg kg−1), or the oxygen free radical scavenger vitamin E (50 mg kg−1 in castor oil and polyethyleneglycol) by gavage the afternoon after surgery, and twice daily for the following 6 days, with the last dose given ∼16 h before being taken into the final experiment. To study the effect of additional activity on capillary fine structure, one group of animals had Teflon-coated stainless steel multistranded electrodes implanted close to the peroneal nerve at the time of the ligation of the iliac artery. In these animals the extensor digitorum longus and tibialis anterior muscles were stimulated for 2 days starting ∼24 h after surgery. The stimulation was performed 7 times per day in periods of 15 min with a rest of 85 min at 10 Hz, pulse width 0.3 ms, and up to 5 V intensity using a Neurotech Multichannel Stimulator (Bio-Medical Research, West Galway, Ireland), as previously described (Hudlickáet al. 1994). A group of animals with iliac artery ligation were taken into experiment 3 days later for studies of leukocyte adhesion (cf.Anderson et al. 2006) and capillary fine structure. All other animals were taken into experiment 7 days after unilateral ligation of the iliac artery. For acute experiments, animals were anaesthetised by intraperitoneal injection of sodium pentobarbitone (Sagatal; May & Baker, Dagenham, UK) 50 mg (kg body mass)−1 diluted 1 : 1 with saline. At the end of observations, animals were killed by anaesthetic overdose.
Table 1.
Body mass, EDL/TA muscle mass, peak tension development, activity-induced muscle blood flow, and fatigue index following iliac artery ligation
| Days ligation (n) | Body mass (g) | Ischaemic muscle mass (g) | I/CL | Ipsilateral tension (g/g) | I/CL | Ischaemic MBF (ml min−1 (100 g)−1) | I/CL | Ipsilateral FI (%) | I/CL |
|---|---|---|---|---|---|---|---|---|---|
| 0 (7) | 391.9 ± 17.0 | 0.894 ± 0.054 | 0.966 | 280.0 ± 14.5 | 1.095 | 123.0 ± 18.1 | 0.936 | 66.0 ± 2.7 | 1.023 |
| 3 (5) | 365.2 ± 17.3 | 0.814 ± 0.048 | 0.977 | 186.4 ± 17.3* | 1.416 | 4.1 ± 0.5* | 0.053 | 15.5 ± 0.9* | 0.236 |
| 7 (7) | 349.3 ± 5.2* | 0.786 ± 0.036 | 0.892 | 188.3 ± 12.9* | 1.360 | 4.3 ± 1.2* | 0.069 | 20.0 ± 3.8* | 0.309 |
| 35 (6) | 408.3 ± 15.3 | 0.926 ± 0.051 | 0.894 | 145.3 ± 19.2* | 0.824 | 29.1 ± 5.6* | 0.498 | 52.2 ± 4.3* | 0.841 |
| 3d SL (6) | 349.2 ± 6.2* | 0.816 ± 0.043 | 0.993 | 119.6 ± 29.7* | 0.739 | 6.7 ± 0.8* | 0.100 | 36.9 ± 7.0* | 0.572 |
Means ±s.e.m. (number of animals).
P < 0.05 versus control (ANOVA). Abbreviations: I, ischaemic muscle; CL, contralateral muscle; MBF, muscle blood flow; FI, fatigue index; 3d SL, 3 days ligation and 2 days stimulation.
Muscle performance and blood flow measurement
The right jugular vein was cannulated using vinyl tubing to supplement anaesthesia as necessary, and a tracheal cannula assisted spontaneous breathing. Both brachial arteries were cannulated, for the recording of blood pressure via a pressure transducer (Bell & Howell, Basingstoke, UK) and withdrawal of blood samples for measurement of blood flow. The left ventricle was cannulated via the right carotid artery, with the pressure pulse used to identify when the tip of the cannula was in the ventricle. For recording of muscle tension the thighs were fixed to a specially designed board where a U-shaped lead restraining bar was bent around the groin to prevent movement of the thigh and knee joint, thus providing a stable position of the calf from which muscle contractions could be measured. Feet were clamped into special holders, and the tendons of EDL and TA were exposed in both hindlimbs, cut and attached to strain gauges to record isometric muscle tension in response to indirect stimulation of the peroneal nerve at 4 Hz and a pulse width of 0.3 ms. The stimulation voltage and muscle length were adjusted to give maximum force of contraction. Tension output and blood pressure recordings were displayed on a six-channel recorder (Lectromed, Letchworth Garden City, UK). Muscle performance was assessed as peak tension and fatigue index (tension in g (g muscle)−1 at the end of the period of contractions/peak tension × 100).
Radioactive microspheres (15 μm diameter and labelled with 46Sc, 57Co, or 113Sn; PerkinElmer (NEN), Groningen, the Netherlands) were used to measure muscle blood flow (MBF) at rest and at the end of 5 min isometric twitch contractions in the EDL and TA as previously described (Hudlickáet al. 1994). Each animal received 1.5–1.8 × 105 microspheres. The blood flow was measured in tibialis anterior and extensor digitorum longus muscles, where the number of microspheres was at least 400 (estimated on the basis of specific activity), which is sufficient to ensure reasonable accuracy (Buckberg et al. 1971). A reference blood sample was taken at a withdrawal rate of 0.5 ml min−1 using a precision withdrawal pump (Braun, Melsungen, Germany) from the brachial artery simultaneously with injection of the microspheres into the left ventricle, to calculate MBF by scaling. The withdrawn blood volume was replaced with an intravenous infusion of 1% w/v bovine serum albumen in saline. At the end of the experiment radioactivity of EDL and TA muscles was determined (Packard Auto-Gamma Counter), along with samples from right and left kidney cortex (to confirm homogeneous distribution of microspheres) and from the lung (to assess the percentage of shunting). Any animal where the kidney samples had greater than a 10% discrepancy in blood flow, or where the lung sample showed greater than 5% shunting, was discounted.
Intravital microscopy
Another group of rats were surgically prepared with a jugular vein cannula for delivery of supplementatal anaesthetic as required, a carotid artery cannula to record blood pressure, and a tracheal cannula to allow spontaneous breathing. Two coiled stainless steel multistranded Teflon-coated electrodes were implanted in the vicinity of the peroneal nerve and exteriorised on the back of the animal for later stimulation. The EDL was exposed for intravital observation, as described by Anderson et al. (1997). The muscle was superfused with warm (37°C) deoxygenated (95% N2–5% CO2) physiological salt solution (131.9 mm NaCl, 4.7 mm KCl, 2.0 mm CaCl2, 1.2 mm MgSO4 and 22 mm NaHCO3, pH 7.35–7.45) during dissection, and for the whole time of observation under the intravital microscope (ACM Zeiss, Oberkochen, Germany). The muscle was viewed using water immersion objective (×25, NA 0.6) and fibre optic epi-illumination (equipped with a green filter), and images of vessels were recorded as described in detail previously (Thomson et al. 1994; Anderson et al. 2006). The diameter of venules, together with the branching pattern, helped to classify them as collecting venules (V4), V3 draining them, or V2 draining V3. The final magnification on the monitor was ×1000, and monitor resolution was calculated as 0.45 μm pixel−1 from measurements using a graticule. Once the animal was transferred under the microscope, the hindlimb muscles were stimulated at 1 Hz (0.3 ms pulse width at 3–5 V; Grass S8 stimulator) for 30 min. This procedure increased leukocyte adhesion (Thomson et al. 1994) and thus enabled a better assessment of interventions. Five to 10 venules in different categories in each animal were observed for 1 min intervals to measure leukocyte rolling and adhesion offline, usually over a total period of 2 h. Leukocyte rolling or stationary adhesion was estimated as number of cells per 100 μm of each individual vessel adhering, or rolling for 10 s.
Capillary supply
Sections of EDL were mounted on cork discs using OCT medium and rapidly frozen in isopentane precooled in liquid nitrogen. Cryostat sections 8 μm thick were stained for alkaline phosphatase using the indoxyl-tetrazolium method, and capillary supply was estimated by counting number of fibres and capillaries in two areas of each muscle (each 0.25 mm2) with the results expressed as capillary to fibre ratio (C : F).
Capillary fine structure
EDL from both legs were dissected free and thin strips fixed at resting length in 2.5% glutaraldehyde, 1.5% sucrose, 0.1 m phosphate buffer (350 mosmol l−1) pH 7.4 at room temperature for 1 h. We adopted immersion in isotonic fixative despite the use of high osmolarity and perfusion fixation in other studies, as the latter tends to obscure structural changes in the endothelium of ischaemic tissue (Egginton et al. 1993). The strips were then sliced into small blocks ∼2 mm3 and placed in fresh fixative for 24–48 h at 4°C. Post-fixation was carried out using 1% OsO4 in phosphate buffer for 1 h at room temperature. Samples were dehydrated in a graded series of alcohols and embedded in Agar 100/Epon substitute resin. Four blocks were prepared from each of four animals, and one per animal chosen at random for analysis. Semi-thin (0.5 μm) sections were used to ensure true transverse sections of muscle. Ultrathin sections were cut at 60–80 nm, stained with uranyl acetate for 7 min and Reynolds lead citrate for 7 min, and viewed in a Jeol 100 CX II transmission electron microscope at 80 kV. A 35 mm camera was used to capture images of capillaries (approximately 40 per muscle) at an initial magnification of ×640, using an unbiased sampling protocol. Negatives were then projected on a film reader (×17.5) and scanned into an Apple Macintosh computer, using NIH Image with purpose-written macros or Optilab software (Graftex, Meudon-la-Foret, France) to quantify capillary structure by means of stereological measurements. As perfect circular profiles only exist with vessels that are fixed in situ whilst perfused (immersion fixation removes hydrostatic pressure and reveals unperfused vessels as collapsed profiles), and tortuosity of the capillary bed would also produce oblique profiles, both cross-sectional area and perimeter were determined. The former will be sensitive to section angle and degree of opening, but the latter will be less sensitive to such differences in in vivo status. The absolute and relative area of lumen and nucleus, area of endothelial cytoplasm and surface/volume ratio of lumen were used as indices of endothelial swelling.
Statistical analysis
All results are presented as means ±s.e.m. Statistical evaluation was carried out by ANOVA with Fisher's PLSD post hoc test used for intergroup comparisons. The distribution of data for both capillary endothelial swelling and leukocyte adherence was left skewed, and was normalised by a log10 transformation before statistical processing. The distribution of capillary ultrastructural values among groups were also analysed using the Mann–Whitney U test. For all tests, P < 0.05 was taken as a significant difference.
Results
Body, muscle mass and blood pressure
The mean body and muscle mass (Table 1) and blood pressure (controls, 100.8 ± 8.7 mmHg) of all animals used in this study were similar.
Muscle performance
Twitch, rather than tetanic tension was used to assess muscle performance since the use of tetanic contractions to elicit fatigue was considered to be too severe in ischaemic muscles. The right EDL and TA muscles in control animals produced a peak isometric twitch tension of 280.0 ± 14.5 g g−1, with significantly lower values in muscles 3, 7 and 35 days following ligation (P < 0.05; Table 1). Lower peak tension was also developed by the contralateral muscles at 3 days following ligation, and a reduction was apparent throughout the whole time course following ligation (Table 1). Fatigue index (FI) was 66.0 ± 2.7% in the EDL and TA muscles of the right limb, and 64.5 ± 4.1% in the left limb in control animals. Fatiguability increased dramatically in animals 3 days following ligation, gradually reaching a value approaching that of control muscles after 35 days. The FI in contralateral muscles was not significantly different from control after ligation up to 35 days (see Table 1).
Muscle blood flow
Muscle blood flow (MBF) in EDL and TA at rest was significantly reduced at all time intervals following ligation, compared with muscles in control animals. Although some improvement was observed at 5 week after ligation (5.03 ± 1.14 ml min−1 (100 g)−1), doubling that seen at 7 days (2.60 ± 0.41 ml min−1 (100 g)−1), flow was still significantly lower than in control muscles (7.27 ± 1.13 ml min−1 (100 g)−1 in control animals, P < 0.05). Resting blood flow in the contralateral EDL and TA showed no difference at any time point after ligation (data not shown; see also Milkiewicz et al. 2006).
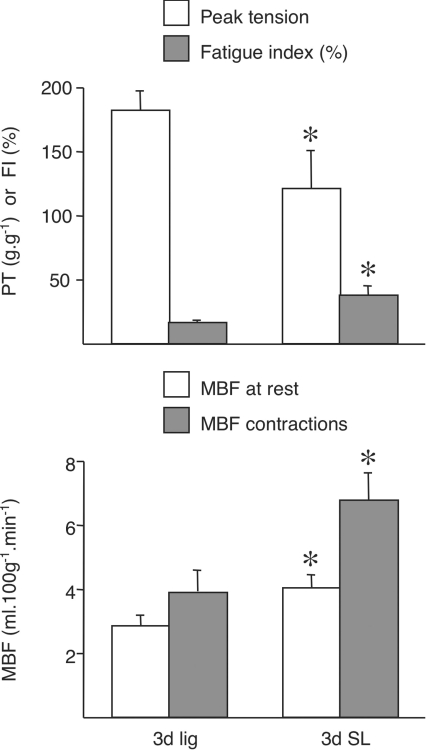
MBF at the end of a 5 min period of muscle contractions at 4 Hz increased to 123.04 ± 18.11 ml min−1 (100 g)−1 in control animals. It was limited in all animals with iliac artery ligation, with the greatest reduction seen in muscles that had been ischaemic for 3 days (4.06 ± 0.50 ml min−1 (100 g)−1 (P < 0.01 versus control; Table 1). The improvement was gradual, but MBF was still significantly lower 5 week after ligation (29.10 ± 5.62 ml min−1 (100 g)−1, P < 0.05 versus control). Flow in the contralateral muscles (always on the left side) of animals 7 days after ligation was significantly lower than in the left EDL and TA muscles in control animals (60.97 ± 6.93 ml min−1 (100 g)−1versus 117.10 ± 16.88 ml min−1 (100 g)−1, P < 0.05) but there were no differences from controls in contralateral muscles at any other time point (Table 1; see also Milkiewicz et al. 2006). The fatigue resistance of ischaemic muscle fell in line with the reduced MBF (Figs 1 and 7).
Figure 1. Muscle blood flow (MBF) and fatigue in combined EDL + TA muscles at rest and at the end of 5 min isometric contractions in 3 days ischaemic (3d lig) and 3 days ischaemic with 2 days stimulation (3d SL).
Fatigue index (FI) is the percentage of the peak tension (PT) at the end of the 5 min period of contractions. * denotes values significantly different from 3 days lig (P < 0.05). For control data, please refer to Table 1.
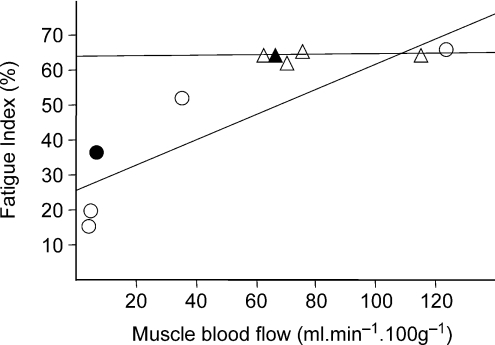
Figure 7. The relationship between muscle fatigue resistance and muscle blood flow during contractions.
Mean values are given for groups of animals at different times after ligation. Circles, ipsilateral muscle; triangles, contralateral muscle. Filled symbols represent 3d SL. Linear regression of group means give FI = 31.482 + 0.301 × MBF, R2= 0.725 (ipsilateral), and FI = 63.645 + 0.008 × MBF, R2= 0.017 (contralateral).
Leukocyte rolling and adhesion
The behaviour of leukocytes was observed in postcapillary venules of rats with unilateral iliac artery ligation, and in those also treated with allopurinol, amiloride and vitamin E. Evaluation was performed in 45–50 collecting venules (V4), 60–70 V3 and 35–45 V2 in the various groups of rats. Resting venular diameters were V4= 7.0 ± 0.3 μm, V3= 11.4 ± 0.9 μm and V2= 19.0 ± 0.8 μm at 7 days, similar to those previously described (Anderson et al. 2006), and did not appear to have been affected by drug treatment. There was, however, a transient, though modest, dilatation at 3 days, possibly due to surgical trauma, with venule diameters of 8.7 ± 0.5, 12.1 ± 0.9 and 24.6 ± 2.8 μm, respectively (n.s.).
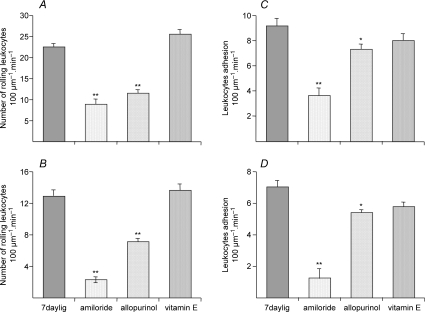
No rolling or adhering leukocytes were found in the smallest collecting venules (V4) either in control or in muscles ischaemic for 7 days. Both rolling and adherent leukocytes were observed in V3 with 12.8 ± 0.8 and 7.0 ± 0.4 (100 μm segment)−1 (60 s)−1, respectively (4× and 17× increase from control values, P < 0.05). Values in V2 were 22.3 ± 0.79 and 9.1 ± 0.6 (100 μm segment)−1 (60 s)−1, respectively (2.4× and 6.4× increase from controls). The values in muscles ischaemic for 3 days only were similar to controls, but were increased by mild electrical stimulation, as seen previously (Anderson et al. 2006). Amiloride practically eliminated both rolling and adherence in V3, and significantly decreased it in V2 (to 8.7 ± 1.2 and 3.6 ± 0.6 (100 μm segment)−1 (60 s)−1, respectively, P < 0.01 versus ischaemia alone; Fig. 2). The effect of allopurinol was more modest (11.4 ± 0.8 and 7.2 ± 0.26 (100 μm segment)−1 (60 s)−1, respectively), with vitamin E having little or no effect (n.s., Fig. 2).
Figure 2. Leukocyte rolling and adhesion in untreated 7 days ischaemic muscles, and ischaemic muscles in animals treated with amiloride, allopurinol or vitamin.
*P < 0.05, **P < 0.01 versus ischaemia alone. For comparison, in untreated control muscles the corresponding vales are: V3 rolling 3.0 ± 1.7, stationary 0.4 ± 0.4; V2 rolling 9.3 ± 3.0, stationary 1.4 ± 0.6 min−1 (Anderson et al. 2006).
Capillary supply
Capillary to fibre ratio (C : F) in the EDL muscles of control animals was 1.32 ± 0.07. No significant differences were observed in the ischaemic muscles following ligation at 3 or 7 days but increased to 1.70 ± 0.1 (P < 0.05 versus control) 35 days after ligation. All contralateral muscles had C : F values not significantly different from those seen in control animals.
Capillary fine structure
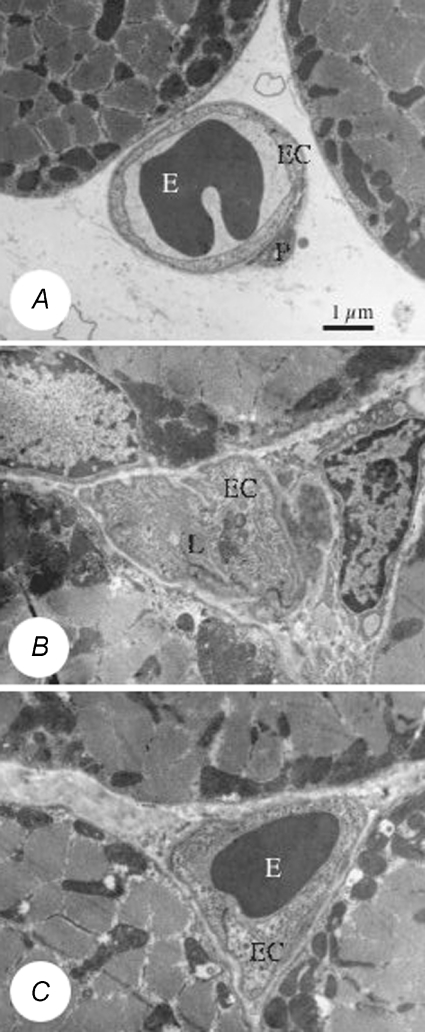
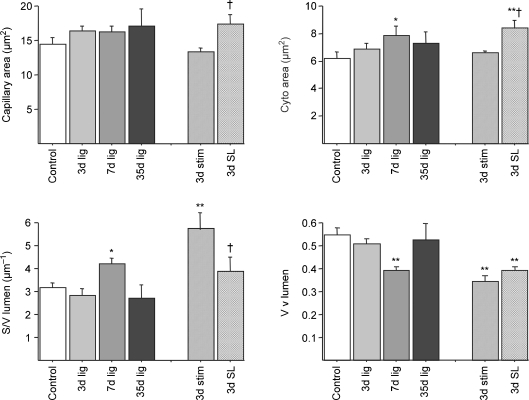
Stereological analysis of the total capillary and luminal cross sectional area or surface/volume ratio (S/V) revealed differential changes in capillary fine structure according to length of ischaemia and drug treatment. Muscle ischaemia per se did not significantly alter the cross sectional area of capillaries, but resulted in swelling of the capillary endothelium. The cytoplasm was more translucent in capillaries that showed swelling, and many capillaries had protrusions into the lumen (Fig. 3). The values for cytoplasmic area (i.e. volume of endothelium – volume of nucleus), volume density and S/V of the capillary lumen – which are indications of capillary endothelium swelling – are given in Fig. 4 for ischaemic muscles, with other parameters measured included in Table 2. Capillaries in control muscles had an endothelial cell (EC) area of 7.52 ± 0.87 μm2 and the lumen occupied 54.3 ± 3.1% of the total capillary area (cross sectional area of capillary profiles, i.e. lumen + endothelium). A significant increase in relative EC area was observed at 7 days following ligation (P < 0.05, Mann–Whitney U test), but values were not significantly different from controls after 5 week (Fig. 4). Surprisingly, there was no evidence of endothelial swelling in muscles 3 days after ligation. The increased percentage of EC cytoplasm resulted in a smaller luminal area and, reflecting this, luminal S/V was increased from 3.2 ± 0.2 μm−1 in control muscles up to 4.2 ± 0.3 μm−1 7 days after ligation. The values for cytoplasmic area and lumen S/V were significantly greater in muscles that had been ischaemic for 7 days or in those ischaemic for 3 days with increased activity (Fig. 4).
Figure 3. Changes in capillary fine structure with ischaemia.
A, electron micrograph illustrating the normal capillary phenotype from a control muscle showing the thin endothelium and smooth luminal and abluminal surfaces. B, a capillary with a swollen endothelium from a muscle 7 days after ligation of the iliac artery, where endothelial protrusions obscure the lumen. C, a capillary with a swollen endothelium from a muscle after 7 days ligation treated with amiloride, where the endothelial swelling is reduced sufficiently to allow likely passage of an erythrocyte. Abbreviations: E, erythrocyte; EC, endothelial cell; L, lumen; P, pericyte. Scale bar = 1.0 μm.
Figure 4. Capillary indices used to evaluate endothelial swelling on the basis of quantitative electron microscopy in control and ischaemic rat EDL.
Abbreviations: cyto, endothelial cytoplasm; S/V, surface to volume ratio; Vv, volume density. *P < 0.05, **P < 0.01 versus control; †P < 0.05 versus 3d stimulation alone.
Table 2.
Morphometric analysis of the capillary response to ischaemia and imposed activity in rat EDL
| Control | 3d lig | 7d lig | 35d lig | 3d stim | 3d SL | |
|---|---|---|---|---|---|---|
| Cap perim (μm) | 16.33 ± 0.27 | 16.89 ± 0.20 | 17.37 ± 0.70 | 17.82 ± 1.04 | 16.34 ± 0.50 | 18.70 ± 0.54*† |
| S/V (capillary) (μm−1) | 2.59 ± 0.32 | 2.57 ± 0.17 | 2.02 ± 0.08* | 2.36 ± 0.15 | 2.19 ± 0.08 | 2.12 ± 0.07 |
| Endo area (μm2) | 7.52 ± 0.87 | 7.82 ± 0.31 | 9.90 ± 0.72* | 8.58 ± 1.91 | 8.63 ± 0.40 | 10.45 ± 0.72* |
| Lum area (μm2) | 6.92 ± 0.23 | 8.57 ± 0.69* | 6.31 ± 0.28 | 8.56 ± 1.48 | 4.73 ± 0.36* | 6.93 ± 0.69† |
| Vv(endo,cap) | 0.457 ± 0.021 | 0.496 ± 0.024 | 0.611 ± 0.015** | 0.479 ± 0.072 | 0.660 ± 0.024** | 0.612 ± 0.017** |
| Lum perim (μm) | 13.96 ± 0.20 | 15.37 ± 0.36 | 14.03 ± 0.51 | 15.31 ± 0.81 | 13.60 ± 0.54 | 15.68 ± 0.40*† |
| Sv(lum,cap) (μm−1) | 1.068 ± 0.077 | 1.079 ± 0.065 | 0.953 ± 0.059 | 1.025 ± 0.087 | 1.097 ± 0.029 | 0.988 ± 0.071 |
Abbreviations: Cap, capillary; perim, perimeter; S/V, surface to volume ratio; Endo, endothelium; Lum, lumen; Vv, volume density; Sv, surface density.
P < 0.05
P < 0.001 versus control
P < 0.05 versus 3 days stimulation.
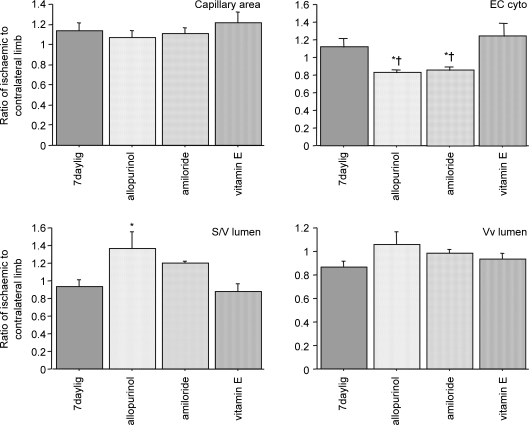
As there were minimal changes in the morphological parameters described above in contralateral muscles (see online supplemental material, Supplementary Table 1), the effect of drugs was evaluated as a proportion of change related to the contralateral side. Neither drug altered the capillary profile area. Allopurinol and amiloride, but not vitamin E decreased the cytoplasmic area, indicating their beneficial effect on reducing capillary endothelial swelling (Fig. 5 and Table 3). There was a significant correlation between resting MBF and the proportion of the capillary occupied by endothelium (R2= 0.603, P < 0.05).
Figure 5. Several parameters used for evaluation of capillary endothelial swelling expressed as a ratio between the values for contralateral and ischaemic muscles following drug treatment.
*P < 0.05 versus control; †P < 0.05 versus vitamin E.
Table 3.
Relative capillary response in rat EDL to 7 days ligation, as a ratio of ischaemic to contralateral limb
| Untreated | Allopurinol | Amiloride | Vitamin E | |
|---|---|---|---|---|
| S/V(capillary) | 0.91 ± 0.04 | 0.92 ± 0.03 | 0.90 ± 0.02 | 0.92 ± 0.06 |
| Endo area | 1.08 ± 0.11 | 0.83 ± 0.05† | 0.90 ± 0.03† | 1.34 ± 0.16 |
| Lum area | 1.25 ± 0.05 | 1.70 ± 0.26† | 1.62 ± 0.19† | 1.12 ± 0.12 |
| Cyto:Lum | 0.92 ± 0.09 | 0.54 ± 0.10*† | 0.56 ± 0.06*† | 1.14 ± 0.14 |
P < 0.05 versus untreated
P < 0.05 versus vitamin E. Cyto, endothelial cytoplasm
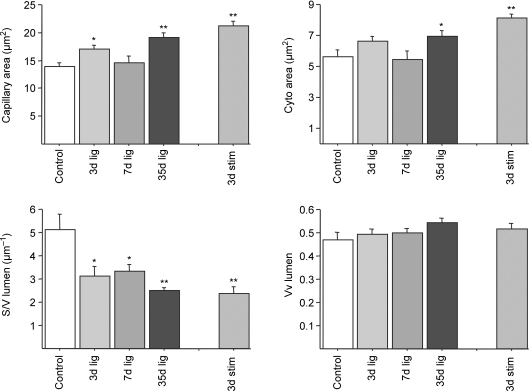
Changes in capillary fine structure were also observed in papillary muscle (Fig. 6 and Table 4). Unlike in skeletal muscles, capillary area in the papillary muscle was increased as a result of ischaemia, and although the changes in the cytoplasmic area was smaller the surface to volume ratio was decreased in all muscles from ischaemic animals, even 35 days after ligation of the common iliac artery. Other parameters (capillary and luminal cross-sectional area, proportion of lumen, and lumen radius) showed a gradual change with time after ligation. Lumen S/V gradually decreased, indicating that the increase in lumen volume was greater that the increase in the luminal perimeter, possibly as a result of fewer luminal protrusions (Table 4).
Figure 6. Capillary indices used to evaluate endothelial swelling in papillary muscle.
*P < 0.05, **P < 0.01 versus control.
Table 4.
Morphometric analysis of the capillary response to ischaemia in rat papillary muscle
| Control | 3d lig | 7d lig | 35d lig | 3d stim | |
|---|---|---|---|---|---|
| Cap perim (μm) | 21.30 ± 1.69 | 18.94 ± 0.65 | 17.96 ± 0.81* | 21.56 ± 0.68 | 21.33 ± 0.55 |
| S/V(capillary) (μm−1) | 3.357 ± 0.061 | 2.637 ± 0.040* | 2.890 ± 0.102* | 2.867 ± 0.192* | 2.527 ± 0.090** |
| Endo area (μm2) | 7.18 ± 0.59 | 8.50 ± 0.34 | 7.10 ± 0.63 | 8.70 ± 0.78 | 10.26 ± 0.48** |
| Lum area (μm2) | 6.72 ± 0.57 | 8.46 ± 0.22 | 7.43 ± 0.88 | 10.35 ± 0.46* | 10.85 ± 1.05** |
| Vv(endo,cap) | 0.541 ± 0.037 | 0.529 ± 0.022 | 0.512 ± 0.027 | 0.474 ± 0.009 | 0.495 ± 0.024 |
| Vv(cyto,cap) | 0.423 ± 0.029 | 0.413 ± 0.017 | 0.393 ± 0.021 | 0.378 ± 0.007 | 0.391 ± 0.019 |
| Lum perim (μm) | 20.97 ± 0.86 | 17.49 ± 0.74* | 16.75 ± 1.34* | 22.65 ± 1.20 | 19.36 ± 0.52 |
| Sv(lum,cap) (μm−1) | 1.624 ± 0.108 | 1.148 ± 0.049** | 1.258 ± 0.092* | 1.240 ± 0.019* | 0.980 ± 0.054** |
Abbreviations as per Table 2.
P < 0.05
P < 0.001 versus control
P < 0.05 versus 3 days stimulation.
Effect of activity on capillary fine structure, blood flow and performance in ischaemic muscles
Morphometric data based on quantitative electron microscopy showed that marked capillary EC swelling was absent at 3 days but appeared 7 days after ligation, i.e. at a time when the animals were observed first using the ischaemic limb, suggesting that structural changes could be due to a combination of ischaemia with muscle activity. To test this hypothesis, we increased muscle activity by electrical stimulation for 2 days starting 24 h after ligation of the iliac artery. This resulted in EC swelling at 3 days that was greater than with ligation alone (P < 0.05) and similar to that found in ischaemic muscles at 7 days (n.s., Fig. 4), and induced modest changes in the contralateral muscles (Supplementary Table 1, some data not shown). Although the total capillary area was greater than in controls (17.4 ± 1.4 μm2versus 14.4 ± 1.0 μm2, P < 0.05) and in 3 days ischaemic muscles (13.4 ± 0.58 μm2, P < 0.05), the luminal area did not change and thus the relative area of lumen (0.39 ± 0.02 versus 0.34 ± 0.03) as well as the luminal radius (1.38 ± 0.09 μm versus 1.56 ± 0.62 μm in 3 day ischaemic muscles) were significantly smaller (P < 0.05; Table 2, Fig. 4).
In spite of the changes in capillary fine structure that would limit capillary perfusion, animals which received muscle stimulation showed a modest improvement in resting MBF when compared with those ischaemic for 3 days (3.83 ± 0.07 ml min−1 (100 g)−1 in stimulated animals compared with 2.81 ± 0.32 ml min−1 (100 g)−1 with ligation alone, P < 0.05), as well as during muscle contractions (6.73 ± 0.84 ml min−1 (100 g)−1versus 4.06 ± 0.50 ml min−1 (100 g)−1, respectively, P < 0.05). There was no difference in MBF in contralateral limbs at rest, with only a slight improvement in contraction-induced hyperaemia (data not shown). The peak tension in the stimulated ischaemic muscles was lower than with ligation alone, but muscle stimulation resulted in a significant improvement in the FI when compared to the 3 days ligated animals without stimulation (P < 0.05), albeit with a lower peak tension (Fig. 1). The contralateral muscles of animals receiving muscle stimulation in conjunction with 3 days of ligation showed no significant differences in either peak tension or in fatiguability (data not shown).
Discussion
This study shows that increased activity during recovery of chronically ischaemic skeletal muscles parallels changes in microvasculature, demonstrated as swelling of capillary endothelium and increased rolling and adhesion of leukocytes in venules, which are attenuated by use of the xanthine oxidase inhibitor allopurinol or the Na+/H+ exchange inhibitor amiloride. The addition of muscle activity by electrical stimulation accentuated the structural changes (this study), and also increased leukocyte adhesion (Anderson et al. 2006). An important question is how these two responses may be linked. Various agents liberated from ischaemic tissues could explain increased capillary permeability and leukocyte adhesion (Ley, 1992), the latter induced primarily by changes in venules followed by modification of the capillary endothelium (Mayrovitz et al. 1987), as described during inflammatory reactions in the rat cremaster (Joris et al. 1992). Subsequently, increased leukocyte rolling and adhesion would increase the resistance to flow in capillaries (Harris & Skalak, 1993) and accentuate ischaemia-induced hypoxia, leading to capillary endothelial swelling.
Muscle performance and blood flow
There was a striking reduction in peak tension (i.e. tension within 1–20 s after the beginning of muscle contractions) in ischaemic muscle, which we reason is due to a lower content of glycogen, and possibly ATP or phosphocreatine. Chronic stimulation leads to depletion of glycogen with very slow repletion (Maier & Pette, 1987), and this repletion is less complete in ischaemic muscles (Hudlickáet al. 1994). The content of ATP in muscles at rest that were chronically stimulated was lower than in controls (Hudlickáet al. 1986), and ATP content in resting, stimulated ischaemic muscles was lower than in untreated ischaemic muscles (Elander et al. 1985). Interestingly, the systemic effect of muscle ischaemia was seen in a reduction in tension developed in the contralateral limbs, although the cause of this is unknown.
We have shown previously (Milkiewicz et al. 2006) that blood flow was reduced to 60% of control values (7.5 ± 0.9 ml (100 g)−1 min−1) in muscles ischaemic for 7 days, with a gradual return towards control values after 35 days. A similar reduction of blood flow to that seen after 7 days was observed after 3 days. Mild chronic stimulation of these muscles increased blood flow at rest and during contractions relative to ischaemia alone, and improved fatigue resistance without recovery of peak muscle tension (Fig. 1). It is unclear whether there is any direct relationship between peak tension and muscle blood flow, although both decline after ligation, but muscle perfusion is more likely to dictate the relative endurance, indicated by the fatigue index. This includes 3 days ischaemia, when there is low MBF but capillary swelling has not yet occurred (see below). Indeed, the resistance to fatigue was related to MBF during contractions (Fig. 7). Indirect electrical stimulation is likely to have improved fatigue resistance because it improved blood flow, and it has been shown to restore vasoreactivity of arterioles which is lost in ischaemic muscle (Hudlickáet al. 1994). It also decreased the proportion of capillaries with intermittent flow, but increased the time red cells spent stationary in individual capillaries, thus enabling greater extraction of oxygen (Anderson et al. 1997).
Improved MBF in this model is likely to involve some formation of collateral vessels, as demonstrated by measurements of perfusion pressure below the site of ligation in the femoral artery at its branch to the saphenous artery, i.e. well below the site of ligation. This dropped to 20% of the original value shortly after ligation, recovering by only 10% after 7 days but recovering to 50% of the original value 35 days after ligation (Hudlickáet al. 1994).
Leukocyte adhesion
The changes in capillary fine structure were linked with increased leukocyte rolling and adhesion, although it is not clear to what extent events affecting capillaries would also alter properties of the venular endothelium (Mayrovitz et al. 1987). One possible link might be that low shear stress favours expression of some adhesion molecules in tissue cultures (Ando et al. 1994; Tsou et al. 2008). In addition, low shear stress in venules may induce aggregation of red blood cells, thus forcing leukocytes towards the vessel wall (Nazziola & House, 1992) favouring increased rolling and adhesion. However, Nazziola & House (1992) consider the most important factor enabling leukocyte adhesion in venules (as opposed to arterioles) to be the different quality of venular endothelium. This may be modified by chronic ischaemia and explain greater adhesion of leukocytes in patients with peripheral vascular diseases, which was accentuated by treadmill exercise (Ciuffetti et al. 1994). Indeed, chronic ischaemia in conjunction with increased activity led to increased expression of ICAM-1 in small venules (Anderson et al. 2006). However, after 7 days stimulation alone there was an increase in leukocyte flux from 12.3 ± 4.6 to 34.9 ± 6.3 min−1, but without significant change in adhesion (JM. Dawson & O. Hudlická, unpublished observation). Similar data may be found in contralateral muscles (Anderson et al. 2006). The pH in chronically ischaemic muscles is reduced (Angersbach et al. 1988), while chronic stimulation increased the lactate content 3-fold (Elander et al. 1985). While such changes are unlikely to be responsible for the responses observed in contralateral muscles, they may modify leukocytes leaving ischaemic muscles in the venular drainage (Anderson et al. 1997), providing a mechanism by which signals are transferred to the endothelium in non-ischaemic muscles.
Capillary fine structure
Capillary endothelial cell swelling occurs in acutely ischaemic muscles (Strock & Majno, 1969), in conjunction with reperfusion (Gidlöf et al. 1988) and during haemorrhage (in skeletal muscle, Mazzoni et al. 1992; in the mesentery Kretchmar & Engelhardt, 1994). It has been linked with increased permeability (Suval et al. 1987), which could be prevented by calcium entry blockers (Paul et al. 1990), suggesting that swelling may be due to increased Ca2+ influx, possibly mediated by accumulation of adenosine in ischaemic muscles. Muscle ischaemia results, of course, in acidosis that is increased during muscle activity, and could induce increased sodium entry into the cells as suggested by Mazzoni et al. (1992, 1994). The fact that amiloride decreased swelling in ischaemic muscles favours the second explanation. Moreover, inhibition of Na+/H+ exchanger decreased injury in the heart caused by reperfusion (Masereel et al. 2003). Amiloride and its analogues also decreased the swelling of cultured endothelial cells exposed to low pH (Hamada et al. 2000). Similar changes in capillary swelling and fatigue resistance may imply an additional resistance to tissue perfusion, due to vessel stenosis or blockade, and this may contribute to heterogeneity within the muscle at a finer scale than would be the case with alteration of individual arteriolar reactivity previously reported in ischaemic muscle. In particular, the swollen endothelial cells were shown to impair capillary perfusion: red blood cells spent more time stationary than flowing in individual capillaries 1 week after ligation, and the proportion of capillaries with intermittent flow increased from 60% in control muscle to 85% 3 days after ligation, whereas stimulation of ischaemic muscles reduced this proportion to 70% (Anderson et al. 1997). In the absence of compensatory changes in autonomic tone, the shear stress-mediated NO response may be dominant. Although we know of no evidence for changes in ascending dilatation, modulation of autonomic tonus and/or humoral regulators of vascular smooth muscle cells may influence the relative importance of our findings.
Interestingly, capillary endothelial swelling was also observed in muscle remote from the ischaemic ones, and it is unlikely that muscles such as spinotrapezius (Hickey et al. 1993) or the papillary muscle (this study; Egginton et al. 1993) would have lower pH as a result of ischaemia produced in the hindlimb. Although the degree of swelling was considerably smaller than in ischaemic muscles, it persisted for longer with the area of cytoplasm still elevated even 35 days after ligation, at which time capillaries in the ischaemic muscles were not different from controls. As white blood cells leaving the ischaemic limbs are morphologically modified (Anderson et al. 1997) they might conduct some so far unknown signals to capillaries in other parts of the body. Changes in papillary muscle may thus be an indicator of generalized changes resulting from muscle ischaemia, and patients suffering from limb ischaemia frequently experience altered coronary vasculature (Rothwell et al. 2005).
Amelioration of systemic injury
Some systemic effects of muscle ischaemia are observed (e.g. papillary muscle capillaries), but as we used systemic drug treatment the limitation of using contralateral muscles as reference may be similar to that using a separate set of animals, given the inherent biological variability and its effect on the sensitivity of cross-sectional studies. The issue of a reduced maximum tension in contralateral muscles may be related to spinal reflexes, as discussed elsewhere (Hudlickáet al. 2003), but as the fatigue index did not change from that of control muscle (e.g. 65% at 7 days versus 66% in controls, Shiner, 1994), and capillary structure was largely unaffected, we consider the responses as described to be valid as relative, if not absolute, comparisons.
Reperfusion after acute ischaemia initiates a number of events that act as pro-inflammatory stimuli, including release of leukotriene B4 (Gute et al. 1998). A stable prostacyclin analogue (iloprost) which antagonises the effect of leukotriene B4 reduced leukocyte rolling and adhesion under these conditions (Thomson et al. 1994). However, these stimuli are unlikely to play a major role in the observed changes in microcirculation, as there were no overt signs of inflammation in our model of muscle ischaemia (Anderson et al. 2006).
Breakdown of high-energy phosphates in ischaemic muscles, particularly while contracting, results in the formation of hypoxanthine, which xanthine oxidase (XO) coverts to xanthine and oxygen free radicals in the presence of oxygen during reperfusion (Carden & Granger, 2000). As XO is present in capillary endothelium in skeletal muscles (Hellstenwesting, 1993), this process may be responsible for the capillary endothelium swelling, as well as leukocyte adhesion. Allopurinol, an inhibitor of xanthine oxidase, attenuated swelling in acute reperfusion (Ferrari et al. 1996) and was as effective in our experiments as amiloride. However, its effect on leukocyte adhesion was less than that of amiloride. The improvement of microcirculation by allopurinol might explain the drugs beneficial effect on muscle function (McCutchan et al. 1990) and blood flow (Hardy et al. 1992) during acute reperfusion.
Local ischaemia may result in the release of endothelin 1 from hypoxic endothelium as described in cultured rat aortic endothelial cell (Bodin et al. 1992). Although there are no data on the release of endothelin in either peripheral vascular diseases, or in animal models of muscle ischaemia, increased plasma levels were found in coronary ischaemia resulting in myocardial infarction (Miyauchi et al. 1989). Endothelin 1 is a potent activator of the Na+/H+ antiport (Khandoudi et al. 1994) and this could explain capillary endothelial cell swelling observed in the ischaemic muscles. Inhibitors of Na+/H+ antiport also decreased swelling of cultured endothelial cell exposed to low pH (Huck et al. 2007), and in endothelium in femoral arteries after ischaemia–reperfusion (Hamada et al. 2000).
The effect of allopurinol in this study is in agreement with those obtained by others in acute ischaemia and reperfusion. In contrast, data regarding efficacy of vitamin E under these conditions is varied and controversial, and in common with the literature we are unable to explain the lack of effect in our experiments. Vitamin E may prevent the vascular damage after acute reperfusion, as it inhibited superoxide production from rat macrophages (Hiramatsu et al. 1991) and in skeletal muscle (Novelli et al. 1997; Formigli et al. 1997), and improved walking distance in patients with peripheral vascular disease (Teoh et al. 1992). In contrast, we saw no attenuation of either endothelial swelling or leukocyte behaviour, in agreement with another report showing vitamin E had no effect on leukocyte adhesion (Lehr et al. 1995). Whereas we used scavengers to reduce the effect of ROS, in a review of complimentary ischaemia studies Gute et al. (1998) demonstrate leukocyte adhesion and endothelial permeability that was ameliorated by reduced ROS generation following xanthine oxidase inhibition. Similarly, Suzuki et al. (1995) used dimethylurea as a scavenger, but we have been unable to uncover any data that would allow a comparison of its influence on muscle performance with the scavengers used in this study. Interestingly, damage was predominantly restricted to glycolytic fibres (Suzuki et al. 1995), consistent with their earlier glycogen depletion compared to oxidative fibres, and increased depletion in contracting ischaemic muscles (Hudlickáet al. 1994).
Effect of activity
The period of recovery after ligation includes gradual restoration of blood flow and muscle metabolism, as well as recuperation of muscle activity. However, from extant data it is unclear whether the imposition of additional muscle activity may aid the recovery process. In spite of the changes in microcirculation caused by electrical stimulation in muscles 3 days after ligation of the iliac artery, stimulation improved muscle blood flow and performance assessed as fatigue resistance (though not peak twitch tension). This apparent discrepancy can be explained by reference to the regulation of muscle blood flow. Arterioles determine the amount of blood entering muscle and their dilatation is under the influence of metabolites released from skeletal muscle (e.g. lactic acid and adenosine). Interestingly, arterioles in ischaemic muscles do not dilate (Kelsall et al. 2004), but their dilatation was restored by chronic electrical stimulation (Hudlickáet al. 1994). Muscle contractions result in increased shear stress and generation of NO which could help dilatation of arterioles but little, if anything, is known about its effect on the ultrastructure of microvessels, although it is known that it is essential in the prevention of leukocyte adhesion in cremaster muscle (Akimitsu et al. 1995). Although the NO response may be dominant, modulation of autonomic tonus and/or humoral regulators of vascular smooth muscle may influence the relative importance of these findings. Stimulation of non-ischaemic muscle did not cause such changes to the same extent, as the fatigue index was the same as controls at 3 days and 7 days in contralateral muscles, although it did increase in ipsilateral muscles despite the presence of capillary swelling (Egginton & Hudlická, 1999). Hence, while increased muscle activity imposes an additional insult on capillary structure to that elicited by ischaemia alone, this was outweighed by the beneficial effect on blood flow (Fig. 7). It also decreased the proportion of capillaries with intermittent flow, but increased the time red cells spent stationary in individual capillaries, thus enabling greater extraction of oxygen (Anderson et al. 1997).
Conclusions
This paper demonstrates that ischaemic muscle exposed to mild, ambulatory activity shortly after the ischaemic insult has impaired microcirculation function and structure (increased number of rolling and adhering leukocytes in venules, and swollen capillary endothelium). These symptoms were alleviated by inhibition of the formation of oxygen free radicals with allopurinol or by the Na+/H+ channel blocker amiloride. However, an oxygen free radical scavenger, vitamin E, was not effective. Although the microcirculation showed signs of deterioration, additional activity imposed by electrical stimulation improved muscle blood flow and fatigue resistance, probably because it restores the dilatation of arterioles otherwise lost in ischaemic muscles. As activation of capillary endothelium may be one cause of leukocyte adhesion, the implication of this work is that combination treatment may attenuate impaired function of the microcirculation in working ischaemic muscles.
Acknowledgments
A. Garnham was a British Heart Foundation Research Fellow. We would like to acknowledge the generous support of Glaxo Plc.
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.158055/DC1
References
- Akimitsu T, Gute DC, Korthuis RJ. Leukocyte adhesion induced by inhibition of nitric oxide production in skeletal muscle. J Appl Physiol. 1995;78:1725–1732. doi: 10.1152/jappl.1995.78.5.1725. [DOI] [PubMed] [Google Scholar]
- Anderson SI, Hudlická O, Brown MD. Capillary red blood cell flow and activation of white blood cells in chronic muscle ischemia. Am J Physiol Heart Circ Physiol. 1997;272:H2757–H2764. doi: 10.1152/ajpheart.1997.272.6.H2757. [DOI] [PubMed] [Google Scholar]
- Anderson SI, Shiner R, Brown MD, Hudlická O. ICAM-1 expression and leukocyte behaviour in the microcirculation of chronically ischemic rat skeletal muscle. Microvascular Res. 2006;75:205–211. doi: 10.1016/j.mvr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Ando J, Tsuboi H, Korenaga R, Takeda Y, Toyamasorimachi N, Miasaka M, Kamiya A. Shear stress inhibits adhesion of cultured mouse endothelial cells to lymphocytes by down-regulating VCAM-1 expression. Am J Physiol Cell Physiol. 1994;267:C679–C687. doi: 10.1152/ajpcell.1994.267.3.C679. [DOI] [PubMed] [Google Scholar]
- Angersbach D, Jukna JJ, Nicholson CD, Ochlich P, Wilke R. The effect of short-term and long-term femoral artery ligation on rat calf muscle oxygen tension, blood flow, metabolism and function. J Microcirc Clin Exp. 1988;7:15–30. [PubMed] [Google Scholar]
- Bodin P, Milner P, Winter R, Burnstock G. Chronic hypoxia changes the ratio of endothelin to ATP release from rat aortic endothelial cells exposed to high flow. Proc Biol Sci. 1992;247:131–135. doi: 10.1098/rspb.1992.0019. [DOI] [PubMed] [Google Scholar]
- Buckberg GD, Luck JC, Payne DB, Hoffman JI, Archie JP, Fixler DE. Some sources of error in measuring regional blood flow with radioactive microspheres. J Appl Physiol. 1971;31:598–604. doi: 10.1152/jappl.1971.31.4.598. [DOI] [PubMed] [Google Scholar]
- Carden DL, Granger DN. Pathophysiology of ischemia- reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Challis RA, Hayes DJ, Petty RFH, Radda GK. An investigation of arterial insufficiency in rat hindlimb. Biochem J. 1986;236:461–467. doi: 10.1042/bj2360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffetti G, Lombardini R, Paltriccia R, Santambrogio L, Mannarino E. Human leukocyte–endothelial interaction in peripheral arterial occlusive disease. Eur J Clin Invest. 1994;24:65–68. doi: 10.1111/j.1365-2362.1994.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Dawson JM, Okyayuz-Baklouti I, Hudlická O. Skeletal muscle microcirculation: the effects of limited blood flow and torbafylline. Int J Microcirc Clin Exper. 1990;9:385–400. [PubMed] [Google Scholar]
- Egginton S, Hudlická O. Early changes in performance, blood flow and capillary fine structure in rat fast muscle induced by indirect electrical stimulation. J Physiol. 1999;515:265–275. doi: 10.1111/j.1469-7793.1999.265ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egginton S, Hudlická O, Glover M. Fine structure of capillaries in ischaemic and non-ischaemic rat striated muscle. Effect of torbafylline. Int J Microcirc Clin Exper. 1993;12:33–44. [PubMed] [Google Scholar]
- Elander A, Idström JP, Holm S, Scherstén T, Bylund-Fellenius AC. Metabolic adaptation to reduced muscle blood flow. II. Mechanisms and beneficial effects. Am J Physiol Endocrinol Metab. 1985;249:E70–E76. doi: 10.1152/ajpendo.1985.249.1.E70. [DOI] [PubMed] [Google Scholar]
- Ferrari RP, Battison B, Brunelli G, Casella A, Caimi L. The role of allopurinol in preventing oxygen free radical injury to skeletal muscle and endothelial cells after ischemia-reperfusion. J Reconstr Microsurg. 1996;12:447–450. doi: 10.1055/s-2007-1006617. [DOI] [PubMed] [Google Scholar]
- Formigli L, Ibba Maneschi L, Tani AS, Gardini E, Adembri C, Pratesi C, Novelli GP, Orlandini S. Vitamin E prevents neutrophil accumulation and attenuates tissue damage in ischemic-reperfused skeletal muscle. Histol Histopathol. 1997;12:663–669. [PubMed] [Google Scholar]
- Gidlöf A, Lewis DH, Hammersen F. Fine structure of the human skeleta1 muscle capillaries. A morphometric analysis. Int J Microcirc Clin Exp. 1988;7:43–66. [PubMed] [Google Scholar]
- Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Moll Cell Biochem. 1998;179:169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kanda T, Ishikawa S, Imai S, Nagai R, Matsui K, Ohashi N, Morishita Y. Na+/H+ exchange inhibitor reduces vascular injury caused by ischemia-reperfusion in the rat hind limb. Res Comm Mol Pathol Pharmacol. 2000;107:259–267. [PubMed] [Google Scholar]
- Hardy SC, Homervanniasinkam S, Gough MJ. Effect of free radical scavenging on skeletal muscle blood flow during postischaemic reperfusion. Br J Surg. 1992;79:1289–1292. doi: 10.1002/bjs.1800791214. [DOI] [PubMed] [Google Scholar]
- Harris AG, Skalak TC. Effects of leukocyte activation on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol. 1993;264:H909–H916. doi: 10.1152/ajpheart.1993.264.3.H909. [DOI] [PubMed] [Google Scholar]
- Hellstenwesting Y. Immunohistochemical localization of xanthine oxidase in human cardiac and skeletal muscle. Histochemistry. 1993;100:215–222. doi: 10.1007/BF00269094. [DOI] [PubMed] [Google Scholar]
- Hickey NC, Hudlická O, Gosling P, Shearman CP, Simms MH. Intermittent claudication incites systemic neutrophil activation and increased vascular permeability. Br J Surg. 1993;80:181–184. doi: 10.1002/bjs.1800800215. [DOI] [PubMed] [Google Scholar]
- Hickey NC, Hudlická O, Simms MH. Claudication induces systemic capillary endothelial swelling. Eur J Vasc Surg. 1992;6:36–40. doi: 10.1016/s0950-821x(05)80092-1. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Velasco RD, Packer L. Decreased carbon centered and hydrogen radicals in skeletal muscles of vitamin E supplemented rats. Biochem Biophys Res Commun. 1991;179:859–864. doi: 10.1016/0006-291x(91)91897-l. [DOI] [PubMed] [Google Scholar]
- Hou X, Baudry N, LeNoble M, Vicaut E. Leukocyte adherence in ischemic muscle perfused by a collateral circulation. J Cardiovasc Pharmacol. 1995;25(Suppl. 2S):119–123. doi: 10.1097/00005344-199500252-00025. [DOI] [PubMed] [Google Scholar]
- Huck V, Nimeyer A, Goerge T, Schnaeker EM, Ossig R, Rogge P, Schneider MF, Oberleithner H, Schneider SW. Delay of acute intracellular pH recovery after acidosis decreases endothelial cell activation. J Cell Physiol. 2007;211:399–400. doi: 10.1002/jcp.20947. [DOI] [PubMed] [Google Scholar]
- Hudlická O, Brown MD, Egginton S, Dawson JM. Effect of long-term electrical stimulation on vascular supply and fatigue in chronically ischemic muscles. J Appl Physiol. 1994;77:1317–1324. doi: 10.1152/jappl.1994.77.3.1317. [DOI] [PubMed] [Google Scholar]
- Hudlická O, Cotter MA, Cooper J. Effect of long-term electrical stimulation on capillary supply and metabolism in fast skeletal muscle. In: Nix WA, Vrbova G, editors. Electrical Stimulation and Neuromuscular Diseases. Berlin: Springer Verlag; 1986. pp. 21–32. [Google Scholar]
- Hudlická O, Graciotti L, Fulgenzi G, Brown MD, Egginton S, Milkiewicz M, Granata A-L. The effect of chronic skeletal muscle stimulation on capillary growth in the rat: are sensory nerve fibres involved? J Physiol. 2003;546:813–822. doi: 10.1113/jphysiol.2002.030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA, Hudlická O. Changes in capillary ultrastructure, blood flow and muscle performance during development of collateral circulation in rat fast muscles. J Physiol. 1992;452:115P. [Google Scholar]
- Jerome SN, Akimitsu T, Korthuis RJ. Leukocyte adhesion, edema, and development of postischemic capillary no-reflow. Am J Physiol Heart Circ Physiol. 1994;267:H1329–H1336. doi: 10.1152/ajpheart.1994.267.4.H1329. [DOI] [PubMed] [Google Scholar]
- Joris I, Cuenoud HF, Doern GV, Underwood JM, Majno G. Endothelial permeability in inflammation – the role of capillaries versus venules. In: Simionescu N, Simionescu M, editors. Endothelial Cell Dysfunction. New York: Plenum Press; 1992. pp. 233–242. [Google Scholar]
- Kelsall CJ, Brown MD, Kent J, Kloehn M, Hudlická O. Arteriolar endothelial dysfunction is restored in ischaemic muscles by chronic electrical stimulation. J Vasc Res. 2004;41:241–251. doi: 10.1159/000078301. [DOI] [PubMed] [Google Scholar]
- Khandoudi N, Ho J, Karmazyn M. Role of Na+-H+ exchange in mediating effects of endothelin-1 on normal and ischemic reperfusion. Circ Res. 1994;75:369–378. doi: 10.1161/01.res.75.2.369. [DOI] [PubMed] [Google Scholar]
- Kretchmar K, Engelhardt T. Swelling of capillary endothelial cells contributes to traumatic hemorrhagic shock-induced microvascular injury: a morphologic and morphometric analysis. Int J Microcirc Clin Exper. 1994;14:45–49. doi: 10.1159/000178205. [DOI] [PubMed] [Google Scholar]
- Lehr HA, Frei B, Olofsson AM, Carew TE, Arfors KE. Protection from oxidised LDG-induced leukocyte adhesion to microvascular endothelium in vivo by vitamin C but not vitamin E. Circulation. 1995;91:1525–1532. doi: 10.1161/01.cir.91.5.1525. [DOI] [PubMed] [Google Scholar]
- Leng GC, Lee AJ, Fowkes FG, Whitemen M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic arterial disease in the general population. Int J Epidemiol. 1996;25:1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- Ley K. Leukocyte adhesion – molecular basis and physiological consequences. Clin Hemorheol. 1992;12:93–108. [Google Scholar]
- Maier A, Pette D. The time course of glycogen depletion in single fibers of chronically stimulated rabbit fast-twitch muscle. Pflugers Arch. 1987;408:338–342. doi: 10.1007/BF00581126. [DOI] [PubMed] [Google Scholar]
- Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Mayrovitz HN, Kang S, Herscovici B, Sampsell RN. Leukocyte adherence initiation in skeletal muscle capillaries and venules. Microvasc Res. 1987;33:22–34. doi: 10.1016/0026-2862(87)90004-5. [DOI] [PubMed] [Google Scholar]
- Mazzoni MC, Cragoe EJ, Arfors KE. Systemic blood acidosis in low-flow ischemia induces capillary luminal narrowing. Int J Microcirc Clin Exp. 1994;14:144–150. doi: 10.1159/000178822. [DOI] [PubMed] [Google Scholar]
- Mazzoni MC, Intaglietta M, Cragoe EJ, Arfors KE. Amiloride sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol. 1992;73:1467–1473. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- McCutchan HJ, Schwappach JR, Enquist EG, Walden DL, Terada LS, Reiss OK, Leff JA, Repine JE. Xanthine oxidase-derived H2O2 contributes to reperfusion injury of ischemic skeletal muscle. Am J Physiol Heart Circ Physiol. 1990;258:H14145–H11459. doi: 10.1152/ajpheart.1990.258.5.H1415. [DOI] [PubMed] [Google Scholar]
- Milkiewicz M, Hudlická O, Shiner R, Egginton S, Brown MD. VEGF mRNA and protein do not change in parallel during non-inflammatory skeletal muscle ischaemia in rat. J Physiol. 2006;577:671–678. doi: 10.1113/jphysiol.2006.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T, Yanagisawa M, Tomizawa T, Sugushita Y, Suzuki N, Fujino M, Ajisaka R, Goto K, Masaki T. Increased plasma concentration of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet. 1989;2:53–54. doi: 10.1016/s0140-6736(89)90303-6. [DOI] [PubMed] [Google Scholar]
- Nazziola E, House SD. Effects of hydrodynamics and leukocyte-endothelium specificity on leukocyte–endothelium interactions. Microvasc Res. 1992;44:127–142. doi: 10.1016/0026-2862(92)90076-2. [DOI] [PubMed] [Google Scholar]
- Nolte D, Bayer M, Lehr HA, Becker M, Krombach F, Kreimeier U, Messmer K. Attenuation of postischemic microvascular disturbances in striated muscle by hyperosmolar saline dextran. Am J Physiol Heart Circ Physiol. 1992;263:H1411–H1416. doi: 10.1152/ajpheart.1992.263.5.H1411. [DOI] [PubMed] [Google Scholar]
- Novelli GP, Adembri C, Gandini E, Orlandini SZ, Papucci L, Formogli L, Manneschi LI, Quatrone A, Pratesi C, Capaccioli S. Vitamin E protects human skeletal muscle from damage during surgical ischemia-reperfusion. Am J Surg. 1997;173:206–209. doi: 10.1016/s0002-9610(97)89593-1. [DOI] [PubMed] [Google Scholar]
- Paul J, Bekker AY, Duran WN. Calcium entry blockade prevents leakage of macromolecules induced by ischemia reperfusion in skeletal muscle. Circ Res. 1990;66:1636–1642. doi: 10.1161/01.res.66.6.1636. [DOI] [PubMed] [Google Scholar]
- Perry MA, Granger DN. Leukocyte adhesion in local venous hemorrhage-induced ischemia. Am J Physiol Heart Circ Physiol. 1992;263:H810–H815. doi: 10.1152/ajpheart.1992.263.3.H810. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CF, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality and mortality for all acute vascular events in all arterial territories. (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- Shiner RA. PhD Thesis. University of Birmingham; Chronic muscle ischaemia: local and remote effects. [Google Scholar]
- Strock PE, Majno G. Microvascular changes in acutely ischemic rat muscle. Surg Gynecol Obstet. 1969;129:1215–1224. [PubMed] [Google Scholar]
- Suval WD, Duran WN, Boric MP, Hobson RW, Berendsen PB, Ritter AB. Microvascular transport and endothelial cell alterations preceding skeletal muscle damage in ischemia/reperfusion injury. Am J Surg. 1987;154:211–218. doi: 10.1016/0002-9610(87)90181-4. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Poole DC, Zweifach BW, Schmidt-Schoenbaib GW. Temporal correlation between maximum titanic force and cell death in postischemic rat skeletal muscle. J Clin Invest. 1995;96:2892–2897. doi: 10.1172/JCI118360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh MK, Chong MK, Jamludin M. In: Lipid-soluble antioxidants: Biochemistry and clinical applications. Ong AS, Packer L, editors. Basel, Switzerland: Birkhauser-Verlag; 1992. pp. 606–621. [Google Scholar]
- Thomson IA, Egginton S, Hudlická O, Simms MH. Iloprost reduces leukocyte adhesion in skeletal muscle venules following ischemia in a rat model of femorodistal bypass. Eur J Vasc Surg. 1994;8:335–341. doi: 10.1016/s0950-821x(05)80152-5. [DOI] [PubMed] [Google Scholar]
- Tsou JK, Gower RM, Ting HJ, Schaff UY, Insana MF, Passerini AG, Simon SI. Spatial regulation of inflammation by human aortic endothelial cells in a linear gradient of shear stress. Microcirculation. 2008;15:311–323. doi: 10.1080/10739680701724359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.