Abstract
It is found that dark reduction of photooxidized primary electron donor P870+ in reaction centres from purple anoxygenic bacteria (two non-sulphur Fe-oxidizing Rhodovulum iodosum and Rhodovulum robiginosum, Rhodobacter sphaeroides R-26 and sulphur alkaliphilic Thiorhodospira sibirica) is accelerated upon the addition of Mn2+ jointly with bicarbonate (30–75 mM). The effect is not observed if Mn2+ and HCO3− have been replaced by Mg2+ and HCO2−, respectively. The dependence of the effect on bicarbonate concentration suggests that formation of Mn2+–bicarbonate complexes, Mn(HCO3)+ and/or Mn(HCO3)2, is required for re-reduction of P870+ with Mn2+. The results are considered as experimental evidence for a hypothesis on possible participation of Mn–bicarbonate complexes in the evolutionary origin of oxygenic photosynthesis in the Archean era.
Keywords: manganese–bicarbonate complexes, reaction centres, purple bacteria
1. Introduction
It is known that bicarbonate is required for maximal activity of both electron acceptor and electron donor sides of photosystem II (PSII; for recent review, see Van Rensen & Klimov (2005)). The stimulating effect of bicarbonate on electron transfer on the donor side of PSII is especially pronounced during reassembly of the inorganic core of the Mn-containing water-oxidizing complex (WOC; Klimov et al. 1995a,b, 1997; Allakhverdiev et al. 1997; Baranov et al. 2000, 2004; Klimov & Baranov 2001). It was shown that after the removal of all four manganese ions from subchloroplast PSII preparations, an effective reactivation of electron transfer (Allakhverdiev et al. 1997; Klimov et al. 1995a,b, 1997; Hulsebosch et al. 1998) and oxygen evolution (Allakhverdiev et al. 1997; Baranov et al. 2000, 2004) was observed only if a nearly stoichiometric amount of Mn2+(2–4 atoms of Mn2+ per PSII reaction centre) was added together with bicarbonate. Changes in redox properties of manganese ions upon formation of complexes with bicarbonate evidently play a key role in the effect described above. In fact, upon the addition of NaHCO3 to an aqueous solution of Mn2+, the potential for oxidation of Mn2+ to Mn3+ was shifted from 1.18 to 0.52–0.68 V at pH 8.3 as a result of formation of bi(carbonate) complexes (Kozlov et al. 1997, 2004; Dasgupta et al. 2006). Formation of Mn complex with carboxylates (formate, acetate) induced similar lowering of the oxidation potential; however, only bicarbonate stimulated the electron transfer from Mn2+ to PSII reaction centres (RCs; Kozlov et al. 2004). On the basis of the electrochemical and electron paramagnetic resonance (EPR) data, it was proposed that the unique capability of Mn2+–bicarbonate complexes to be photooxidized by PSII could be due to four possible reasons: (i) significantly larger decrease in the oxidation potential of Mn2+ (down to 0.52 V), (ii) electroneutrality of the functional electron transfer complex, (iii) the more favourable energetics reflected in the two pKa values for H2CO3/HCO3− and HCO3−/CO32− and greater number of proton transfer sites, and (iv) multiple composition possibilities for the Mn3+ photoproduct such as Mn3+(HCO3−)3, Mn3+(HCO3−)(CO32−) and Mn3+(HCO3−)2(OH−) (due to the high Lewis acidity of Mn3+; pKa<1; Kozlov et al. 2004; Dasgupta et al. 2006).
The oxidation potential of Mn–bicarbonate complex (0.52–0.67 V) is close to the midpoint redox potential of the primary electron donor in the RCs of anoxygenic bacteria (Kozlov et al. 2004) and therefore it has been suggested (Dismukes et al. 2001) that the capability of bicarbonate to form easily oxidizable complexes with manganese ions might be critical to the evolutional origin of the first O2-evolving cyanobacteria from a non-oxygenic bacterial precursor in the Archean period (>2.2 Gyr ago) when the concentration of bicarbonate dissolved in water was 30–30 000 times greater than today.
Recently, it has been shown that as a result of a set of single mutations of amino acid residues near P870, the primary electron donor in the RCs of purple anoxygenic bacterium Rhodobacter sphaeroides R-26, the P+/P midpoint potential (equal to 0.5 V in the initial strain) was increased to 0.58–0.765 V, and exogenous Mn2+ added together with bicarbonate enabled electron donation to P+ in RCs isolated from these mutants (Kalman et al. 2003). In the present work, we investigated the possibility of electron donation from Mn2+–bicarbonate complexes to oxidized P in wild-types of anoxygenic photosynthesizing bacteria. Three types of purple bacteria (belonging to the class of Proteobacteria containing type II of RCs; Gupta et al. 1999) were used: (i) two non-sulphur Fe-oxidizing bacteria Rhodovulum iodosum and Rhodovulum robiginosum (isolated from sediment of the North Sea, Germany) that use Fe2+ as an electron donor for anoxygenic photosynthesis (Straub et al. 1999) and so can be good candidates as possible users of the easily oxidizable Mn2+–bicarbonate complexes as electron donors, (ii) the purple sulphur alkaliphilic bacterium Thiorhodospira sibirica (received from the collection of Prof. V.M. Gorlenko, Institute of Microbiology, RAS, Moscow), found in ‘soda’ lake Malyi Kasitui (pH of water is 9.5); its optimal growth is at pH 9.0, at a carbonate concentration of 45 mM; the bacterium is resistant to high concentrations of NaCl (up to 1 M) and Na2CO3 up to 740 mM (Bryantseva et al. 1999), and (iii) the widely used non-sulphur bacterium Rh. sphaeroides R-26.
The results presented in this work show that the formation of Mn2+–bicarbonate complexes favours electron donation from Mn2+ to type II RCs from at least four contemporary purple bacteria.
2. Material and methods
(a) Isolation of pigment–protein complexes B890
Cell cultures of Rh. iodosum and Rh. robiginosum, and Th. sibirica were grown as described earlier (Straub et al. 1999; Bryantseva et al. 1999; respectively) at 25–30°C and continuous irradiance of 2000 lux. The cells were washed twice in distilled water, suspended in 10 mM HEPES (pH 7.8) and after sonication pigment–protein complexes B890 were isolated as described earlier (Qian et al. 2000) with some modifications. The chromatophore membranes (OD890=50 cm−1) were incubated in 10 mM HEPES buffer (pH 7.8) with 2% dodecyl-β-d-maltoside (DM) for 30 min at 4°C in the dark. The suspension was centrifuged (10 000g), and the supernatant was loaded onto a stepwise sucrose density gradient (0.3, 0.5, 0.6 and 1.2 M) and centrifuged (200 000g) at 4°C for 20 h. The pigment–protein complexes B890 appearing between the 0.5 and 0.6 M sucrose layers were collected and loaded onto a 20 mm×200 mm DEAE 650s column that had been equilibrated with 10 mM HEPES (pH 7.8) containing 0.1% DM (eluent). The crude pigment–protein complexes B890 were applied to the column, washed with the eluent without and then with 0.05 M NaCl to remove free pigments. The salt concentration was then increased to 0.25 M to remove the LH2 component. Finally, the remaining pigment–protein complexes B890 were eluted with the eluent containing 0.5 M NaCl. The pigment–protein complexes B890 were stored at −20°C. The RCs from Rh. sphaeroides R-26 were isolated as described earlier (Shuvalov & Duysens 1986). The concentration of RCs was determined as described previously (Clayton & Wang 1971).
(b) Measurements of absorbance changes related to reversible photooxidation of P870
Kinetics of photoinduced absorbance changes, related to reversible photooxidation of P870, were measured in a 10 mm cuvette at 20°C using a phosphoroscopic set-up (Klimov et al. 1982). Accumulation of the oxidized primary electron donor, P870+, was reached by illumination of pigment–protein complexes B890 with red light (λ>600 nm, 1900 μmol photon s−1 m−2) for 30 s. The difference ‘light−dark’ absorption spectrum was measured by a spectrophotometer (Hitachi-U-2800, Japan).
(c) Removal of bicarbonate from complexes B890
Partial removal of bicarbonate from pigment–protein complexes was achieved by a 50-fold dilution of the samples in the medium depleted of the endogenous bicarbonate by 60 min flushing with air freed of CO2 by passage through a solution of 50% NaOH and a 20 cm layer of ascarite.
3. Results
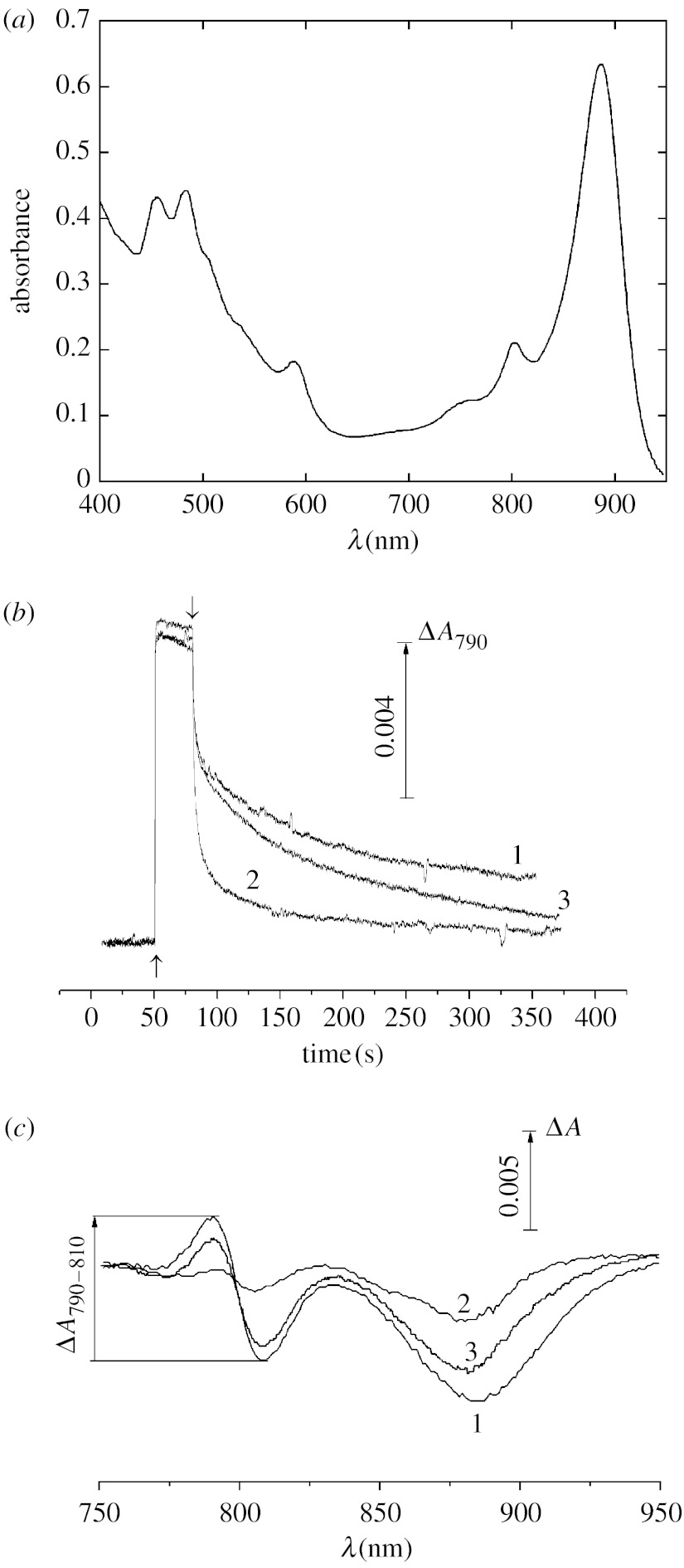
To study the possibility of redox interaction of Mn2+–bicarbonate complexes with RCs of purple bacteria, we used the pigment–protein complexes B890 isolated from Rh. iodosum, Rh. robiginosum and Th. sibirica. The complex B890 from purple bacteria is known to be the light-harvesting complex (that is termed LH1) tightly associated with the RC in fixed stoichiometry 1 : 1 (Law et al. 2004). Figure 1a illustrates the absorption spectrum of the pigment–protein complexes B890 isolated from Rh. iodosum which is similar to that isolated previously from other types of purple bacteria (Law et al. 2004; Moskalenko et al. 2005).
Figure 1.
(a) Absorption spectrum, kinetics of photoinduced absorbance changes at 790 nm (ΔA790) (b) related to photooxidation of the primary electron donor P870 in pigment–protein complexes B890 isolated from the non-sulphur Fe-oxidizing purple bacterium Rh. iodosum and (c) difference light−dark absorption spectra. (c) Spectra were measured after a 30 s illumination with actinic light (λ>600 nm, 1900 μmol photon s−1 m−2) and 2.5 min incubation in the dark. The measurements of ΔA790 and difference light−dark absorption spectra were made without additions (1) and after the addition of 0.1 mM K4[Fe(CN)6] (2), 0.5 mM MnCl2 and 50 mM NaHCO3 (3). Up and down arrows indicate actinic light on and off, respectively. The measurements were done in the medium containing 50 mM HEPES (pH 8.2) depleted of CO2/HCO3− (see §2).
Kinetics of photoaccumulation of the primary electron donor P870 in the oxidized form P+ and its dark re-reduction, measured at 790 nm (ΔA790; corresponding to the electrochromic blue shift of absorption band at 800 nm due to oxidation of P870), are shown in figure 1b. After a 30 s illumination at pH 8.2, nearly 50% of RCs remain in a ‘long-lived’ oxidized state: their re-reduction occurs in minute range. Using redox titration of the amplitude of the photoinduced ΔA790 (upon the addition of K3[Fe(CN)6] and K4[Fe(CN)6]), the midpoint redox potential (Em) of the pair P+/P in Rh. iodosum, Rh. robiginosum and Th. sibirica was found to be equal to 450±30 mV (which is close to Em of P in other purple bacteria; Prince et al. 1976; Klimov et al. 1977; Lin et al. 1994).
The dark reduction of P+ is considerably accelerated if 0.1 mM K4[Fe(CN)6] (a known artificial electron donor to P+) is present in the medium so that only 15% of P were in the oxidized state after 30 s of darkness (figure 1b, curve 2). Interestingly, an acceleration of P+ reduction is also observed upon the addition of 0.5 mM MnCl2 together with 50 mM NaHCO3 (figure 1b, curve 3). The effect was clearly seen for the long-lived component of P+ relaxation: a 50% decay of this component was at 110, 63 and 5 s for the samples without additions, in the presence of 0.5 mM MnCl2 together with 50 mM NaHCO3, and in the presence of 0.1 mM K4[Fe(CN)6], respectively. In further experiments on the effect of Mn2+ on the redox state of P+, the difference light−dark absorption spectra of P+ corresponding to the long-lived state of photooxidized P870 were measured. A similar approach for revealing the redox interaction of Mn2+ with bacterial RCs was used earlier (Kalman et al. 2003). The spectra were measured after a 2.5 min dark incubation of the samples illuminated with red light (λ>600 nm, 1900 μmol photon s−1 m−2) for 30 s.
The spectrum (figure 1c) is typical of photooxidation of P in pigment–protein complexes B890 of purple bacteria (Shuvalov & Klimov 1976; Moskalenko et al. 2005). It includes the bleaching of a broad absorption band of P with maximum at 870 nm accompanied by a characteristic shift of the absorption band at 800 nm. The amplitudes of both the bleaching at 870 nm and the shift at 800 nm are decreased upon the addition of either 0.1 mM ferricyanide (figure 1c, curve 2) or 0.5 mM MnCl2 added jointly with 50 mM NaHCO3 (figure 1c, curve 3) indicating the acceleration of dark re-reduction of P+. For a detailed investigation of the specificity of the pair Mn2++bicarbonate in the acceleration of re-reduction of P+ in purple bacteria, the difference ΔA790−ΔA810 (ΔA790−810; corresponding to the blue shift of the absorption band at 800 nm due to oxidation of P) was used as a measure of the fraction of RCs with oxidized P870 (to avoid possible absorbance changes related to photobleaching of the antenna BChl).
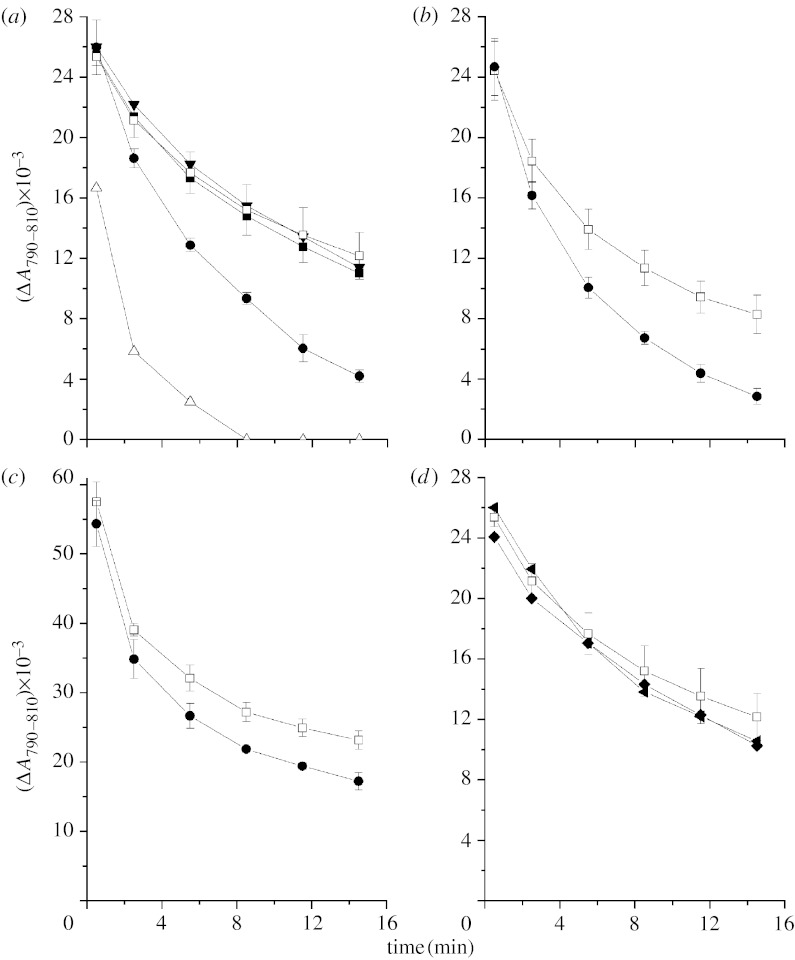
Figure 2a shows that the addition of 0.5 mM MnCl2 together with 50 mM NaHCO3 to pigment–protein complex B890 from Rh. iodosum accelerates re-reduction of P+ (closed circles), while 0.5 mM MnCl2 or bicarbonate added alone does not increase the rate of dark relaxation of ΔA790–810 (filled inverted triangles and filled squares in figure 2a, respectively). The bicarbonate requirement for reduction of P+ with added Mn2+ is in agreement with the data reported earlier (Kozlov et al. 2004) on lowering of the oxidation potential of Mn2+ upon formation of complexes with bicarbonate.
Figure 2.
Kinetics of the long-lived component of dark relaxation of photoinduced ΔA related to the reversible photooxidation of P870 in pigment–protein complexes isolated from (a) Rh. iodosum, (b) Rh. robiginosum, (c) Th. sibirica in the absence of additions (open squares) and in the presence of 0.1 mM K4Fe(CN)6 (open triangles), 0.5 mM MnCl2 (filled inverted triangles), 50 mM NaHCO3 (filled squares), 0.5 mM MnCl2 and 50 mM NaHCO3 (filled circles). The difference ΔA790−ΔA810 (corresponding to the blue shift of absorption band at 800 nm due to oxidation of P870) taken from difference light−dark absorption spectra measured after a 30 s illumination (for details see figure 1) was used as a measure of the fraction of RCs with oxidized P870.The first spectrum was measured 30 s after switching the actinic light off. (d) Kinetics of the long-lived component of dark relaxation of photoinduced ΔA related to the reversible photoreduction of P870 in pigment–protein complexes isolated from Rh. iodosum in the absence of additions (open squares) and presence of 0.5 mM MgCl2 and 50 mM NaHCO3 (filled diamond); 0.5 mM MnCl2 and 50 mM NaHCO2 (right-faced filled triangle). The results are an average of three experiments.
Mg2+ (in contrast to Mn2+) added jointly with bicarbonate does not increase the rate of re-reduction of P+ (figure 2d). Similarly, replacement of bicarbonate by formate (without change in Mn2+ concentration) also leads to the loss of the effect observed in the presence of Mn2+ and bicarbonate (figure 2d). This clearly demonstrates that Mn2+–bicarbonate complexes are important for the acceleration of P+ re-reduction.
A similar effect of the increase of the rate of dark reduction of P+ upon addition of the Mn2+ jointly with bicarbonate is observed in pigment–protein complex B890 isolated from two other purple bacteria: Fe-oxidizing Rh. robiginosum (figure 2b) and sulphur alkaliphilic Th. sibirica (figure 2c).
The acceleration of dark reduction of P+ upon the addition of Mn2+ depended on the concentration of added bicarbonate: at an Mn2+ concentration equal to 0.5 mM, the effect was not observed at bicarbonate concentrations of 10 and 15 mM; it is clearly seen starting from a bicarbonate concentration of 30 mM and is saturated at 50–70 mM (figure 3a). The dependence of the effect on Mn2+ concentration shows that in the presence of 50 mM NaHCO3, the acceleration of reduction of P+ is observed already at Mn2+ concentration of 10 μM, and the effect is maximal at 0.3–0.5 mM MnCl2 (figure 3b).
Figure 3.
Effect of Mn2+–bicarbonate complexes on the re-reduction of P+ in the pigment–protein complexes B890 from Rh. iodosum at various concentrations of (a) bicarbonate and (b) MnCl2.The experiments shown in (a) were done in the presence of 0.5 mM MnCl2 without other additions (filled squares) and after the addition of 30 (open triangles), 40 (open diamond), 50 (filled circles) and 75 mM (open inverted triangles) of NaHCO3.The experiments shown in (b) were done in the presence of 50 mM NaHCO3 without other additions (filled squares) and after the addition of 0.01 (open triangles), 0.1 (open diamond), 0.3 (filled circles) and 0.5 mM (open inverted triangles) of MnCl2.The results are an average of three experiments.
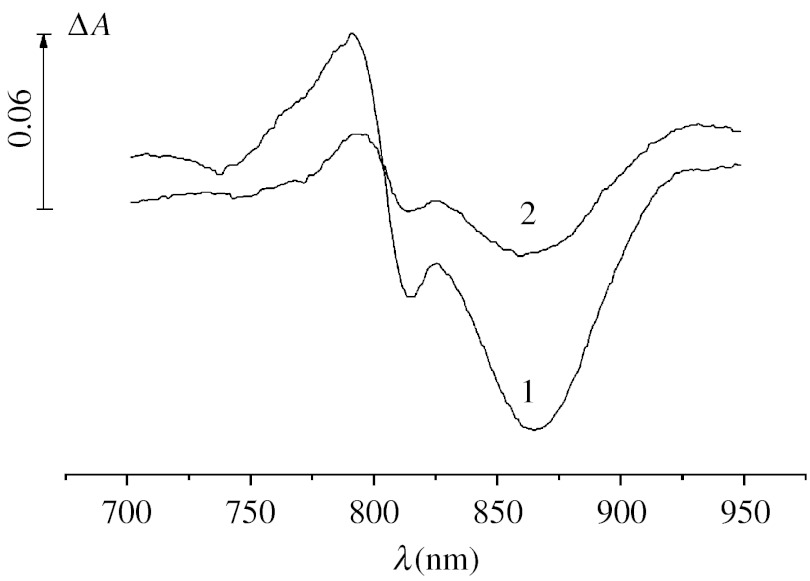
It has been shown earlier (Kalman et al. 2003) that in RCs isolated from the initial strain of Rh. sphaeroides R-26 (the P+/P midpoint potential=0.5 V), Mn2+ added jointly with 15 mM bicarbonate is not able to accelerate the dark reduction of P870+. Figure 4 demonstrates that under the conditions used in our experiments (photoaccumulation of P+ at pH 8.2, addition of 0.5 mM MnCl2 together with 50 mM NaHCO3), the rate of re-reduction of P+ in RCs isolated from the initial strain of Rh. sphaeroides is considerably increased upon the addition of the pair Mn2++bicarbonate so that in their presence the concentration of P+ remaining after a 30 s incubation of illuminated samples in the dark becomes 3.5 times lower.
Figure 4.
Difference light−dark absorption spectra of isolated RCs from Rh. sphaeroides R-26 measured after a 30 s illumination with actinic light (λ>600 nm, 1900 μmol photon s−1 m−2) and a 30 s incubation in the dark in the absence (1) and presence (2) of 0.5 mM MnCl2 and 50 mM NaHCO3.
4. Discussion
Dismukes et al. (2001) put forward a hypothesis that Mn2+–bicarbonate complexes could play a key role in the evolutionary origins of the WOC due to the use of Mn complexes as transient electron donors (and ‘building blocks’) for anoxygenic photosynthetic bacteria in the Archean Ocean when the content of CO2 and HCO3− was high enough (a few orders higher than nowadays) to produce easily oxidizable Mn2+ complexes.
The results obtained in our work show that the formation of Mn2+–bicarbonate complexes favours electron donation from Mn2+ to the RC of purple bacteria. Such a conclusion has been made on the basis of spectrophotometric measurements of ΔA related to reversible photooxidation of the primary electron donor, P. As in the case with ferrocyanide (a known exogenous electron donor to P+), Mn2+ in the presence of bicarbonate accelerated the re-reduction of P+. The midpoint potential for the P+/P of anoxygenic purple bacteria is 0.45–0.50 V (Prince et al. 1976; Klimov et al. 1977; Lin et al. 1994) and a similar Em is found in our work for the pair P+/P in RCs isolated from Rh. iodosum. Therefore, the RC of these bacteria cannot act as an oxidant for Mn2+ (the oxidation potential for aqua ions of Mn2+ is 1.18 V). On the basis of electrochemical measurements, it was demonstrated that the formation of complexes of Mn2+ with bicarbonate greatly favours the electrochemical oxidation of Mn2+ to Mn3+ so that the oxidation potential of Mn2+ becomes 0.52–0.67 V (Kozlov et al. 2004). Therefore, in response to the formation of Mn2+–bicarbonate complexes, the oxidation potential of Mn2+ becomes so low that it is possible to expect the oxidation of Mn2+ by RCs of anoxygenic purple bacteria, and that is what we see in our experiments. The replacement of Mn2+ by Mg2+ or bicarbonate with formate leading to the loss of the effect observed with the Mn–bicarbonate system confirms the specificity of Mn–bicarbonate complexes in this reaction (figure 3d). Recently, it has been found that carboxylates (formate and acetate) are also capable of producing complexes with Mn2+ that lead to a lowering of the oxidation potential of Mn2+; the oxidation potential of Mn2+–formate complexes is approximately 0.76 V (Kozlov et al. 2004). The relatively high oxidation potential of Mn2+–formate complexes (in comparison with Mn2+–bicarbonate complexes) can explain the inability of the pair Mn2++formate to accelerate re-reduction of P+ in purple bacteria. In addition, bicarbonate (in contrast to formate) can form electroneutral complexes with Mn2+ and Mn3+ (MnII(HCO3)2 and MnIII(HCO3)3 (Kozlov et al. 2004) that can also favour the redox interaction of Mn2+ with the RC of purple bacteria. It is interesting that PSII RCs are also unable to oxidize Mn–formate complexes (Kozlov et al. 2004) though the oxidation potential of P680+(>1.1 V) is enough for their oxidation. Evidently, the multiple composition possibilities for the Mn3+ photoproduct, such as Mn3+(HCO3−)3, Mn3+(HCO3−)(CO32−) and Mn3+(HCO3−)2(OH−) (Kozlov et al. 2004), are important for the redox interaction of Mn–bicarbonate complex with RCs of both PSII and anoxygenic bacteria.
The acceleration of re-reduction of P+ in the presence of Mn2+ begins from 30 mM of added bicarbonate and the effect is maximal at 50 mM. Electrochemical and EPR measurements (Kozlov et al. 2004; Dasgupta et al. 2006) revealed the presence of 1 : 1([Mn(HCO3)]+) and 1 : 2 (Mn(HCO3)2) complexes in water solution of Mn2+ and bicarbonate. The oxidation potentials for the 1 : 1 and 1 : 2 Mn2+–bicarbonate complexes (0.61 and 0.52 V, respectively; Kozlov et al. 2004) are much lower than those for the aqua complex of Mn2+(1.18 V). Using the equilibrium constants of Mn2+–bicarbonate complexes presented earlier (Kozlov et al. 2004; Dasgupta et al. 2006), we estimated the ratio of Mn2+–bicarbonate complexes in the solution at different concentrations of added bicarbonate. In the presence of 15 mM bicarbonate at pH 8.2, the total Mn2+ in the solution is represented by a mixture of 69% of ions Mn2+ aqua, approximately 23% of [Mn(HCO3)]+ and 8% of Mn(HCO3)2. The content of Mn(HCO3)2 increases with increase in the bicarbonate concentration and at 50 mM the mixture of total Mn2+ is 29% of Mn2+ aqua, approximately 31% of [Mn(HCO3)]+ and 40% of Mn(HCO3)2. Most probably, the bicarbonate concentration dependence of the acceleration of re-reduction of P+ with Mn2+ (figure 3a) reflects the increase in the content of the complex Mn(HCO3)2 most active in the redox interaction with P+. It was shown (Kalman et al. 2003) that only RCs from the mutant cells of Rh. sphaeroides in which the P+/P midpoint potential was increased to 0.58–0.765 V were able to oxidize Mn2+ in the presence of 15 mM bicarbonate, while oxidation of Mn2+ by RCs from the initial strain (where the P+/P midpoint potential was 0.45–0.5 V) was not observed. (Our experiments confirm that if the concentration of added bicarbonate is lower than 30 mM, Mn2+ does not accelerate the reduction of P+.) One can suggest that the domination of the complex [Mn(HCO3)]+ and, as a consequence, the low the concentration of the complex Mn(HCO3)2, were responsible for the lack of redox interaction of Mn–bicarbonate complex with the RCs of Rh. sphaeroides in the experiments reported earlier (Kalman et al. 2003).
The effect of Mn2+–bicarbonate complexes on the redox state of P+ was also obtained in pigment–protein B890 isolated from another non-sulphur Fe-oxidizing purple bacterium Rhodovullum robiginosum and from a purple sulphur alkaliphilic bacterium Th. sibirica (figure 2b,c). Thus, the results demonstrate that even without pre-modifications of the redox properties of the RCs, at least four contemporary purple bacteria (containing type II RCs) are capable of using Mn–bicarbonate complexes as electron donor for oxidized P and this capability could be typical of most purple bacteria with a P+/P midpoint potential near 0.45–0.5 V. Therefore, under certain conditions (appropriate pH, enhanced concentration of bicarbonate) favourable for the formation of the easily oxidizable Mn2+–bicarbonate complexes, the purple bacteria could use the Mn2+–bicarbonate complexes as a source of electrons; according to the hypothesis (Dismukes et al. 2001) this eventually could have led to the origin of the WOC in the Archean era.
Discussion
V. Batista (Yale University, USA). What is the experimental evidence that bicarbonate would be involved in the proton exit channel?
V. V. Klimov. Our data on the increase of the stimulating effect of bicarbonate on both oxygen evolution and variable fluorescence upon the addition of PSII-associated carbonic anhydrase CAH3 to PSII preparations isolated from a CAH3 lacking mutant of Chlamydomonas reinhardtii and similar stimulation of the activities upon the addition of an amphiphilic buffer neutral red to these preparations allow us to suggest that bicarbonate and CAH3 can be involved in the process of removal of protons released during water oxidation (Shutova et al. Submitted). There is no direct evidence for involvement of bicarbonate in the proton exit channel.
A. W. Rutherford (CEA Saclay, France). How close is carbonic anhydrase to PSII? Is it bound and if so where?
V. V. Klimov. In Chlamydomonas reinhardtii, PSII-associated carbonic anhydrase CAH3 is located on the lumenal side of PSII (judging from the amino acid sequence characteristics of other lumenal PSII proteins), and upon isolation of PSII core complex from PSII particles, it remains bound to PSII and its content (per Chl basis) increases (Villarejo et al. 2002).
H. Dau (Freie University, Berlin, Germany). I have a comment. Tatiana Shutova (Laboratory of Klimov, Pushinov, Russia, and G. Samuelsson, Umeå, Sweden) carried out measurements in our laboratory using a time-resolved delayed fluorescence approach (Grabolle & Dau 2005; Buchta et al. 2007) to study the donor-side processes in PSII preparations from wild-type cells and mutants lacking the PSII-associated carbonic anhydrase (CA; Villarejo et al. 2002). Interestingly, the results obtained for the CA-free mutant suggest that proton release in the S2→S3 and S3→S4 transition is indeed accelerated by bicarbonate addition. This finding supports that bicarbonate facilitates more efficient proton removal from the Mn complex.
W. Hillier (Australian National University, Australia). I would like to comment. PSII is often found to contain carbonic anhydrase activity. We have quantified this activity in a number of different samples, and surprisingly for the PSII from Th. elongatus there is practically no carbonic anhydrase activity associated with PSII.
J. Barber (Imperial College London). I would like to point out that the electron density tentatively assigned to carbonate in the OEC by Ferreira et al. (2004) is in part occupied by D1Asp170 in the Loll et al. model (2005).
S. Styring (Uppsala, Sweden). I have two questions. Was the effect of carbonic anhydrase specific to the enzyme from Chlamydomonas and can you relate any role of bicarbonate to a specific S-state transition?
V. V. Klimov. Yes. The effect of carbonic anhydrase was specific for the enzyme from Chlamydomonas; the effect was not seen upon the addition of a bovine carbonic anhydrase. We consider bicarbonate as an available base with an appropriate pKa (6.4) which can be used as an acceptor for protons released from the water oxidation centre (especially for the S3→S4→S0 transition; Shutova et al. Submitted).
Acknowledgments
The authors would like to thank Dr Z. K. Makhneva for assistance in the isolation of pigment–protein complexes B890, Dr K. G. Tikhonov for helpful discussion on the manuscript, V. A. Gorlenko for the gift of alkaliphilic purple bacterium Th. sibirica and A. Ya. Skuropatov for the gift of RCs from Rh. sphaeroides R-26.This work was supported by grants from the Russian Foundation for Basic Research and the US Civilian Research and Development Foundation.
Footnotes
One contribution of 20 to a Discussion Meeting Issue ‘Revealing how nature uses sunlight to split water’.
References
- Allakhverdiev S, Yruela I, Picorel R, Klimov V. Bicarbonate is an essential constituent of the water-oxidizing complex of photosystem II. Proc. Natl Acad. Sci. USA. 1997;94:5050–5054. doi: 10.1073/pnas.94.10.5050. doi:10.1073/pnas.94.10.5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov S.V, Ananyev G.M, Klimov V.V, Dismukes G.C. Bicarbonate accelerates assembly of the inorganic core of the water-oxidizing complex in manganese-depleted photosystem II: a proposed biogeochemical role for atmospheric carbon dioxide in oxygenic photosynthesis. Biochemistry. 2000;39:6060–6065. doi: 10.1021/bi992682c. doi:10.1021/bi992682c [DOI] [PubMed] [Google Scholar]
- Baranov S.V, Tyryshkin A.M, Katz D, Dismukes G.C, Ananyev G.M, Klimov V.V. Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitition of O2 evolution by photoactivation. Biochemistry. 2004;43:2070–2079. doi: 10.1021/bi034858n. doi:10.1021/bi034858n [DOI] [PubMed] [Google Scholar]
- Bryantseva I, Gorlenko V.M, Kompantseva E.I, Imhoff J.F, Suling J, Mityushina L. Thiorhodospira sibirica gen. nov sp. nov., a new alkaliphilic purple sulfur bacterium from a Siberian soda lake. Int. J. Syst. Bacteriol. 1999;49:697–703. doi: 10.1099/00207713-49-2-697. [DOI] [PubMed] [Google Scholar]
- Buchta J, Grabolle M, Dau H. Photosynthetic dioxygen formation studied by time-resolved delayed fluorescence measurements—method, rationale, and results on the activation energy of dioxygen formation. Biochim. Biophys. Acta. 2007;1767:565–574. doi: 10.1016/j.bbabio.2007.04.003. doi:10.1016/j.bbabio.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Clayton R.K, Wang R.T. Photochemical reaction centers from Rhodopseudomonas sphaeroides. Methods Enzymol. 1971;23:696–704. [Google Scholar]
- Dasgupta J, Tyryshkin A.M, Kozlov Y.N, Klimov V.V, Dismukes G.C. Carbonate complexation of Mn2+ in the aqueous phase: redox behavior and ligand binding modes by electrochemistry and EPR spectroscopy. J. Phys. Chem. B. 2006;110:5099–5111. doi: 10.1021/jp055213v. doi:10.1021/jp055213v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes G.C, Klimov V.V, Baranov S.V, Kozlov Y.N, DasGupta J, Tyryshkin A. The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis. Proc. Natl Acad. Sci. USA. 2001;98:2170–2175. doi: 10.1073/pnas.061514798. doi:10.1073/pnas.061514798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira K.N, Iverson T.M, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. doi:10.1126/science.1093087 [DOI] [PubMed] [Google Scholar]
- Grabolle M, Dau H. Energetics of primary and secondary electron transfer in photosystem II membrane particles of spinach revisited on basis of recombination-fluorescence measurements. Biochim. Biophys. Acta. 2005;1708:209–218. doi: 10.1016/j.bbabio.2005.03.007. doi:10.1016/j.bbabio.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Gupta R.S, Mukhta T, Singh B. Evolutionary relationships among photosynthetic prokaryotes (Heliobacterium chlorum, Chloroflexus aurantiacus, cyanobacteria, Chlorobium tepidum and proteobacteria): implications regarding the origin of photosynthesis. Mol. Microbiol. 1999;32:893–906. doi: 10.1046/j.1365-2958.1999.01417.x. doi:10.1046/j.1365-2958.1999.01417.x [DOI] [PubMed] [Google Scholar]
- Hulsebosch R.J, Allakhverdiev S.I, Klimov V.V, Picorel R, Hoff A.J. Effect of bicarbonate on the S2 multiline EPR signal of the oxygen-evolving complex in photosystem II membrane fragments. FEBS Lett. 1998;424:146–148. doi: 10.1016/s0014-5793(98)00163-x. doi:10.1016/S0014-5793(98)00163-X [DOI] [PubMed] [Google Scholar]
- Kalman L, LoBrutto R, Allen J.P, Williams J.C. Manganese oxidation by modified reaction centers from Rhobobacter sphaeroides. Biochemistry. 2003;42:11 016–11 022. doi: 10.1021/bi034747o. doi:10.1021/bi034747o [DOI] [PubMed] [Google Scholar]
- Klimov V.V, Baranov S.V. Bicarbonate requirement for the water-oxidizing complex of photosystem II. Biochim. Biophys. Acta. 2001;1503:187–196. doi: 10.1016/s0005-2728(00)00222-x. doi:10.1016/S0005-2728(00)00222-X [DOI] [PubMed] [Google Scholar]
- Klimov V.V, Shuvalov V.A, Krakhmaleva I.N, Klevanik A.V, Krasnovskii A.A. Photoreduction of bacteriopheophytin b in the primary light reaction of Rhodopseudomonas viridis chromatophores. Biochemistry (Mosc.) 1977;42:519–530. [PubMed] [Google Scholar]
- Klimov V.V, Allakhverdiev S.I, Shuvalov V.A, Krasnovsky A.A. Effect of extraction and re-addition of manganese on light reactions of photosystem II preparations. FEBS Lett. 1982;148:307–312. doi: 10.1016/0014-5793(82)80830-2. doi:10.1016/0014-5793(82)80830-2 [DOI] [PubMed] [Google Scholar]
- Klimov V.V, Allakhverdiev S.I, Baranov S.V, Feyziev Y.M. Effects of bicarbonate and formate on the donor side of photosystem II. Photosynth. Res. 1995a;46:219–225. doi: 10.1007/BF00020434. doi:10.1007/BF00020434 [DOI] [PubMed] [Google Scholar]
- Klimov V.V, Allakhverdiev S.I, Feyziev Y.M. Bicarbonate requirement for the donor side of photosystem II. FEBS Lett. 1995b;363:251–255. doi: 10.1016/0014-5793(95)00327-6. doi:10.1016/0014-5793(95)00327-6 [DOI] [PubMed] [Google Scholar]
- Klimov V.V, Hulsebosch R, Allakhverdiev S.I, Wincencjusz H, van Gorkom H, Hoff A. Bicarbonate may be required for ligation of manganese in the oxygen evolving complex of photosystem II. Biochemistry. 1997;36:16 277–16 281. doi: 10.1021/bi9717688. doi:10.1021/bi9717688 [DOI] [PubMed] [Google Scholar]
- Kozlov Y.N, Kazakova A.A, Klimov V.V. Changes in the redox potential and catalase activity of Mn2+ ions during formation of Mn-bicarbonate complexes. Membr. Cell Biol. (Mosc.) 1997;11:115–120. [PubMed] [Google Scholar]
- Kozlov Y.N, Zharmukhamedov S.K, Tikhonov K.G, Dasgupta J, Kazakova A.A, Dismukes G.C, Klimov V.V. Oxidation potentials and electron to photosystem II of manganese complexes containing bicarbonate and carboxylate ligands. Phys. Chem. Chem. Phys. 2004;6:4905–4911. doi:10.1039/b406569g [Google Scholar]
- Law C.J, Rozack A.W, Southall J, Gardiner A.T, Isaacs N.W, Cogdell R.J. The structure and function of bacterial light-harvesting complexes (review) Mol. Memb. Biol. 2004;21:183–191. doi: 10.1080/09687680410001697224. doi:10.1080/09687680410001697224 [DOI] [PubMed] [Google Scholar]
- Lin X, Murchison H.A, Nagarajan V, Parson W.W, Allen J.P, Williams J.C. Specific alteration of the oxidation potential of the electron donor in reaction centers from Rhodobacter sphaeroides. Proc. Natl Acad. Sci. USA. 1994;91:10 265–10 269. doi: 10.1073/pnas.91.22.10265. doi:10.1073/pnas.91.22.10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. doi:10.1038/nature04224 [DOI] [PubMed] [Google Scholar]
- Moskalenko A.A, Makhneva Z.K, Fiedor L, Scheer H. Effects of carotenoid inhibition on the photosynthetic RC-LH1 complex in purple sulphur bacterium Thiorhodospira sibirica. Photosynth. Res. 2005;86:71–80. doi: 10.1007/s11120-005-4473-9. doi:10.1007/s11120-005-4473-9 [DOI] [PubMed] [Google Scholar]
- Prince R.C, Leigh J.S, Dutton L.P. Thermodynamic properties of the reaction center of Rhodopseudomonas viridis in vivo measurement of the reaction center bacteriochlorophyll–primary acceptor intermediary electron carrier. Biochim. Biophys. Acta. 1976;440:622–636. doi: 10.1016/0005-2728(76)90047-5. doi:10.1016/0005-2728(76)90047-5 [DOI] [PubMed] [Google Scholar]
- Qian P, Yagura T, Koyama Y, Cogdell R.J. Isolation and purification of the reaction center (RC) and the core (RC-LH1) complex from Rhodobium marinum: the LH1 ring of the detergent-solubilized core complex contains 32 bacteriochlorophylls. Plant Cell Physiol. 2000;41:1347–1353. doi: 10.1093/pcp/pcd068. doi:10.1093/pcp/pcd068 [DOI] [PubMed] [Google Scholar]
- Shutova, T. et al. Submitted. The PSII-associated carbonic anhydrase in Chlamydomonas enhances the rate of oxygen production by proton removal.
- Shuvalov V.A, Duysens L.N. Primary electron transfer reactions in modified reaction centers from Rhodopseudomonas sphaeroides. Proc. Natl Acad. Sci. USA. 1986;83:1690–1694. doi: 10.1073/pnas.83.6.1690. doi:10.1073/pnas.83.6.1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvalov V.V, Klimov V.V. The primary photoreactions in the complex cytochrome-P-890–P-760 (bacteriopheophytin760) of Chromatium minutissimum at low redox potentials. Biochim. Biophys. Acta. 1976;440:587–599. doi: 10.1016/0005-2728(76)90044-x. doi:10.1016/0005-2728(76)90044-X [DOI] [PubMed] [Google Scholar]
- Straub K.L, Rainey F.A, Widdel F. Rhodovulum iodosum sp. nov. and Rhodovulum robiginosum sp. nov: two new marine phototrophic ferrous-iron-oxidizing purple bacteria. Int. J. Syst. Bacteriol. 1999;49:729–735. doi: 10.1099/00207713-49-2-729. [DOI] [PubMed] [Google Scholar]
- Van Rensen J.J.S, Klimov V.V. Bicarbonate interactions. In: Wydrzynski T, Satoh K, editors. Photosystem II: the light-driven water : plastoquinone oxidoreductase. Springer; Dordrecht, The Netherlands: 2005. pp. 329–345. [Google Scholar]
- Villarejo A, Shutova T, Moskvin O, Forssén M, Klimov V.V, Samuelsson G. A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J. 2002;21:1930–1938. doi: 10.1093/emboj/21.8.1930. doi:10.1093/emboj/21.8.1930 [DOI] [PMC free article] [PubMed] [Google Scholar]