Abstract
Objective
The primary objective of the trial is to compare survival to hospital discharge with Modified Rankin Score (MRS) ≤3 between a strategy that prioritizes a specified period of CPR before rhythm analysis (Analyze Later) versus a strategy of minimal CPR followed by early rhythm analysis (Analyze Early) in patients with out-of-hospital cardiac arrest.
Methods
Design
Cluster randomized trial with cluster units defined by geographic region, or monitor/defibrillator machine.
Population
Adults treated by Emergency Medical Service (EMS) providers for non-traumatic out-of-hospital cardiac arrest not witnessed by EMS.
Setting
EMS systems participating in the Resuscitation Outcomes Consortium and agreeing to cluster randomization to the Analyze Later versus Analyze Early intervention in a crossover fashion.
Sample Size
Based on a two-sided significance level of 0.05, a maximum of 13,239 evaluable patients will allow statistical power of 0.996 to detect a hypothesized improvement in the probability of survival to discharge with MRS ≤ 3 rate from 5.41% after Analyze Early to 7.45% after Analyze Later (2.04% absolute increase in primary outcome).
Conclusion
If this trial demonstrates a significant improvement in survival with a strategy of Analyze Later, it is estimated that 4,000 premature deaths from cardiac arrest would be averted annually in North America alone.
Keywords: prehospital, cardiac arrest, CPR, defibrillation
1.0 Introduction
A presenting rhythm of ventricular fibrillation (VF) or pulseless ventricular tachycardia (PVT) provides the best chance for survival after a resuscitation attempt for victims of out-of-hospital cardiac arrest. The traditional approach to these patients has been to prioritize the analysis of cardiac rhythm and delivery of defibrillatory shocks, if indicated, as quickly as possible. As a result, administration of external chest compressions with ventilation (CPR) is often delayed until after the initial rhythm evaluation. Some now advocate the opposite strategy of delaying rhythm analysis and electrical shocks until after the provision of a period of CPR. Three clinical studies have each attempted to evaluate the strategy of sustained CPR with deferred rhythm analysis versus early rhythm analysis (CPR only until the defibrillator electrodes can be placed) 1–3. While the findings from two studies supported the strategy of CPR before initial rhythm analysis1;2, one did not.3 Furthermore, none were definitive and all had important limitations.
The Resuscitation Outcomes Consortium (ROC) Investigators have proposed a large clinical trial, using a partial factorial design, entitled ROC PRIMED (Prehospital Resuscitation using an Impedance valve and Early vs Delayed analysis) that will test two strategies. One strategy (Part 1) involves the impedance threshold device (ITD), which enhances venous return and cardiac output by increasing the degree of negative intrathoracic pressure during decompression. The second strategy (Part 2) involves initiating resuscitation with a sustained period of manual compressions and ventilations (Analyze Later), rather than attempting analysis and, if indicated, defibrillation immediately (Analyze Early). The purpose of this paper is to describe the rationale and methodology for Part 2, a comparison of a strategy of Analyze Later versus a strategy of Analyze Early in patients with out-of-hospital cardiac arrest. The rationale and methodology for the use of the ITD (Part 1) is described in a companion paper.
1.1 Background and Significance
1.1.1 Conceptual Framework for Analyze Later
Our current paradigm of cardiac arrest defines VF as “shockable,” with the optimal therapeutic approach being immediate direct countershock.4 Integral to this approach is the concept that defibrillation attempts should occur without delay upon recognition of VF, either by prehospital personnel or the analysis software contained within automated external defibrillators (AED), which can be applied by first responders with limited training or even laypersons.5 This approach has defined current Emergency Cardiac Care (ECC) algorithms, shaped the development of emergency medical service (EMS) systems and has resulted in improved survival for cardiac arrest victims with an initial rhythm of VF in some EMS systems.6–12 Others have questioned whether this current standard of care has measurably improved outcome from out-of-hospital cardiac arrests on a community level.1
One of the major limitations to this cardiac arrest paradigm is its consideration of VF as homogenous, without regard for variability in VF morphology or elapsed time since the arrest. In contrast, experimental models of VF arrest support three distinct phases, each with a different optimal therapeutic approach.13 The early moments following arrest define an “electrical phase” during which little ischemic injury has occurred and rapid defibrillation attempts appear to be most effective. After some time period, probably around 3–4 minutes, the optimal therapeutic approach no longer appears to be immediate countershock but instead includes a period of chest compressions prior to defibrillation attempts. Effective chest compressions, traditionally held to provide approximately 30% of normal cardiac output during the first several minutes, may be sufficient to modify favorably the status of the myocardium during ventricular fibrillation. Immediate defibrillation attempts during the circulatory phase may be unsuccessful due to persistent or recurrent VF or may result in terminal pulseless electrical activity (PEA) or asystole. Interestingly, outcomes in patients “shocked” into PEA or asystole are significantly worse than when these (rather than VF) are the presenting rhythms.14 After some additional elapsed time, even chest compressions prior to defibrillation attempts do not appear to change outcome. This may be due to the initiation of irreversible ischemic changes that ultimately lead to substantial myocyte and neuronal cell death. This “metabolic phase” is thought to start after about 10 minutes of total arrest duration, with no currently available therapies demonstrating efficacy once this phase is reached.
Another consideration relates to the significant proportion of patients who present with asystole or PEA as their initial cardiac arrest rhythm. Early provision of CPR in such patients may be of benefit in promoting the spontaneous return of circulation, or perhaps, fostering the development of a subsequent shockable rhythm.
1.1.2 Preliminary Studies
The effect of early rhythm analysis versus later rhythm analysis has been evaluated in animal and human studies. Animal models demonstrate improved ROSC and neurological outcomes with delayed countershock following a period of chest compressions in VF of moderate duration.15–18 Other animal studies have identified various VF morphologic features as potentially useful in predicting successful defibrillation in animal models of VF.19–21 Limited human data exist to support morphological analysis of VF/PVT as a predictor of successful ROSC.21–23 In addition, animal and human data suggest that chest compressions alone can modulate these morphological features to a more favorable configuration for successful ROSC. 20–22 None of these morphological features have been implemented prospectively into devices in a manner that is adequate to justify their clinical use; however, these data further support the therapeutic value of chest compressions prior to defibrillation in VF of moderate duration. Finally, the duration of time between cessation of chest compressions and direct countershock appears to influence success of ROSC and ultimate survival. 24;25 This suggests that prehospital providers should attempt to minimize delays after pausing chest compressions for rhythm analysis or ventilation prior to defibrillation attempts. These recommendations were incorporated into the 2005 AHA ECC guidelines.26
1.1.3 Clinical Studies
Three clinical studies have compared outcomes from out-of-hospital cardiac arrest due to VF when a period of CPR has or has not been prescribed prior to the first attempts at defibrillation.1–3
Cobb et al.’s prospective observational, population-based study revealed that survival to hospital discharge significantly improved during the intervention period (n=478) when out-of-hospital cardiac arrest patients were treated with 90 seconds of CPR prior to shock compared to the pre-intervention period (n=639) when out-of-hospital cardiac arrest patients were treated immediately with an AED (30% vs. 21%; p=0.04).1 There was a non-significant trend (71% vs. 79%, p = 0.11) toward a more favorable neurological outcome observed during the intervention period. A significant interaction also described a relatively greater survival benefit for CPR before defibrillation as the response interval of the first arriving unit increased, particularly in cases in which the response interval of the first arriving unit was 4 minutes or longer (p=0.04). These findings, although encouraging, could not be considered definitive and confirmative randomized clinical trials were recommended.
Wik et al. conducted a prospective randomized trial of 200 patients with out-of-hospital cardiac arrest due to VF to compare standard care with immediate defibrillation (n=96) or 3 minutes of CPR prior to defibrillation (n=104).2 The primary outcome of survival to hospital discharge did not differ significantly between the two treatment arms nor were there significant differences in ROSC, 1 year survival, and “good neurological recovery” at hospital discharge or 1 year after cardiac arrest. Yet, among the 119 patients with EMS response times longer than 5 minutes, posthoc subgroup analysis revealed that more patients in the CPR first strategy compared to the standard group achieved ROSC (58% vs. 38%; p=0.04), survived to hospital discharge (22% vs. 4%; p=0.006) and survived to 1 year (20% vs. 4%; p=0.01) but did not adjust for multiple comparisons.
Jacobs et al. conducted a prospective prehospital randomized trial in Western Australia which randomized 256 patients to a strategy of 90 seconds of CPR before defibrillation versus immediate defibrillation.3 Results revealed no significant difference in survival to hospital discharge between the two groups or between patients with a response interval of ≤5 minutes versus > 5 minutes.
1.1.4 Summary of Rationale
While it has been recognized for many years that out-of-hospital cardiac arrest patients who received “bystander CPR” have positive outcomes,27 this impact has been relegated to a secondary or even tertiary role in resuscitation sequencing. Small randomized or observational studies suggest that CPR before defibrillation may increase survival but the results to date are inconclusive. Although there is some evidence favoring immediate defibrillation in cases where the response time is < 2 minutes, such response times are rare and the frequent delay in recognition of the out-of-hospital cardiac arrest and calling 911, as well as the complexity of the resuscitation protocol, convince us that response time should not be used as an intervention modifier. Thus, for those who sustain a cardiac arrest before EMS arrival, there is clinical equipoise with regard to the competing strategies of Analyze Later versus Analyze Early. In contrast, patients who sustain a cardiac arrest after EMS arrival will be ineligible for inclusion in this study, in recognition that such a known brief period of arrest is best treated with early defibrillation.
1.2 Aim
The primary aim of the trial is to compare survival to hospital discharge with Modified Rankin Score (MRS) ≤ 3 between a strategy of Analyze Later consisting of a sustained period of CPR first followed by rhythm analysis versus a strategy of Analyze Early consisting of minimal CPR while defibrillator electrodes are attached with early rhythm analysis in patients with out-of-hospital cardiac arrest. The secondary aims of the trial are to compare survival to discharge, functional status at discharge and at 1, 3 and 6 months follow-up as well as Geriatric Depression Scale scores at 3 and 6 months.
2. Methods
2.1 Study Design
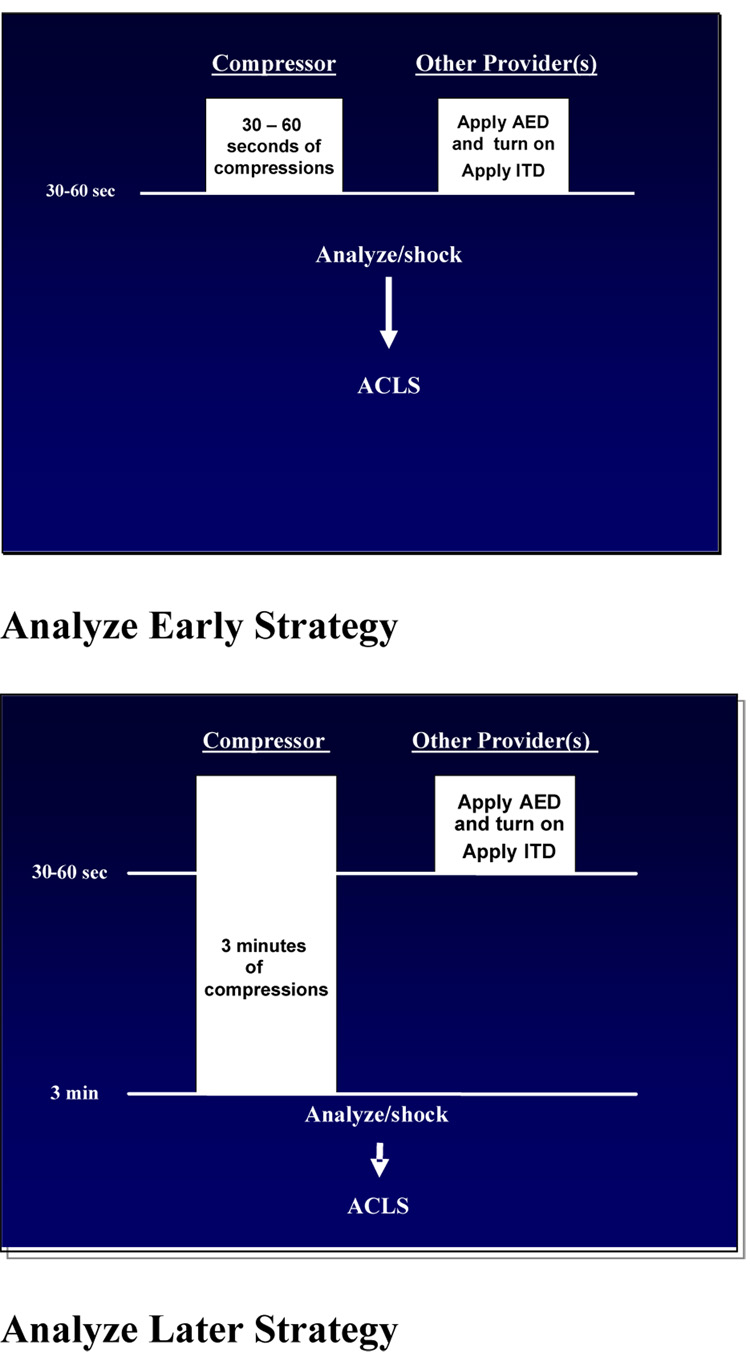
The ROC PRIMED Part 2 study will be a single-blinded cluster randomized crossover controlled trial with two intervention groups: a) an Analyze Early group, and b) Analyze Later group. Subjects in the Analyze Early group will be assigned to receive 30 – 60 seconds of chest compressions (that is, a brief period of standard CPR sufficient to allow time for placement of defibrillation electrodes and assure readiness of the defibrillator for rhythm analysis) prior to ECG analysis and defibrillation shocks if indicated. The Analyze Later group will receive approximately 3 minutes of standard compression – ventilation CPR prior to ECG analysis and rescue defibrillation. The intervention will be implemented by the first qualified provider to arrive at the scene of cardiac arrest. Qualified providers are defined as defibrillation-capable first responders, emergency medical technicians (EMTs), and paramedics. We will include all out-of-hospital locations within the participating ROC study communities. The ITD strategy (Part 1) and the Analyze Later versus Analyze Early strategy (Part 2) will be implemented simultaneously, capitalizing on the common infrastructure necessary to accomplish the study. We do not anticipate a substantial interactive effect between the two strategies. This is a partial factorial design study since the eligibility criteria for the two interventions are not identical.
2.2 Study Population & Primary Comparison Populations
Efficacy Population
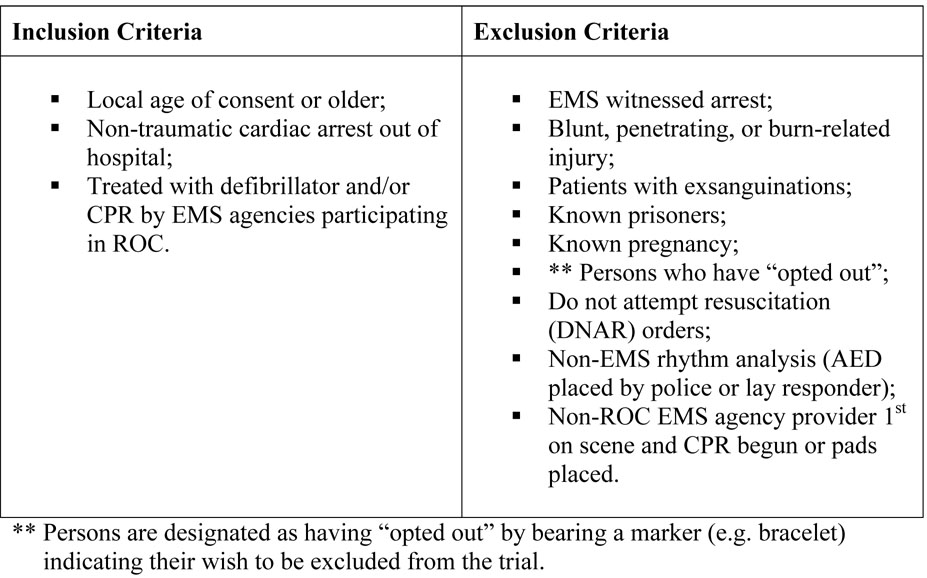
Analysis of primary and secondary efficacy outcomes will be conducted on a modified intent-to-treat basis. In order to be included in the efficacy analyses, patients must meet the inclusion/exclusion criteria for the Analyze Later versus Analyze Early intervention, as described in Figure 1. Furthermore, in order to be evaluable, they must also not have experienced cardiac arrest secondary to drowning, electrocution, or strangulation. All eligible patients are considered enrolled into the Analyze Later versus Analyze Early protocol by intention to treat regardless of how much CPR they actually received and will be included in the primary efficacy analysis.
Figure 1.
Study Population Inclusion and Exclusion Criteria
Safety Population
Evaluation of the safety of the Analyze Later versus Analyze Early strategies will be made using all data from patients who were treated, regardless of whether they are a member of the efficacy population.
2.3 Random Allocation
The intervention will be randomly allocated according to the cluster assignment. Randomization by event or by individual patient was deemed unfeasible because of the potential risk for carry-over from event to event, and because it would add unacceptable complexity for EMS providers. Rather, each ROC site will be subdivided into a goal of at least 20 clusters by the following means: a) according to EMS agency or geographical boundaries, or b) according to individual defibrillator device, rig, or station. All clusters will crossover between intervention assignments at least once (i.e. have at least two distinct treatment periods).
2.4 Intervention
For those clusters allocated to Analyze Later, defibrillator analysis will not be initiated until after delivery of compressions equivalent to approximately 3 minutes of CPR, after which a rescue shock will be administered, if indicated. For those clusters allocated to Analyze Early, defibrillator analysis will not be initiated until after the delivery of 30 – 60 seconds of chest compressions while defibrillator electrodes are attached after which a rescue shock will be administered if indicated. (Fig. 2)
Figure 2.
Intervention Strategies
Chest Compressions
Initiation of chest compressions will not be delayed. Recognition that a patient is in cardiac arrest will immediately prompt activation (“power on”) of the defibrillator and the start of CPR and ventilation with the ITD. This power-on event will initiate the time recording by the device, and serve as a surrogate marker for “time zero” of initiating CPR.
Minimum Interruptions
Training will emphasize uninterrupted chest compressions, except for required ventilations. If endotracheal intubation or other advanced airway procedures are deemed medically necessary, the providers will be encouraged to proceed while ensuring that chest compressions are continued with minimal interruption.
2.5 Adherence to Protocol
As intention-to-treat principles apply, any breach of protocol will not alter the study group to which a patient has been assigned. The time interval from power-on of the defibrillator at the first recognition of a cardiac arrest to the first ECG analysis (i.e. the power-on to analysis interval) and to first rescue shock (i.e. power-on to shock interval) will be calculated from the time annotated from the defibrillator clock on the electronic record. A study monitoring committee will evaluate protocol compliance during the run-in (section 2.12) and active phases of the trial. Feedback from this committee will be provided to sites in order to encourage continuous quality improvement. Explicit criteria will define the successful delivery of the intended therapy and this information will be provided back to the EMS providers (Table 1).
Table 1.
Intervention Compliance Time Interval Targets
| Power-on to analysis interval | Power-on to shock interval | CPR to 1st rhythm analysis interval | |
|---|---|---|---|
| Analyze Early | 30 – 60 seconds | < 90 seconds | < 60 seconds |
| Analyze Later | 180 – 200 seconds | 180 – 220 seconds | 150 – 210 seconds |
2.6 Outcome Measures
The primary outcome is survival to hospital discharge with a MRS ≤ 3.28;29 Patients who are transferred to another acute care facility will be considered to be still hospitalized. Patients transferred to a non-acute ward or facility will be considered discharged. The secondary 30–33 and exploratory 34;35 outcomes are listed in Table 2. In addition, the number of hospital days and time interval from 911 call to patient death will be described for all hospitalized patients as measures of in-hospital morbidity after resuscitation. Additional background information, rationale for selection, and details about specific functional status measures are given in Appendix 1.
Table 2.
Timing and Content of Secondary and Exploratory Outcome Measures
Abbreviations: MRS (Modified Rankin Score), CPC (Cerebral Performance Category), ALFI-MMSE (Adult Lifestyle and Function (ALFI) version of the Mini-Mental Status Exam (MMSE), HUI (Health Utilities Index), GDC (Geriatric Depression Score)
by chart review for hospital discharge and by phone after hospital discharge.
2.7 Sample Size and Analysis
The sample size calculation was based on having 90% power for the ITD (Part 1) portion of the trial yielding 14,154 evaluable patients. Due to the entry criteria differences between the Part 1 and Part 2 (Analyze Later versus Analyze Early) studies, we expect to enrol 13,239 evaluable patients for Part 2. Given 13,239 maximum evaluable patients, we have 99.6% statistical power to detect an improvement in the probability of survival to discharge with MRS ≤3 rate from 5.41% after Analyze Early to 7.45% after Analyze Later. This assumes a two-sided p<0.05 group sequential stopping rule with up to three analyses (two interim and the final analysis) after accruing approximately one-third, two-thirds, and all of the maximum sample size (O’Brien-Fleming Boundaries, Pd = 1.0, Pa =1.0). 36
Further, we did not assume the statistical information at a given analysis was proportionate to the sample size, but instead we assumed that only one-sixth of the statistical information will be available at the first interim look, one-half will be available at the second interim look, and all (assuming a 5% loss of efficiency due to cluster randomization) at the final analysis. The lower amount of statistical information corresponds to the estimated proportion of time a cluster has spent in both crossover periods at the given analysis. A cluster with equal time in each intervention arm corresponds to maximum statistical information.
Data analysis will be conducted in the framework of general linear mixed models which includes a fixed effect for each treatment arm and random effects for each randomization cluster.37 Adjustments for site and other baseline confounders will be incorporated, if necessary.
2.8 Monitoring of CPR Process
Several recent studies have evaluated the quality of CPR 38;39 and the importance of monitoring and improving CPR performance 40;41 in out-of-hospital and in-hospital settings. For this trial, all ROC clinical trial sites will have implemented a high-quality system for monitoring individual components of CPR. The CPR process will be monitored for a minimum of the first analyzable five minutes in all resuscitations. In addition, CPR process will be monitored for a minimum of five minutes after placement of an advanced airway. Determination of whether a resuscitation effort meets minimally acceptable CPR performance standards will be based on chest compression rate and CPR fraction criteria as defined in Table 3. Further rationale for monitoring CPR and additional information regarding the method of monitoring the CPR process is provided in Appendix 2.
Table 3.
CPR Performance Standards
| Parameter | Target | Minimum Acceptable (per minute) | Maximum Acceptable (per minute) | Criterion for Remediation/Retraining |
|---|---|---|---|---|
| Chest compression | 100/minute* | 80 | 120 | Above maximum or below minimum parameters in > 20% of resuscitations |
| CPR fraction∞ | 0.85 | 0.5 | - | Below minimum parameter in >20% of resuscitations |
refers to speed of compressions rather than actual number of compressions per minute
CPR fraction will be defined as = (Total seconds with chest compressions) ÷ (Total seconds with interpretable signal and no evidence of spontaneous circulation).
2.9 Data Collection and Data Entry
Data will be abstracted from collated source documents; the EMS patient care report(s), EMS dispatch times, EMS/fire/first responder electronic ECGs, emergency and hospital records and entered using customized web entry forms with standard data encryption and authentication methods
2.10 Recruitment and Informed Consent
This study qualifies for exception from informed consent for emergency research as outlined in US FDA regulation 21CFR50.24 and the Canadian Tri-Council Agreement for research in emergency health situations (Article 2.8).
2.11 Training
The training objectives for this study include: review of optimal CPR performance, scientific basis for and review of study protocols, practical (hands-on) session, and knowledge assessment test. Some retraining will occur at least every 6 months, including the use of written reminders, web based training modules, etc. Initial and retraining performance criteria will emphasize: optimal chest compression rate and depth, complete chest wall recoil with each compression, minimizing “hands-off” intervals, three minutes of compressions in Analyze Later arm, 30–60 seconds of compressions in Analyze Early arm, rapid placement of defibrillator pads and monitor/defibrillator activation (“power-on”) immediately upon recognition of pulseless arrest.
2.12 Run-in Phase
Compliance with the protocol and timely submission of the data will be required during the run-in phase before the Study Monitoring Committee determines the agency is now in the active phase of the trial. Compliance monitoring includes: correct inclusion/exclusion criteria, adherence to intervention arm, CPR process measures reported, and correct completion of data elements including reporting of adverse events.
3. Conclusion
A large, randomized clinical trial is underway to examine the impact of delayed defibrillation on survival to hospital discharge in patients who are presumed to be without circulation for several minutes. If this trial demonstrates a significant improvement in survival with a strategy of Analyze Later, we estimate the premature demise of 4,000 victims of out-of-hospital cardiac arrests would be averted annually in North America alone.
Supplementary Material
Acknowledgments
This study was supported by a cooperative agreement (5U01 HL077863) with the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, and the Heart and Stroke Foundation of Canada.
We would like to thank Julie Cummins for editing and preparing the manuscript for submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Tthere is no conflict of interest.
Contributor Information
Ian G. Stiell, Ottawa, Ontario, Canada
Clif Callaway, Pittsburgh, Pennsylvania, USA.
Dan Davis, San Diego, California, USA.
Tom Terndrup, Birmingham, Alabama, USA.
Judy Powell, Seattle, Washington, USA.
Andrea Cook, Seattle, Washington, USA.
Peter J. Kudenchuk, Seattle, Washington, USA
Mohamud Daya, Portland, Oregon, USA.
Richard Kerber, Iowa City, Iowa, USA.
Ahamed Idris, Dallas, Texas, USA.
Laurie J. Morrison, Toronto, Ontario, Canada
Tom Aufderheide, Milwaukee, Wisconsin, USA.
References
- 1.Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 2.Wik L, Hansen TB, Fylling F, Steen T, Vaagenes P, Auestad BH, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289(11):1389–1395. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs IG, Finn JC, Oxer HF, Jelinek GA. CPR before defibrillation in out-of-hospital cardiac arrest: a randomized trial. Emerg Med Australas. 2005;17(1):39–45. doi: 10.1111/j.1742-6723.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. International Consensus on Science. Circulation. 2000;102(8) Suppl I:1–291. [Google Scholar]
- 5.Weisfeldt ML, Kerber RE, McGoldrick RP, Moss AJ, Nichol G, Ornato JP, et al. Public Access Defibrillation. A statement for health care professionals from the American Heart Association Task Force on Automatic External Defibrillation. Circulation. 1995;92:2763. doi: 10.1161/01.cir.92.9.2763. [DOI] [PubMed] [Google Scholar]
- 6.Caffrey SL, Willoughby PJ, Pepe PE, Becker LB. Public use of automated external defibrillators. N Engl J Med. 2002;347(16):1242–1247. doi: 10.1056/NEJMoa020932. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, Hardman RG. Outcomes of Rapid Defibrillation by Security Officers after Cardiac Arrest in Casinos. N Engl J Med. 2000;343(17):1206–1209. doi: 10.1056/NEJM200010263431701. [DOI] [PubMed] [Google Scholar]
- 8.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 9.Myerburg RJ, Fenster J, Velez M, Rosenberg D, Lai S, Kurlansky P, et al. Impact of community-wide police car deployment of automated external defibrillators on survival from out-of-hospital cardiac arrest. Circulation. 2002;106(9):1058–1064. doi: 10.1161/01.cir.0000028147.92190.a7. [DOI] [PubMed] [Google Scholar]
- 10.Mosesso VN, Davis EA, Auble TE, Paris PM, Yealy DM. Use of Automated External Defibrillators by Police Officers for Treatment of Out of Hospital Cardiac Arrest. Ann Emerg Med. 1998;32:200–207. doi: 10.1016/s0196-0644(98)70137-4. [DOI] [PubMed] [Google Scholar]
- 11.Stiell IG, Wells GA, Field BJ, Spaite DW, De Maio VJ, Ward R, et al. Improved out-of-hospital cardiac arrest survival through the inexpensive optimization of an existing defibrillation program: OPALS study phase II. Ontario Prehospital Advanced Life Support. JAMA. 1999;281(13):1175–1181. doi: 10.1001/jama.281.13.1175. [DOI] [PubMed] [Google Scholar]
- 12.Nichol G, Stiell IG, Laupacis A, Pham B, De Maio V, Wells GA. A Cumulative Metaanalysis Of The Effectiveness of Defibrillator-Capable Emergency Medical Services For Victims Of Out-Of-Hospital Cardiac Arrest. Annals of Emergency Medicine. 1999;34(4):517–525. [PubMed] [Google Scholar]
- 13.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288(23):3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 14.Niemann JT, Stratton SJ, Cruz B, Lewis RJ. Outcome of out-of-hospital postcountershock asystole and pulseless electrical activity versus primary asystole and pulseless electrical activity. Crit Care Med. 2001;29(12):2366–2370. doi: 10.1097/00003246-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Yakaitis RW, Ewy GA, Otto CW, Taren DL, Moon TE. Influence of time and therapy on ventricular defibrillation in dogs. Crit Care Med. 1980;8(3):157–163. doi: 10.1097/00003246-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Niemann JT, Cruz B, Garner D, Lewis RJ. Immediate countershock versus cardiopulmonary resuscitation before countershock in a 5-minute swine model of ventricular fibrillation arrest. Ann Emerg Med. 2000;36(6):543–546. doi: 10.1067/mem.2000.109441. [DOI] [PubMed] [Google Scholar]
- 17.Menegazzi JJ, Seaberg DC, Yealy DM, Davis EA, MacLeod BA. Combination pharmacotherapy with delayed countershock vs standard advanced cardiac life support after prolonged ventricular fibrillation. Prehosp Emerg Care. 2000;4(1):31–37. doi: 10.1080/10903120090941614. [DOI] [PubMed] [Google Scholar]
- 18.Menegazzi JJ, Davis EA, Yealy DM, Molner RL, Nicklas KA, Hosack GM, et al. An experimental algorithm versus standard advanced cardiac life support in a swine model of out-of-hospital cardiac arrest. Ann Emerg Med. 1993;22(2):235–239. doi: 10.1016/s0196-0644(05)80211-2. [DOI] [PubMed] [Google Scholar]
- 19.Lightfoot CB, Nremt P, Callaway CW, Hsieh M, Fertig KC, Sherman LD, et al. Dynamic nature of electrocardiographic waveform predicts rescue shock outcome in porcine ventricular fibrillation. Ann Emerg Med. 2003;42(2):230–241. doi: 10.1067/mem.2003.264. [DOI] [PubMed] [Google Scholar]
- 20.Berg RA, Hilwig RW, Kern KB, Ewy GA. Precountershock cardiopulmonary resuscitation improves ventricular fibrillation median frequency and myocardial readiness for successful defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Ann Emerg Med. 2002;40(6):563–570. doi: 10.1067/mem.2002.129866. [DOI] [PubMed] [Google Scholar]
- 21.Callaway CW, Sherman LD, Mosesso VN, Jr, Dietrich TJ, Holt E, Clarkson MC. Scaling exponent predicts defibrillation success for out-of-hospital ventricular fibrillation cardiac arrest. Circulation. 2001;103(12):1656–1661. doi: 10.1161/01.cir.103.12.1656. [DOI] [PubMed] [Google Scholar]
- 22.Jekova I, Dushanova J, Popivanov D. Method for ventricular fibrillation detection in the external electrocardiogram using nonlinear prediction. Physiol Meas. 2002;23(2):337–345. doi: 10.1088/0967-3334/23/2/309. [DOI] [PubMed] [Google Scholar]
- 23.Eftestol T, Sunde K, Steen PA. Effects of interrupting precordial compressions on the calculated probability of defibrillation success during out-of-hospital cardiac arrest. Circulation. 2002;105(19):2270–2273. doi: 10.1161/01.cir.0000016362.42586.fe. [DOI] [PubMed] [Google Scholar]
- 24.Berg RA, Hilwig RW, Kern KB, Sanders AB, Xavier LC, Ewy GA. Automated external defibrillation versus manual defibrillation for prolonged ventricular fibrillation: lethal delays of chest compressions before and after countershocks. Ann Emerg Med. 2003;42(4):458–467. doi: 10.1067/s0196-0644(03)00525-0. [DOI] [PubMed] [Google Scholar]
- 25.Koster RW. Limiting 'hands-off' periods during resuscitation. Resuscitation. 2003;58(3):275–276. doi: 10.1016/s0300-9572(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 26.Hazinski MF, Nadkarni VM, Hickey RW, O'Connor R, Becker LB, Zaritsky A. Major Changes in the 2005 AHA Guidelines for CPR and ECC: Reaching the Tipping Point for Change. Circulation. 2005;112(24suppl):IV-206. doi: 10.1161/CIRCULATIONAHA.105.170809. [DOI] [PubMed] [Google Scholar]
- 27.Stiell IG, Wells GA, Field B, Spaite DW, Nesbitt LP, De Maio VJ, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 28.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scottish Medical Journal. 1957;2(5):200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 29.van Alem AP, de Vos R, Schmand B, Koster RW. Cognitive impairment in survivors of out-of-hospital cardiac arrest. Am Heart J. 2004;148(3):416–421. doi: 10.1016/j.ahj.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Roccaforte WH, Burke WJ, Bayer BL, Wengel SP. Validation of a Telephone Version of the Mini-Mental State Examination. J Am Geriatric Soc. 1992;40:697–702. doi: 10.1111/j.1532-5415.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Burke WJ, Roccaforte WH, Wengel SP, Conley DM, Potter JF. The reliability and validity of the Geriatric Depression Rating Scale administered by telephone. J Am Geriatr Soc. 1995;43(6):674–679. doi: 10.1111/j.1532-5415.1995.tb07205.x. [DOI] [PubMed] [Google Scholar]
- 33.Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–128. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 35.The Brain Resuscitation Clinical Trial II Study Group. A randomized clinical trial of calcium entry blocker administration to comatose survivors of cardiac arrest: design, methods, and patient characteristics. Controlled Clin Trials. 1991;12:525–545. doi: 10.1016/0197-2456(91)90011-a. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 37.Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 38.Abella BS, Alvarado JP, Myklebust H, Edelson DP, Barry A, O'Hearn N, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293(3):305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 39.Wik L, Kramer-Johansen J, Myklebust H, Sorebo H, Svensson L, Fellows B, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 40.Abella BS, Sandbo N, Vassilatos P, Alvarado JP, O'Hearn N, Wigder HN, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111(4):428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 41.Aufderheide TP, Sigurdsson G, Pirrallo RG, Yannopoulos D, McKnite S, von Briesen C, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.