Abstract
Rheumatologists have long been focused on developing novel immunotherapeutics to manage such prototypic autoimmune disease as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). The ultimate challenge in immune suppressing patients with RA and SLE has derived from the dilemma that both protective and harmful immune responses result from adaptive immune responses, mediated by highly diverse, antigen-specific T cells and B cells endowed with powerful effector functions and the ability for long-lasting memory. As regulatory/suppressor T cells can suppress immunity against any antigen, including self-antigens, they emerge as an ideal therapeutic target. Several distinct subtypes of CD8+ suppressor cells (Ts) have been described that could find application in treating RA or SLE. In a xenograft model of human synovium, CD8+CD28−CD56+ T cells effectively suppressed rheumatoid inflammation. Underlying mechanisms involve conditioning of antigen-presenting cells (APC). Adoptively transferred CD8+ T cells characterized by IL-16 secretion have also exhibited disease-inhibitory effects. In mice with polyarthritis, CD8+ Ts suppressed inflammation by IFN-γ-mediated modulation of the tryptophan metabolism in APC. In SLE animal models, CD8+ Ts induced by a synthetic peptide exerted suppressive activity mainly via the TGFβ-Foxp3-PD1 pathway. CD8+ Ts induced by histone peptides were found to downregulate disease activity by secreting TGFβ. In essence, disease-specific approaches may be necessary to identify CD8+ Ts optimally suited to treat immune dysfunctions in different autoimmune syndromes.
Introduction
Studying autoimmune diseases has been instrumental in deciphering how the immune system functions to protect and to attack the host and how immune-mediated protection and tissue injury are closely interlinked. With most of the autoimmune syndromes being genetically associated to major histocompatibility complex (MHC) class II haplotypes, much of the interest in understanding pathogenic immune responses has been focused on CD4+ T cells. Inappropriate activation of CD4+ T cells by antigen presented in the context of disease-associated human leukocyte antigen (HLA) molecules provided a framework for a general paradigm applied to many of the autoimmune syndromes. It seemed less obvious that autoimmunity may result from a lack of immunosuppression. The concept of T cells suppressing immune responses was introduced by Gershon and Kondo in a research paper describing CD8+ suppressor T cells (Ts) [1]. CD8+ Ts attracted much interest until the mid 1980’s but fell into disregard when it was difficult to identify the responsible cell populations and the molecular mechanisms underlying the suppressor activity. However, the appeal for T cells with suppressor/regulatory activity was resurrected after a seminal publication by Sakaguchi and colleagues identifying CD25, interleukin (IL)-2 receptor α chain, as a molecular marker of suppressive CD4+ T cells [2]. A large series of studies has now demonstrated the very important role of CD4+CD25+ regulatory T cells (Tregs) for immune homeostasis and the regulation of autoimmunity [3–5]. Currently, it is believed that regulatory T lymphocytes including natural CD4+CD25+Foxp3+ Tregs, adaptive CD4+CD25+Foxp3+ Tregs [6], T regulatory 1 (Tr1) cells [7], and T helper (Th) 3 cells [8] are critically involved in the maintenance of immune homeostasis and the prevention of autoimmune diseases. Recent publications have established that the regulatory/suppressor T-lymphocyte family consists not only of the CD4+ T-cell population but also includes CD8+ T-cell populations. Notably, several types of CD8+ Ts with several phenotypes (Fig. 1) seem to exist in humans and experimental animals (Table 1), whose modes of suppressive action can be categorized into three mechanisms: cell-cell contact-mediated suppression, anti-inflammatory cytokine secretion, and cytotoxicity to the target cells.
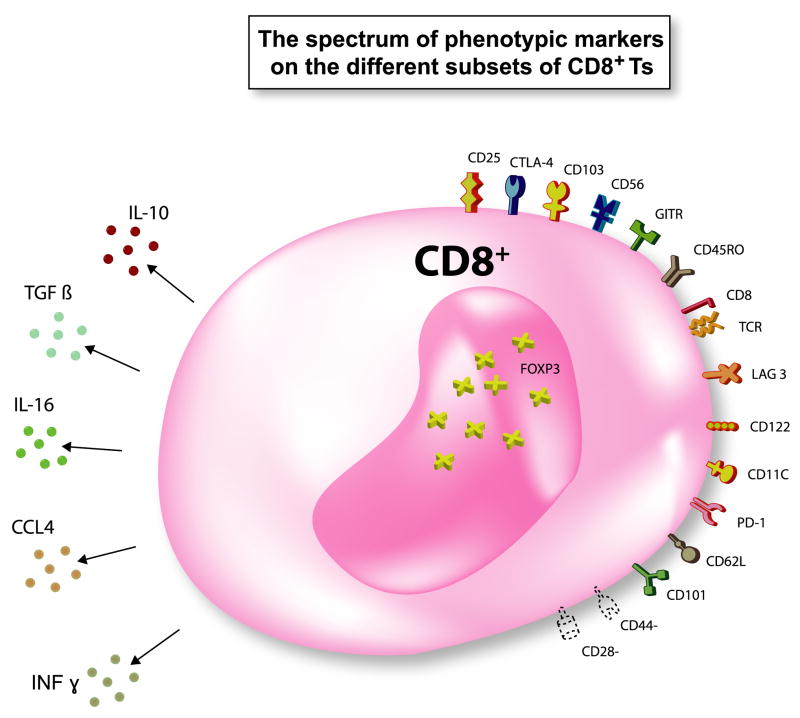
Figure 1. The spectrum of phenotypic markers on the different subsets of CD8+ suppressor T cells (Ts).
Various cell surface markers, cytokines, and Foxp3 have been reported to be expressed by CD8+ Ts. Not all cell surface markers are expressed on one subset of CD8+ Ts. Not all cytokines are produced by one particular CD8+ Ts subset. Foxp3 expression was not determined in some CD8+ Ts.
Table 1.
Known subtypes of inhibitory CD8+ T cells
| Described CD8+ suppressor T cell | Species | Natural or Adaptive | Induction | Mechanism of suppression | Reference |
|---|---|---|---|---|---|
| CD8+CD28−Foxp3+ | human | adaptive | Allogeneic, xenogeneic, or antigen-pulsed autologous APC | Induction of ILT3 and ILT4 on APC | [10–12] |
| CD8+CD103+ | human | adaptive | Alloantigen | Cell contact dependent | [13] |
| CD8+CD25+ CD28+Foxp3+ | human | adaptive | Staphylococcal Enterotoxin B or Anti-CD3 | Cell contact dependent | [14] |
| CD8+CD25+CTLA-4+Foxp3+ | human | adaptive | Modified anti -CD3 | Cell contact dependent | [15] |
| CD8+CD25+CD28−CTLA-4+Foxp3+ | human | adaptive | Autologous LPS-activated DC | CTLA-4 dependent | [16] |
| CD8+IL-10+ | human | adaptive | CD40 ligand-activated plasmacytoid DC | IL-10 secretion | [17] |
| CD8+CCR7+CD45RO+IL-10+ | human | adaptive | Tumor-ascites derived plasmacytoid DC | IL-10 secretion | [18] |
| CD8+ | human | adaptive | IL-2 and TGFβ | [19] | |
| CD8+CD103+CTLA-4+Foxp3+GITR+ | human | adaptive | Virus peptide-pulsed APC | Cell contact dependent | [20] |
| CD8+CD25+Foxp3+LAG3+ | human | adaptive | Bacillus Calmette-Guérin’s stimulation | CCL4 secretion | [21] |
| Nonantigen-specific CD8+CD28− | human | adaptive | Monocytes, GM-CSF, and IL-2 or IL-10 and IL-2 | IL-10 secretion | [23, 24, 55] |
| CD8+CD25+CTLA-4+GITR+Foxp3+ | human | natural | TGFβ- and CTLA-4-dependent | [25] | |
| CD8+CD28−CD56+ | human | adaptive | Allogeneic APC | Downregulation of CD80 and CD86 expression on APC | [48] |
| CD8+IL-16+ | human | in RA synovium | IL-16 secretion | [49] | |
| CD8+CD122+ | mouse | natural | IL-10 secretion | [26–28, 75] | |
| CD8+CD11c+ | mouse | adaptive | Anti-4-1 BB mAb | IFNγ-mediated induction of IDO in monocytes and DC | [53] |
| CD8+Foxp3+PD-1+TGFβ+ | mouse | adaptive | Antigen specific stimulation | Major mechanism: TGFβ-Foxp3-PD1 axis; Minor mechanism: Cytotoxicity via Granzyme B | [60–62] |
| CD8+CD62L+GITR+TGFβ+ | mouse | adaptive | Antigen-specific stimulation | TGFβ secretion | [63] |
| CD8+ | mouse | adaptive | IL-2 and TGFβ | Partially TGFβ dependent | [64] |
| CD8+CD45RO+CD101+CD103+ | mouse | adaptive | Allogeneic intestinal epithelial cells | Cell contact dependent | [68, 69] |
| CD8+CD44−CD103+ | mouse | adaptive | in TNF ΔARE mice | TGFβ secretion | [72] |
| CD8+CD28− | mouse | adaptive | in vitro: cell contact dependent in vivo: IL-10 and TGFβ dependent | [73] | |
| CD8+CD28− Foxp3+ | mouse | adaptive | Antigen-specific stimulation | [77] |
Abbreviations: APC = antigen presenting cells; ILT = immunoglobulin-like transcript; DC = dendritic cells; RA = rheumatoid arthritis; IDO = indoleamine 2,3-dioxygenase; ARE = AU-rich region
A. The multiplicity of CD8+ Ts
-
Ts induced by stimulation of CD8+ T cells
A major breakthrough in the field of suppressive T cells was the discovery that a distinct lineage of CD4+ T cells, CD4+CD25+ T cells, arises in the thymus and is characterized by the expression of the transcription factor Foxp3. However, it is now accepted that Tregs can emerge after stimulation of peripheral T cells. Especially in the human, most inhibitory CD8+ T cells are adaptive, induced after one time or several rounds of stimulation. Our knowledge of the family of induced peripheral regulatory/suppressor T lymphocytes has been growing, but it is currently not understood whether the different types of inducible CD8+ Ts are distinct cell populations, overlapping, or essentially derive from one source. Mostly, they emerge after T-cell receptor (TCR) stimulation and exhibit their downregulatory function by impairing the responsiveness of other T cells. A well-studied type of CD8+ Ts are CD8+CD28− T cells which are induced by stimulating peripheral blood mononuclear cells (PBMC) with either allogeneic [9] or xenogeneic stimulator cells [10] or, alternatively, are enriched by multiple rounds of stimulating with antigen-pulsed autologous antigen-presenting cells (APC) [11]. Suciu-Foca and colleagues have studied in detail how such CD8+CD28− Ts suppress autologous and heterologous CD4+ T-cell proliferation and have discovered that such Ts function by rendering APC tolerogenic. As the underlying molecular mechanism, these authors identified the induction of receptors that transmit negative signals, specifically upregulation of immunoglobulin-like transcript (ILT) 3 and ILT4 [12]. Alloantigen-induced CD8+CD103+ Ts have been reported to suppress T-cell proliferation in mixed lymphocyte culture via a cell to cell contact-dependent mechanism [13].
Continuous antigen stimulation in the presence of CD14+ monocytes has emerged as a means to generate CD8+CD25+Foxp3+ Ts which then suppress T-cell proliferation by a contact-dependent mechanism [14]. An alternative mode of inducing CD8+CD25+CTLA-4+Foxp3+ Ts involves TCR stimulation with modified anti-CD3 monoclonal antibodies (mAb); such CD8+ Ts require cell contact to mediate their inhibitory function [15]. Autoreactive CD8+CD25+CTLA-4+Foxp3+ Ts clones have been isolated from healthy and ankylosing spondylitis patients using autologous LPS-activated dendritic cells (DC); suppression of T-cell proliferation by such autoreactive CD8+ Ts involves a CTLA-4-dependent mechanism [16]. Naïve CD8+ T cells primed with allogeneic CD40 ligand-activated plasmacytoid DC differentiate into CD8+ Ts, which inhibit allospecific proliferation of naïve CD8+ T cells by producing significant amounts of IL-10 [17]. Plasmacytoid DC derived from tumor ascites induce CD8+CCR7+CD45RO+ IL-10+ suppressor T cells, which suppress myeloid DC-mediated tumor-associated antigen-specific T-cell effector function through IL-10 [18]. Ts induced by activating CD8+ T cells in the presence of both IL-2 and TGFβ downregulate IgM and IgG production from B cells cultured with CD4+ Th cells [19]. Human PBMC stimulated with hepatitis C virus or flu virus-specific peptides give rise to CD8+CD103+CTLA-4+Foxp3+GITR+ T cells displaying cell to cell contact-dependent suppressive activity [20]. Bacillus Calmette-Guérin’s stimulation results in the differentiation of CD8+CD25+LAG3+Foxp3+ T cells, which suppress T-cell proliferation partly through the secretion of CCL4 [21]. Notably, Dhodapkar and Steinman have provided direct experimental evidence that CD8+ Ts are functional in humans in vivo [22]. In a clinical trial, the authors injected immature DC pulsed with influenza matrix peptide into healthy human subjects. These in vivo stimulation conditions led to antigen-specific silencing of effector T-cell function, and the suppression was mediated by cell-cell contact.
Nonantigen-specific CD8+ Ts have also been reported [23, 24]. This type of CD8+ Ts can be generated in vitro without TCR stimulation from CD8+CD28− T cells, but not CD8+CD28+ T cells when cultured with autologous monocytes, GM-CSF, and IL-2 or IL-10 and IL-2. Functionally, these CD8+ Ts are capable of inhibiting the proliferation of antigen-specific T lymphocytes as well as the antigen-specific cytotoxicity of cytotoxic T lymphocytes through IL-10 secretion.
-
Natural CD8+ suppressor T cells
Naturally occurring Tregs constitute an endogenous long-lived population of T cells that develop in the thymus. They are believed to have specificity for self antigens and are poised to prevent autoimmunity. As an overriding theme, CD8+CD25+CTLA-4+GITR+Foxp3+ T thymocytes have similarities with natural CD4+CD25+ regulatory thymocytes [25]. Like CD4+CD25+ regulatory thymocytes, CD8+CD25+ thymocytes restrain the proliferative expansion of autologous CD4+CD25− T cells in a contact-dependent manner, which can be reversed by a mixture of anti-CTLA-4 and anti-TGFβ antibodies (Ab). In mice, CD8+CD122+ T cells have received special attention. As naturally occurring CD8+ Ts, they are regarded as a functional T-cell subset that impacts immunity through the release of the anti-inflammatory cytokine IL-10 [26]. Mice genetically deficient for CD122, the IL-2/IL-15 receptor β chain, spontaneously develop severe hyperimmunity by expanding abnormally activated T cells [27]. When CD122-deficient neonates receive adoptively transferred CD8+CD122+ T cells, the development of these abnormal T-cell populations is totally prevented [28]. Furthermore, adoptive transfer of CD8+CD122+ Ts ameliorates established experimental autoimmune encephalomyelitis (EAE) [29]. However, so far there has been no report on human CD8+CD122+ Ts.
-
Inhibitory CD8+ T cells recognizing MHC class Ib molecules on CD4+ T cells
The original observation that the interaction between CD8+ and CD4+ T cells resulted in inhibition if activated CD4+ T cells expressed the MHC class 1b molecule Qa-1 supported the notion that a third class of CD8+ suppressor cells may exist that communicates through defined restriction elements. The equivalent to Qa-1 in humans is the HLA-E molecule [30–33]. This class of MHC class Ib-restricted CD8+ Ts downregulates disease activity in multiple sclerosis (MS) [34, 35] and EAE [36–38] at least in part by killing antigen-activated autologous CD4+ T cells expressing the HLA-E/Qa-1-peptide complex as reviewed elsewhere in this issue.
In essence, it appears that nature relies on a multitude of approaches to inhibit unwanted immune responses. As an overarching theme, CD8+ Ts suppress by directly killing immune cells, an elegant mode of disrupting an immune response, by mediating negative signals, or by coopting other cells to elaborate suppressive molecules. TGFβ and IL-10 represent important players in immunosuppressive regulation (Fig. 2). Altering metabolic activities, such as inducing tryptophan catabolism through the induction of indoleamine 2,3-dioxygenase (IDO) provides a wide field for immunosuppressive impact with CD8+ Ts emerging as important players [39].
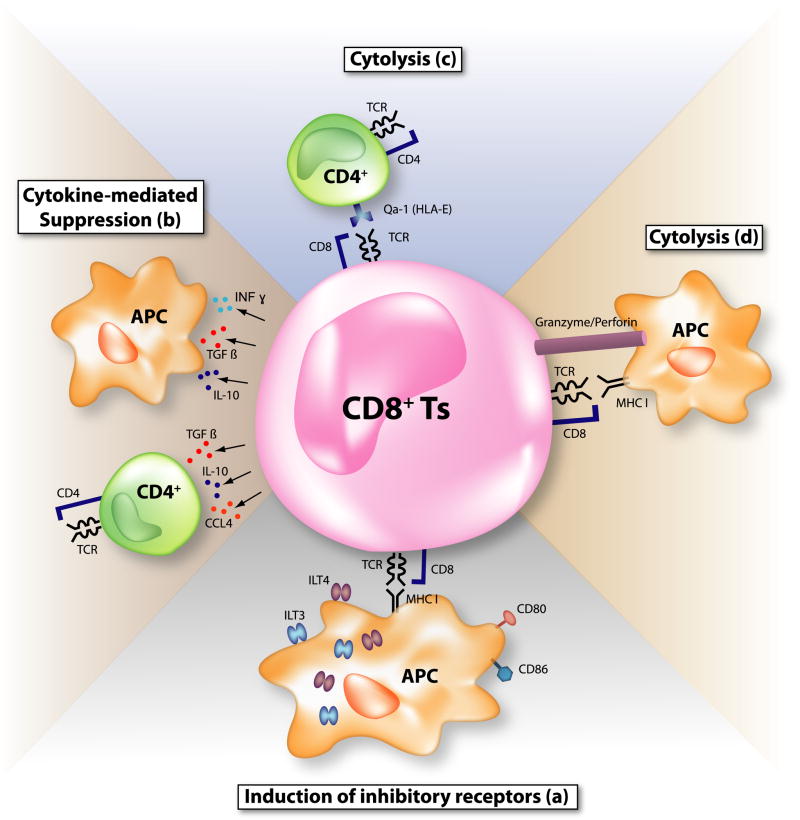
Figure 2. Possible mechanisms of suppression by CD8+ suppressor T cells (Ts).
Various suppression mechanisms by CD8+ Ts have been proposed so far. (a) CD8+CD28−Foxp3+ Ts upregulate inhibitory receptors, immunoglobulin-like transcript (ILT) 3 and ILT4 on antigen-presenting cells (APC), rendering APC tolerogenic. Costimulatory molecules on APC, CD80, and CD86 are also downregulated. (b) Immunosuppressive cytokines such as interleukin (IL)-10, TGFβ, IFNγ, and CCL4 are secreted by CD8+ Ts. (c) Major histocompatibility complex (MHC) class Ib molecule (Qa-1 in mice and HLA-E in humans)-restricted CD8+ Ts kill activated effector CD4+ T cells and dampen immune reactions. (d) CD8+ cytotoxic lymphocytes (CTL) work as suppressive T cells. When CTL encounter antigen-bearing APC, CTL may kill APC and attenuate immune responses.
B. Inhibitory CD8 T+ cell in autoimmune syndromes –Potential new therapeutic targets
Obviously, the great promise is that regulatory/suppressor T cells can eventually be therapeutically used to ameliorate chronic inflammatory autoimmune disease, including prototypic rheumatic autoimmune disease, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Data in murine models suggest that Tregs can be induced by essentially any antigen. Additionally, in experimental systems, antigen-specific Tregs can prevent as well as reverse already established autoimmunity. Much less is known about the potential role that regulatory/suppressor T cells have in human autoimmune disease. We will focus the rest of this review on CD8+ Ts and summarize the current information we have for human autoimmune syndromes and for autoimmune models in experimental animals, with a special emphasis on rheumatic and connective tissue disease.
-
Rheumatoid arthritis and murine models of rheumatoid-like polyarthritis
RA is a quintessential immune-mediated disease which targets small and large joints in a symmetrical pattern. Affected patients are disabled by pain and stiffness, and eventually suffer from irreversible loss of joint function and mobility. The systemic inflammatory response is associated with extraarticular manifestations, such as rheumatoid lung and nerve disease and rheumatoid nodule formation. The recent recognition that RA leads to premature atherosclerotic disease has markedly broadened the spectrum of disease manifestations and the challenges in managing this chronic disorder [40]. In the joint, the underlying pathology is that of dense inflammatory infiltrates accumulating in the synovial membrane. T cells and B cells acquire a highly sophisticated lymphoid organization, at times resulting in the formation of ectopic germinal centers [41–43]. Besides lymphocytes, inflammatory and tissue destructive networks involve a series of immune cell types, such as macrophages, DC, and mast cells. Non-immune resident cells, particularly synovial fibroblasts, endothelial cells, and osteoclasts contribute critically to the immunopathogenesis, specifically by forming tissue invasive pannus, cartilage, and bone erosions [44, 45]. Over the last decade, cytokines, in particular TNFα, have emerged as excellent targets for therapeutic interventions. Other successful treatment strategies include depletion of B cells and disrupting costimulatory pathways [46, 47].
Immunoregulatory functions of CD8+ Ts have been investigated in a chimera model of human RA. In this model, synovial tissues from RA patients are engrafted into severe combined immunodeficiency (SCID) mice and defined T-cell populations are adoptively transferred. Immuno-stimulatory and inhibitory effects are measured by analyzing T-cell and macrophage function in the explanted grafts. Davila et al. [48] have approached the topic of anti-inflammatory CD8+ T cells by generating CD8+CD28−CD56+ T cells. The induction protocol involved stimulating PBMC from HLA-A2 negative donors with irradiated HLA-A2+ myelomonocytic THP-1 cells. Such CD8+CD28−CD56+ T cells suppressed both autologous and heterologous T-cell responses. Subsequently, the authors isolated CD8+CD28−CD56+ T-cell clones from synovial tissues of RA patients, expanded them in vitro, and adoptively transferred them into nonobese diabetic (NOD)-SCID mice engrafted with RA synovial tissues. In the synovial lesions, CD8+CD28−CD56+ T cells displayed strong anti-inflammatory activities, whereas CD8+CD28+CD56− T-cell clones had proinflammatory function. Specifically, CD8+CD28−CD56+ Ts inhibited production of IFNγ, TNFα, CXCL10, and CXCL9 in autologous and HLA class I-matched heterologous synovitis. Adoptively transferred CD8+ Ts downregulated the costimulatory molecules CD80 and CD86 on synovial fibroblasts, suggesting that immunosuppression in the tissue lesions was predominantly mediated by altering APC function. CD8+CD28−CD56+ Ts failed to upregulate ILT3 and ILT4, both in vitro as well as in vivo. Another CD8+ Ts population has been demonstrated to exhibit disease-suppressing activities in RA synovial lesions. Klimiuk et al. [49] have reported that IL-16-producing CD8+ T cells had profound disease-suppressing effects when adoptively transferred into human synovium-SCID chimeras. In contrast to CD4+ T cells which amplified the tissue production of pro-inflammatory cytokines, IL-16-secreting CD8+ T cells successfully disrupted inflammatory networks in the synovial implants. Activation of the CD8+ Ts was accompanied by the induction of IL-16 mRNA. Adoptive transfer of the CD8+ Ts enhanced IL-16 transcription in the synovial grafts. Administration of anti-IL-16 Ab partially reversed T cell-mediated suppression of tissue IFN-γ production. Partial reversal suggests either incomplete neutralization of tissue IL-16 or the contribution of another mediator. Daily treatment with recombinant IL-16 suppressed IFN-γ, TNF-α, and IL-1β expression in synovium-SCID mouse chimeras. Therefore, IL-16-producing CD8+ T cells residing in the RA synovium appear to exert anti-inflammatory activity typically assigned to Tregs. As IL-16 is a natural ligand for the CD4 molecule, the anti-inflammatory effect of IL-16 may be mediated by CD4+ T cells. Possible mechanisms include induction of energy in CD4+ effector-T cells [50, 51]. Alternatively, it has been proposed that IL-16 could have a role in enriching CD4+CD25+Foxp3+ Tregs in the tissue (Fig. 3) [52].
In murine collagen type II (CII)-induced arthritis (CIA), a model frequently utilized to study innate and adaptive immune responses causing destructive polyarthritis, CD11c+CD8+ T cells seems to function as effective Ts. Seo et al. [53] found that administration of agonistic anti-4-1 BB mAb inhibits CIA development and improves even established CIA. Anti-4-1 BB mAb administration suppressed serum antibodies to CII in CIA mice and CD4+ T-cell recall responses to CII in in vitro proliferation assays. Injection of anti-4-1 BB mAb caused a massive and CII-dependent clonal expansion of CD11c+CD8+ T cells and was associated with the accumulation of IDO in CD11b+ monocytes and CD11c+ DC in the spleen and lymph nodes. CD11c+CD8+ T cells produced substantial amounts of IFNγ but not TGFβ, IL-4, or IL-10. Adoptive transfer of CD11c+CD8+ T cells collected from CII, complete Freund’s adjuvant (CFA), and anti-4-1 BB-treated mice suppressed CII-specific CD4+ T-cell recall responses and disrupted the development of CIA. The effect of anti-4-1 BB administration in CIA was reversed by anti-IFNγ mAb or co-administration of the IDO inhibitor 1-methyltryptophan. These data suggest that IFNγ-producing CD11c+CD8+ T cells are selectively expanded by triggering the costimulatory receptor 4-1 BB. Such CD8+ T cells function to suppress antigen-specific CD4+ T cells and ameliorate CIA through an IDO-dependent mechanism.
-
Systemic lupus erythematosus and murine models of tissue inflammation initiated by autoantibodies to DNA and RNA.
SLE is often referred to as a classical systemic autoimmune syndrome. Almost any organ system can be affected by chronic inflammatory attack. Although the etiology remains elusive, several major immune defects have been identified in SLE patients which play an important role in diagnosing the syndrome. Specifically, SLE patients form autoantibodies to a broad range of nuclear antigens. Autoantibodies to DNA and RNA are now considered pathogenically relevant in driving innate immune responses with a bias towards overproduction of type I interferons [54]. Defective clearance of apoptotic cells has also been considered a critical disease mechanism [55]. Many aspects of SLE are explained by the deposition of immune complexes that initiate vascular damage and tissue inflammation. SLE, like RA, is a risk factor for accelerated atherosclerosis; underlying pathomechanisms are not understood [56]. With no new therapy approved for SLE in several decades, Tregs hold great hope for a therapeutic innovation.
Filaci et al. [57] have generated CD8+ Ts in vitro by incubating purified CD8+ T cells and autologous monocytes from SLE patients and healthy subjects with IL-2 and GM-CSF. They assessed the suppressive activity of induced CD8+ Ts by adding them to anti-CD3-stimulated autologous PBMC proliferation assays. Although the phenotypes of CD8+ Ts from healthy controls, those from SLE patients in remission, and those from patients with active SLE were comparable, only CD8+ Ts from patients with SLE in remission and healthy controls functioned as effective suppressors. CD8+ T cells from patients with active SLE failed to suppress committed CD4+ T cells. Such functionally defective CD8+ Ts produced lower levels of IL-6 and higher levels of IL-12 compared with healthy controls. IL-4, IL-10, IFNγ, and TGFβ concentrations were comparable between SLE patients and healthy subjects. Adding anti-INFγ mAb to the cultures and pretreating CD8+ Ts from healthy donors with IL-6 antisense oligonucleotides counteracted the inhibitory activity. Recombinant IL-6 inhibited anti-CD3 mAb-induced PBMC proliferation, whereas IL-12 stimulated T-cell expansion. However, an alternative mechanism may explain the functional differences between the impaired CD8+ Ts isolated from active SLE patients and controls. Notably, CD8+ Ts from patients with SLE in remission also produced lower levels of IL-6 than healthy controls, had comparable levels of IL-12 to patients with active SLE, and IFNγ production for all experimental groups was essentially indistinguishable.
New Zealand Black (NZB)/New Zealand White (NZW) F1 female (BWF1) mice spontaneously develop autoantibodies, including anti-DNA antibodies and have been widely used as a model to study immunoregulatory defects in SLE. Hahn and colleagues [58] have made the important observation that high dose administration of an artificial synthetic peptide based on an amino acid sequence containing MHC class I and MHC class II determinants in the VH region of Ig anti-DNA called pConsensus (pCons, FIEWNKLRFRQGLEW) to either young or diseased mice results in decreased serum levels of autoantibodies and prolonged survival. The mechanism of this protection from disease involves multiple mechanisms, including anergy induction in CD4+ Th cells [59], induction of CD4+CD25+ Tregs [59], and induction of CD8+ Ts [60–62]. CD8+ T cells from pCons-treated mice suppressed anti-dsDNA Ab production in vivo [60]. Both CD8+CD28+ and CD8+CD28− T-cell populations from the spleen of pCons-injected mice suppressed production of anti-DNA antibodies [60] and inhibited the proliferation of CD4+ Th cells in vitro [62]. Naïve CD8+ T cells lacked these functional activities. CD8+ Ts induced in this system succeeded in suppressing CFSE-labeled B-cell proliferation [61]. Characterization of the CD8+ Ts revealed expression of Foxp3 and TGFβ, whose mRNA were further induced by secondary polyclonal stimulation with anti-CD3 and anti-CD28 mAb. Anti-TGFβ Ab addition abrogated suppression of anti-DNA Ig production [60] and the proliferation of naïve CD4+ T and B cells [61]. Based on transwell experiments, the CD8+ Ts did not require cell-cell contact for their regulatory interference [61]. In vitro addition of recombinant TGFβ induced Foxp3 expression in a dose-dependent manner. Small interfering (si) RNA of Foxp3 treatment of the CD8+ Ts abrogated the ability to inhibit anti-DNA production, the proliferation of CD4+ T cells, and TGFβ secretion [62]. Foxp3 and TGFβ may regulate the expression of each other, creating an autocrine loop in the induction and maintenance of Ts. However, it is likely that TGFβ secretion is not the only factor that directly suppresses autoreactivity in this experimental model, because naïve CD4+ T cells that give help to B cells secrete almost the same quantities of TGFβ [62]. Notably, blocking PD1/PDL1 with anti-PD1 or anti-PDL1 mAb abrogated the suppressive activity of the CD8+ Ts [61]. CD8+CD28−T cells from pCons-treated mice could kill B cells from nephritic animals, which expressed B-cell receptors with anti-DNA specificity. One cytotoxic effector molecule, granzyme B, was increased in pCons-treated CD8+ T cells compared with naïve CD8+ T cells [61]. In summary, pCons-induced CD8+ Ts may utilize diverse strategies to counteract disease-inducing immunity, most significantly utilizing the TGFβ/Foxp3/PD1 axis as well as cytotoxicity.
Kang et al. [63] have reported similar results to the above data; by administering a very low-dose of nucleosomal histone peptide autoepitopes to lupus prone (SWR × NZB) F1 (SNF1) mice, the authors were able to reduce autoantibody levels and extend life span, mostly by deferring nephritis. Mechanistic studies demonstrated that this low-dose peptide therapy induced CD8+ Ts as well as CD4+CD25+ Tregs. Splenic CD8+ T cells from peptide-tolerized mice were positive for the surface expression of TGFβ, CD62L, and GITR, typical markers of mature Tregs. The generated CD8+ Ts suppressed the production of INFγ and IgG autoantibodies to dsDNA, ssDNA, and anti-nucleosome. Adoptive transfer of the CD8+ Ts from low-dose peptide-tolerized SNF1 mice significantly suppressed IgG autoantibodies to dsDNA, ssDNA, nucleosomes, and histone in SNF1 mice immunized with pathogenic H1′22–42 peptide. In a helper-suppression assay with CD4+ Th and B cells, the suppression of IgG autoantibody production by CD8+ Ts was almost completely abrogated by the addition of anti-TGFβ Ab. Upon antigen-specific or anti-CD3 stimulation, the CD8+ Ts released increased amounts of total TGFβ. Furthermore, in transwell experiments, CD8+ Ts placed in the upper chamber suppressed IgG autoantibody production from CD4+ Th cells and B cells cocultured in the lower chamber. These data indicate that soluble TGFβ from the CD8+ Ts mediated the immunosuppression, at least in the in vitro helper-suppression assay.
Zheng et al. [64] have generated CD4+ and CD8+ suppressive T cells ex vivo with the intention to explore their therapeutic efficacy in a murine model of stimulatory graft-vs-host disease (sGVHD) associated with a lupus-like syndrome. Induction of DBA/2 T cells with irradiated APC from C57BL/6 in the presence of TGFβ and IL-2 induces CD4+ and CD8+ suppressive T cells. Injection of DBA/2 T cells into (DBA/2 × C57BL/6) F1 mice causes sGVHD. Adoptive transfer of ex vivo-generated suppressive T cells could essentially prevent or ameliorate sGVHD. Both coadministration of suppressive and stimulatory T cells together and injection of suppressive T cells two weeks after disease induction had beneficial therapeutic effects. Unfortunately, the authors did not assess CD4+ and CD8+ suppressive T-cell populations separately in the in vivo experiments. The same authors also showed that the ex vivo-generated CD8+ T cells suppressed naïve DBA/2 T-cell proliferation in culture with irradiated B6 splenic APC. In this in vitro culture, neutralizing anti-TGFβ Ab partially reversed inhibitory effects, opening the possibility that additional pathways of immunosuppression were functional in this experimental system.
-
Inflammatory bowel disease
Like most autoimmune syndromes, inflammatory bowel diseases (IBD) are considered to represent sequel of aberrant immune responses in genetically predisposed hosts. For Crohn’s disease (CD) and ulcerative colitis (UC) gut luminal antigens, in particular the anaerobic microbiota of the distal ileum and colon, have attracted great attention, and the current paradigm proposes that interactions of such bacteria with the host’s epithelial cells and the mucosal immune system eventually result in continuous microbial antigenic stimulation and associated tissue damage [65]. Formerly categorized as Th1- and Th2-mediated diseases, CD and UC are currently re-investigated for the contribution of the IL-23/Th17 axis [66]. Regulatory T cells are believed to be crucial in adjusting response thresholds to microbial antigen as well as modulating tissue-damaging immune reactions and as such are regarded as promising new therapeutic targets [67].
Limited information is available about the phenotypic and functional characteristics of such regulatory/suppressor T cells in humans. There is, however, evidence that T cells from the CD8+ Ts family may warrant further exploration. Allez et al. [68] have generated CD8+ Ts by stimulating peripheral blood T cells with irradiated allogeneic intestinal epithelial cells (IEC). Phenotypic markers of such CD8+ Ts included CD101 and CD103; functionally, they suppressed Ig production by pokeweed mitogen (PWM)-stimulated PBMC and the proliferation of CD4+ T cells in an unrelated mixed lymphocyte reaction, whereas CD8+CD101−CD103− T cells did not. The same group [69] has shown that lamina propria (LP) CD8+ T cells derived from normal controls possess regulatory activity, whereas both unfractionated LP lymphocytes and purified LP CD4+ T cells do not. Either LP CD8+CD28+ or CD8+CD28− T cells inhibit Ig production by PWM-stimulated PBMC in a cell contact-dependent manner. LP CD8+ Ts express the CD101+, CD103+, and CD45RO+ phenotype. Whereas CD8+ T cells isolated from non-inflamed mucosa displayed suppressive capabilities, LP CD8+ T cells derived from CD and UC specimens left Ig production unchanged, suggesting a deficiency of CD8+ Ts in IBD. Previous studies have indicated that CD8+ Ts expandable by IEC stimulation in vitro may be enriched in the TCR Vβ5.1+ subset [70]. Interestingly, the frequency of Vβ5.1+ CD8+ T cells was diminished amongst IBD LP when compared to normal controls [69].
Guided by the clinical observation that blockade of TNF is highly successful in inducing and even maintaining remission in some IBD patients, animal models have been developed that utilize TNF overexpression to induce disease. Deletion of a 69 bp stretch within the AU-rich region (ARE) of the TNF gene heightens mRNA stability and increased TNF protein synthesis. TNF overexpression in TNFΔARE mice leads to the development of transmural CD-like chronic inflammation in the terminal ileum [71]. Ho et al. [72] have examined CD8 Ts function in such mice and have found that CD8+CD44−CD103+ T cells produce TGFβ, but not IL-10 or IFNγ CD8+CD44−CD103+ T cells derived from TNFΔARE mice downregulate the proliferation of CD4+ T cells in culture with APC and anti-CD3 compared to CD8+CD44−CD103+ T cells isolated from wild-type mice. Adoptive transfer of CD4+ T cells from TNFΔARE mice into immunodeficient RAG−/− mice induces ileitis but co-transfer of CD8+CD44−CD103+ Ts from wild type mice as well as TNFΔARE mice attenuates the ileitis histology. An alternate experimental model mimicking IBD utilizes injection of CD4+CD45RBhigh cells into syngeneic RAG-2-deficient mice to generate gut mucosal inflammation. In this model, Ménager-Marcq et al. [73] have demonstrated that CD8+CD28− but not CD8+CD28+ T cells freshly isolated from the spleen or gut efficiently prevent the development of colitis as assessed by body weight and histology. CD8+CD28− T cells derived from IL-10-deficient mice lack the functional ability to avert colitis. Moreover, the inflammation induced by CD4+CD45RBhigh cells derived from mice transgenic for the dominant negative form of the TGFβ receptor II (dnTbRII-Tg) was resistant to the inhibitory effect transmitted by CD8+CD28− Ts. In this study, the in vitro behavior of CD8+CD28− Ts was different from that seen in in vivo experiments. In allogenic mixed lymphocyte cultures, CD8+CD28− Ts from wild-type mice inhibited proliferation and IFNγ production by CD4+ T cells. If MHC-deficient APC plus anti-CD3e antibody were applied to drive CD4+ T-cell proliferation, wild type, but also IL-10-deficient, Ts were effective inhibitors. Proliferation of dnTbRII-Tg CD4 responder T cells was also significantly inhibited by wild-type Ts, although suppression of T-cell proliferation was less efficient than in the presence of IL-10 or TGFβ signaling. These cytokines may play a minor role in in vitro suppressor assay in contrast to the in vivo experiments.
-
Other autoimmune diseases
Graves’ disease (GD) is a thyroid-specific autoimmune disease in which the autoantigen is known. Patients with GD built stimulatory autoantibodies against the thyroid stimulating hormone receptor (TSHR) [74]. Clinical consequences of this aberrant immune response include hyperthyroidism. In a murine model of autoimmune thyroid disease induced through an adenovirus expressing TSHR, Saitoh et al. [75] have explored the functional contribution of CD8+CD122+ Ts. These authors have also examined how CD4+CD25+ Tregs affect disease induction and disease course by depleting such regulatory cells through administration of anti-CD25 mAb. In both C57BL/6 and BALB/c mice, intramuscular injection of adenovirus expressing the human TSHR-A subunit induces serum T4 and anti-TSHR elevation (indicators for hyperthyroidism). Antibody-mediated depletion of CD8+CD122+ T cells promptly increases the incidence of hyperthyroidism indicating an active role of Tregs in controlling anti-TSHR immune responses.
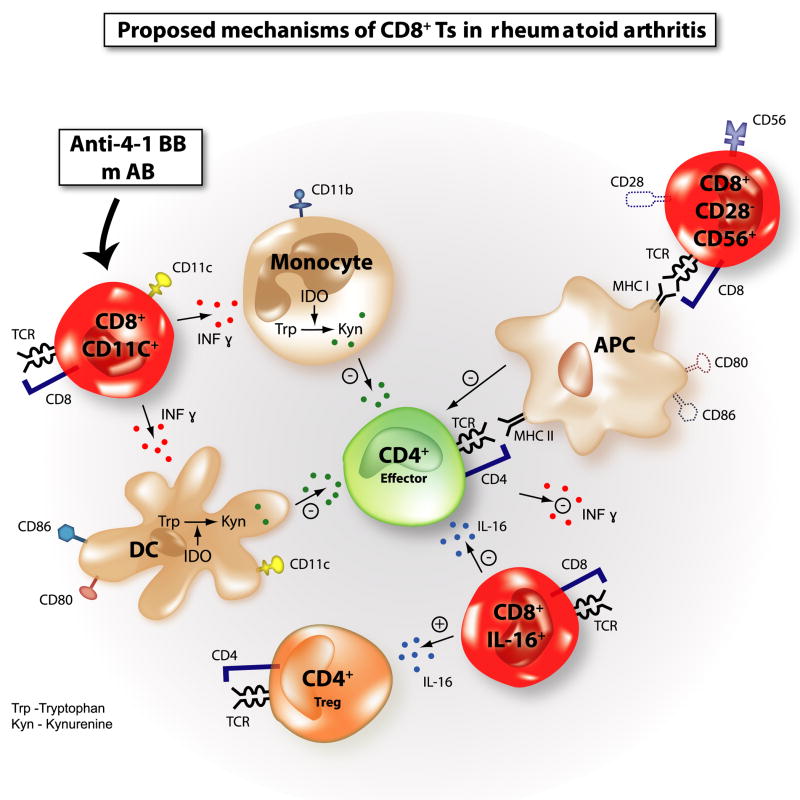
Figure 3. Proposed mechanisms of CD8+ suppressor T cells (Ts) in rheumatoid arthritis (RA).
CD8+CD28−CD56+ Ts transferred into SCID-chimera mice transplanted with synovial tissues from RA patients suppress the tissue inflammation measured by proinflammatory cytokines and chemokine expression, downmodulating the expression of costimulatory molecules CD80 and CD86 on antigen-presenting cells (APC) and synovial fibroblast-like cells (FLS). Interleukin (IL)-16-secreting CD8+ Ts suppress the tissue inflammation in the same SCID-chimera model. IL-16 is a natural ligand of the CD4 molecule and induces CD4+ T-cell anergy. IL-16 also may induce or recruit CD4+CD25+Foxp3+ Tregs in the tissue. In a mice collagen-induced arthritis model (CIA), CD8+CD11c+ Ts induced by the administration of anti-4-1 BB monoclonal antibodies (mAb) secrete IFNγ and induce indoleamine 2,3-dioxygenase (IDO) in CD11b+ monocytes and CD11c+ dendritic cells (DC). IDO catabolizes tryptophan (Trp) into its catabolite such as kynurenine (Kyn). Depletion of Trp and generated Kyn exert immunosuppressive effects on effector CD4+ T cells. Abbreviations: MHC = major histocompatibility complex; T-cell receptor = TCR.
Myasthenia gravis (MG) is an antibody-dependent autoimmune syndrome in which autoantibodies against the acetylcholine receptor (AChR) of skeletal muscles disrupt neurotransmission and leave affected patients with muscle weakness and excessive fatigue. T cells have been implicated as master regulators in the immune pathogenesis. An experimental model of autoimmune MG (EAMG) exists in which mice are rendered sick by immunizing against AChR. [76]. Ben-David et al. [77] have induced EAMG by hyperimmunizing mice with Torpedo AChR (TAChR). Autoimmunity can be abrogated if mice receive an altered peptide ligand (APL) composed of two tandemly arranged single amino acid analogs of myathenogenic peptide. Lymph nodes (LN) from EAMG mice contain T cells that proliferate and secrete IFNγ when restimulated with the antigen. On the other hand, LN cells from mice treated with APL concomitant with TAChR immunization have significantly lower proliferative activity and IFNγ secretion. In CD8−/− mice, the suppressive effects of the dual APL are abolished while CD4+CD25+Foxp3+ Tregs are induced to the same extent as in wild-type mice. Furthermore, approximately 90% of CD8+ T cells from LN in the dual APL-treated mice are CD28− cells, and 28% of CD8+CD28− T cells are Foxp3+ cells. Therefore, CD8+CD28−Foxp3+ T cells may well have a regulatory role for EAMG-associated autoimmune responses.
Summary and Outlook
A wide variety of inhibitory T-cell populations appear to exist within the CD8+ T-cell compartment. Means of induction (natural versus adaptive/induced), phenotypes (CD28+/CD28−), and suppressive mechanisms (cell-cell contact, anti-inflammatory cytokine secretion, or killing) are highly diverse (Table 1). Whether this diversity reflects heterogeneity of cell populations or results from an intricate interplay of inhibitory CD8+ T cells with a unique microenvironment remains to be resolved. Surprisingly, similarities between CD4+ Tregs (natural CD4+CD25+Foxp3+ Tregs versus adaptive/induced CD4+CD25+Foxp3+ Tregs, Tr1, and Th3) are broad, which brings into question how CD4 and CD8 receptors finally contribute to the immunoregulatory potential of these cell populations. A barrier in investigating CD8+ Ts stems from the lack of a specific cell surface marker, again an issue reminiscent of unequivocally defining CD4+ Tregs. Essentially, identification of inhibitory CD8+ T cells requires a functional suppressor assay. CD25, CTLA-4, GITR, LAG3, CD103, and CD122 are all expressed by activated effector T cells. Lack of CD28 expression also cannot be used as a marker to detect CD8+ Ts because CD8+CD28− T cells contain cytotoxic cells [78] and a subset of CD8+CD28+ cells also has suppressor activity [14, 21, 61, 69]. CD56 is expressed on senescent cytotoxic T cells [79, 80]. Although the essential role of Foxp3 expression for the acquisition of regulatory function in CD4+CD25+Foxp3+ Tregs has been demonstrated in mice, this molecule is less reliable in humans. TCR activation transiently induces Foxp3 expression on both CD4+ and CD8+ T cells and such CD4+Foxp3+ and CD8+Foxp3+ cells lack immunosuppressive function [81, 82]. These problems have created a formidable challenge in monitoring inhibitory T-cell populations in humans and deciphering their possible contribution to autoimmunity. Also, efforts of replenishing CD8+ Ts in patients deficient for such protective cell populations are limited by the inability to unequivocally identify the relevant cells. Additionally, we still have limited data on the significance of CD8+ Ts in the spectrum of human autoimmune syndromes. Based on what we know currently on the diversity of CD8+ Ts populations, a significant heterogeneity for different disease entities can be expected.
Here, similarities with CD4+ Tregs may be very helpful. As the field of CD4+ Tregs is rapidly expanding and more and more information is available on how such Tregs affect immune homeostasis and immune-mediated disease, some of the rules may be transferable to the CD8+ T-cell compartment.
It is currently unknown whether CD4+ Tregs and CD8+ Ts have similar or distinct roles in regulating immune responses in vivo. Indeed, recent evidence suggests that the two classes of regulatory/suppressive T cells are functionally connected, adding to the versatility of regulatory T cells as targets of novel immunomodulatory therapies. Vlad et al. [83] have proposed that the potential of antigen-specific MHC class I-restricted CD8+CD28− Ts to render DC tolerogenic translates into the induction of CD4+ Tregs, making a direct interlink between the immunosuppressive capability of CD8+ and CD4+ T cells. One of the intriguing questions arising from the growing field of suppressive T cells relates to the seeming heterogeneity of regulatory/suppressor T-cell populations. A multitude of CD4+ and CD8+ subpopulations have now been described, all of which can effectively disrupt immune responses, including the pathogenic immune responses causing autoimmune disease. The provocative proposal has been made that any T cells may be able to acquire suppressive functions [84]. In that model, the microenvironment in which immune recognition occurs and in which T cells differentiate would possibly be the shaping force. T cells with anti-inflammatory capabilities are, undoubtedly, ideal targets to redirect immune responses, and their therapeutic potential needs further exploration before inhibitory CD8+ T cells can be exploited clinically. Cell-based immunotherapeutics are a demanding approach. Thus, ultimately we need to understand the molecular mechanisms that inhibitory CD8+ T cells use to elegantly interrupt immune responses and utilize that knowledge to design novel therapeutic strategies.
Acknowledgments
Sources of Support: This work was funded in part by grants from the National Institutes of Health (RO1 AR42527, RO1 AR41974, RO1 AI44142, RO1 AI57266, RO1 EY11916, and R01 AG15043) and an ACR Within Our Reach grant.
The authors thank Tamela Yeargin for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723. [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151. [PubMed] [Google Scholar]
- 3.Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Regulatory T cells and human disease. Clin Dev Immunol. 2007;2007:89195. doi: 10.1155/2007/89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouse BT. Regulatory T cells in health and disease. J Intern Med. 2007;262:78. doi: 10.1111/j.1365-2796.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 5.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 6.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- 7.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28− T cells. Int Immunol. 1998;10:775. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 10.Ciubotariu R, Colovai AI, Pennesi G, Liu Z, Smith D, Berlocco P, et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28− regulatory T cells. J Immunol. 1998;161:5193. [PubMed] [Google Scholar]
- 11.Jiang S, Tugulea S, Pennesi G, Liu Z, Mulder A, Lederman S, et al. Induction of MHC-class I restricted human suppressor T cells by peptide priming in vitro. Hum Immunol. 1998;59:690. doi: 10.1016/s0198-8859(98)00073-1. [DOI] [PubMed] [Google Scholar]
- 12.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 13.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177:2775. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 14.Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, et al. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008;38:640. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 15.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35:2896. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 17.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 19.Gray JD, Hirokawa M, Ohtsuka K, Horwitz DA. Generation of an inhibitory circuit involving CD8+ T cells, IL-2, and NK cell-derived TGF-beta: contrasting effects of anti-CD2 and anti-CD3. J Immunol. 1998;160:2248. [PubMed] [Google Scholar]
- 20.Billerbeck E, Blum HE, Thimme R. Parallel expansion of human virus-specific FoxP3- effector memory and de novo-generated FoxP3+ regulatory CD8+ T cells upon antigen recognition in vitro. J Immunol. 2007;179:1039. doi: 10.4049/jimmunol.179.2.1039. [DOI] [PubMed] [Google Scholar]
- 21.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007;104:8029. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 23.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65:142. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Filaci G, Rizzi M, Setti M, Fenoglio D, Fravega M, Basso M, et al. Non-antigen-specific CD8(+) T suppressor lymphocytes in diseases characterized by chronic immune responses and inflammation. Ann N Y Acad Sci. 2005;1050:115. doi: 10.1196/annals.1313.013. [DOI] [PubMed] [Google Scholar]
- 25.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 26.Endharti AT, Rifa IMs, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 28.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YH, Ishida Y, Rifa’i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 30.Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nat Immunol. 2004;5:469. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Goldstein I, Glickman-Nir E, Jiang H, Chess L. Induction of TCR Vbeta-specific CD8+ CTLs by TCR Vbeta-derived peptides bound to HLA-E. J Immunol. 2001;167:3800. doi: 10.4049/jimmunol.167.7.3800. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Werneck MB, Cantor H. The immunoregulatory effects of Qa-1. Immunol Rev. 2006;212:51. doi: 10.1111/j.0105-2896.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 33.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 36.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Kashleva H, Xu LX, Forman J, Flaherty L, Pernis B, et al. T cell vaccination induces T cell receptor Vbeta-specific Qa-1-restricted regulatory CD8(+) T cells. Proc Natl Acad Sci U S A. 1998;95:4533. doi: 10.1073/pnas.95.8.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Ware R, Stall A, Flaherty L, Chess L, Pernis B. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing V beta 8 TCR: a role of the Qa-1 molecule. Immunity. 1995;2:185. doi: 10.1016/s1074-7613(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 39.Niederkorn JY. Emerging concepts in CD8(+) T regulatory cells. Curr Opin Immunol. 2008;20:327. doi: 10.1016/j.coi.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007;26:1228. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- 41.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 42.Weyand CM. Immunopathologic aspects of rheumatoid arthritis: who is the conductor and who plays the immunologic instrument? J Rheumatol Suppl. 2007;79:9. [PubMed] [Google Scholar]
- 43.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 44.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii84. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran CN, Lundy SK, Fox DA. Synovial biology and T cells in rheumatoid arthritis. Pathophysiology. 2005;12:183. doi: 10.1016/j.pathophys.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manadan AM, Block JA. Rheumatoid arthritis: beyond tumor necrosis factor-alpha antagonists, B cell depletion, and T cell blockade. Am J Ther. 2008;15:53. doi: 10.1097/MJT.0b013e31814daf9b. [DOI] [PubMed] [Google Scholar]
- 47.Ostor AJ. Beyond methotrexate: biologic therapy in rheumatoid arthritis. Clin Med. 2005;5:222. doi: 10.7861/clinmedicine.5-3-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, et al. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005;174:7292. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 49.Klimiuk PA, Goronzy JJ, Weyand CM. IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. J Immunol. 1999;162:4293. [PubMed] [Google Scholar]
- 50.Haughn L, Gratton S, Caron L, Sekaly RP, Veillette A, Julius M. Association of tyrosine kinase p56lck with CD4 inhibits the induction of growth through the alpha beta T-cell receptor. Nature. 1992;358:328. doi: 10.1038/358328a0. [DOI] [PubMed] [Google Scholar]
- 51.Theodore AC, Center DM, Nicoll J, Fine G, Kornfeld H, Cruikshank WW. CD4 ligand IL-16 inhibits the mixed lymphocyte reaction. J Immunol. 1996;157:1958. [PubMed] [Google Scholar]
- 52.McFadden C, Morgan R, Rahangdale S, Green D, Yamasaki H, Center D, et al. Preferential migration of T regulatory cells induced by IL-16. J Immunol. 2007;179:6439. doi: 10.4049/jimmunol.179.10.6439. [DOI] [PubMed] [Google Scholar]
- 53.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 54.Krug A. Nucleic acid recognition receptors in autoimmunity. Handb Exp Pharmacol. 2008;129 doi: 10.1007/978-3-540-72167-3_7. [DOI] [PubMed] [Google Scholar]
- 55.Martinez Valle F, Balada E, Ordi-Ros J, Vilardell-Tarres M. DNase 1 and systemic lupus erythematosus. Autoimmun Rev. 2008;7:359. doi: 10.1016/j.autrev.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 56.McMahona M, Hahna BH. Atherosclerosis and systemic lupus erythematosus: mechanistic basis of the association. Curr Opin Immunol. 2007;19:633. doi: 10.1016/j.coi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166:6452. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 58.Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K, Ebling FM. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum. 2001;44:432. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 59.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black × New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173:3542. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 60.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005;175:7728. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 61.Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB × NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J Immunol. 2008;180:2069. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- 62.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007;178:7649. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- 63.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174:3247. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 64.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 65.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 66.Maloy KJ. The Interleukin-23/Interleukin-17 axis in intestinal inflammation. J Intern Med. 2008;263:584. doi: 10.1111/j.1365-2796.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 67.van Driel IR, Ang DK. Role of regulatory T cells in gastrointestinal inflammatory disease. J Gastroenterol Hepatol. 2008;23:171. doi: 10.1111/j.1440-1746.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- 68.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 69.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 70.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 71.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 72.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menager-Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. CD8+CD28− regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rapoport B, McLachlan SM. The thyrotropin receptor in Graves’ disease. Thyroid. 2007;17:911. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- 75.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology. 2007;148:6040. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 76.Atassi MZ, Oshima M. Autoimmune responses against acetylcholine receptor: T and B cell collaboration and manipulation by synthetic peptides. Crit Rev Immunol. 1997;17:481. [PubMed] [Google Scholar]
- 77.Ben-David H, Sharabi A, Dayan M, Sela M, Mozes E. The role of CD8+CD28 regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand. Proc Natl Acad Sci U S A. 2007;104:17459. doi: 10.1073/pnas.0708577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 79.Kelly-Rogers J, Madrigal-Estebas L, O’Connor T, Doherty DG. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Hum Immunol. 2006;67:863. doi: 10.1016/j.humimm.2006.08.292. [DOI] [PubMed] [Google Scholar]
- 80.Michel JJ, Turesson C, Lemster B, Atkins SR, Iclozan C, Bongartz T, et al. CD56-expressing T cells that have features of senescence are expanded in rheumatoid arthritis. Arthritis Rheum. 2007;56:43. doi: 10.1002/art.22310. [DOI] [PubMed] [Google Scholar]
- 81.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:925. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 83.Vlad G, Cortesini R, Suciu-Foca N. License to heal: bidirectional interaction of antigen-specific regulatory T cells and tolerogenic APC. J Immunol. 2005;174:5907. doi: 10.4049/jimmunol.174.10.5907. [DOI] [PubMed] [Google Scholar]
- 84.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]