Abstract
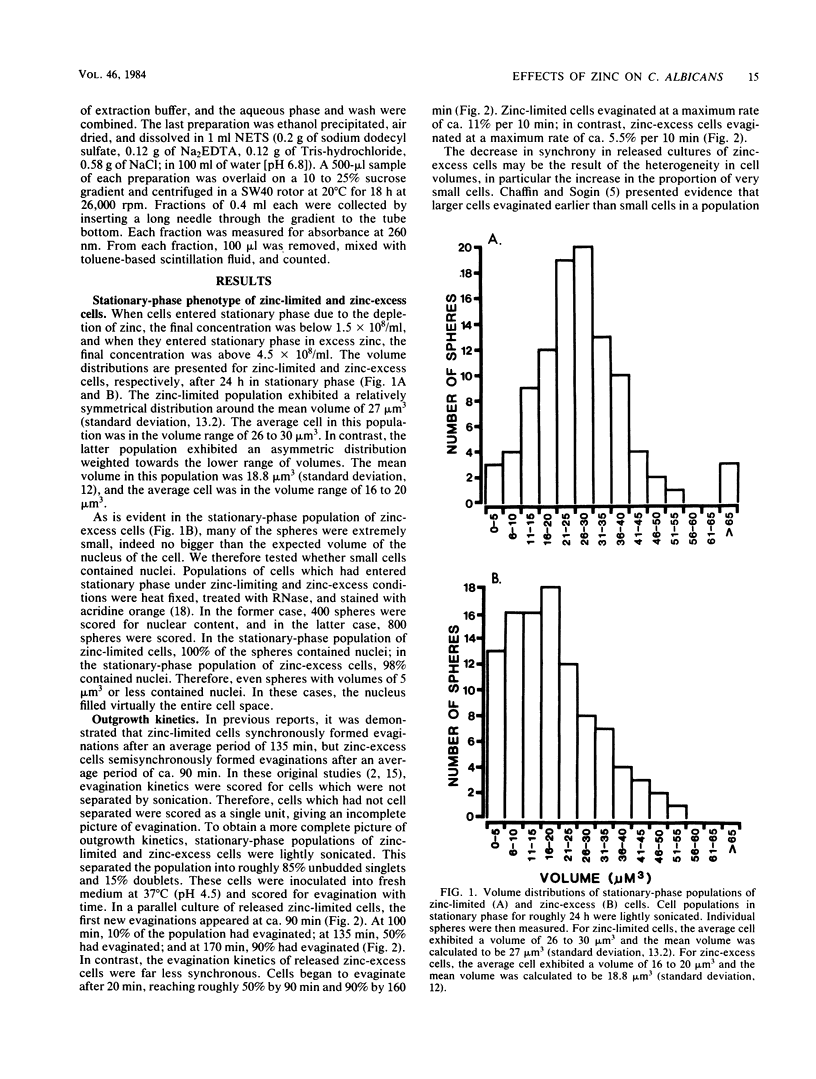
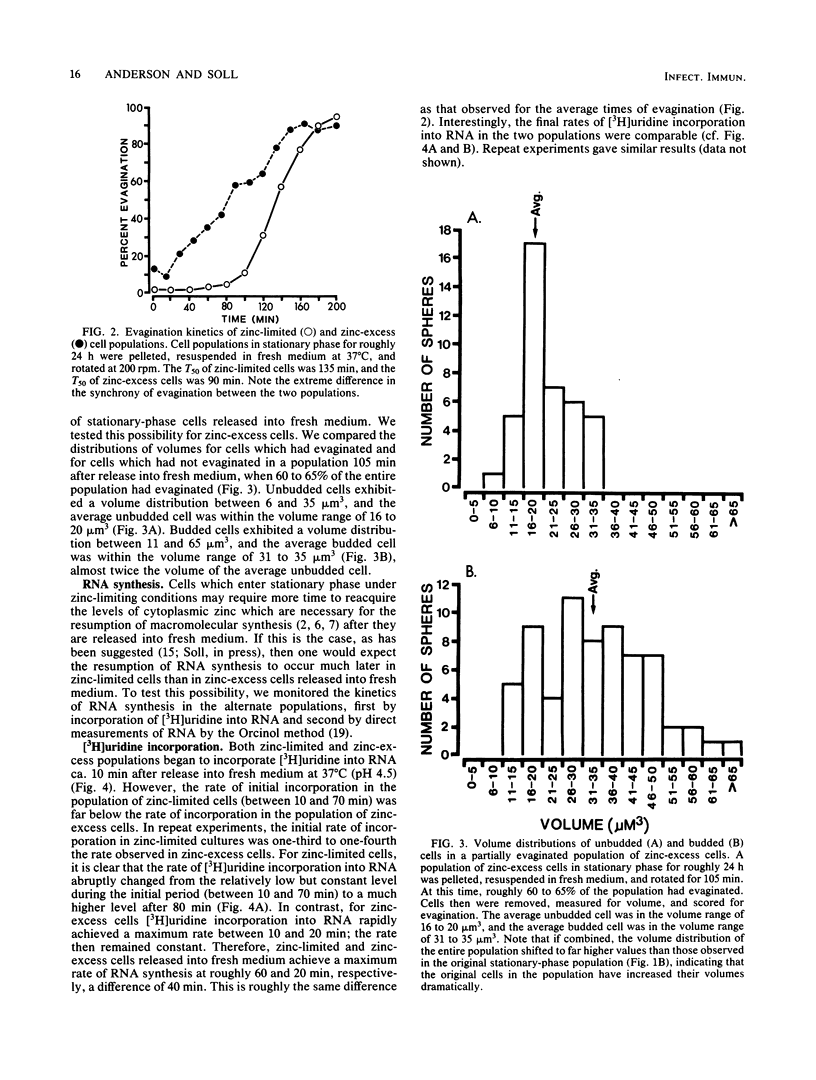
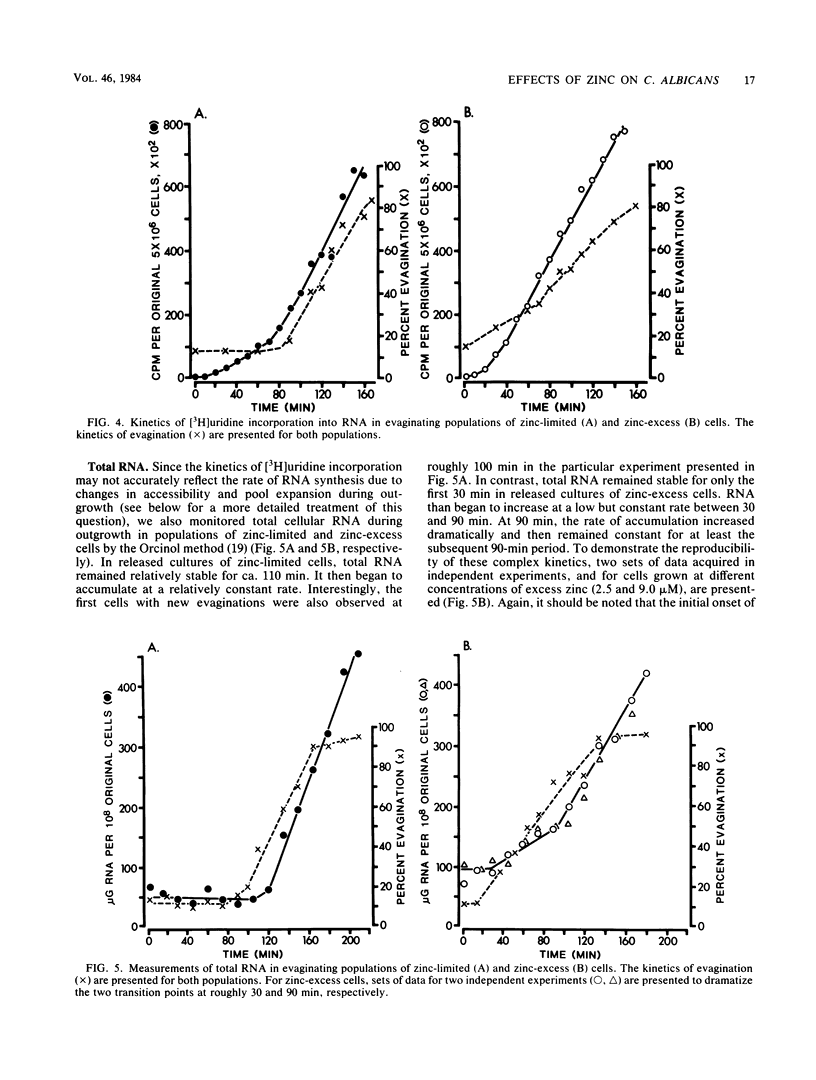
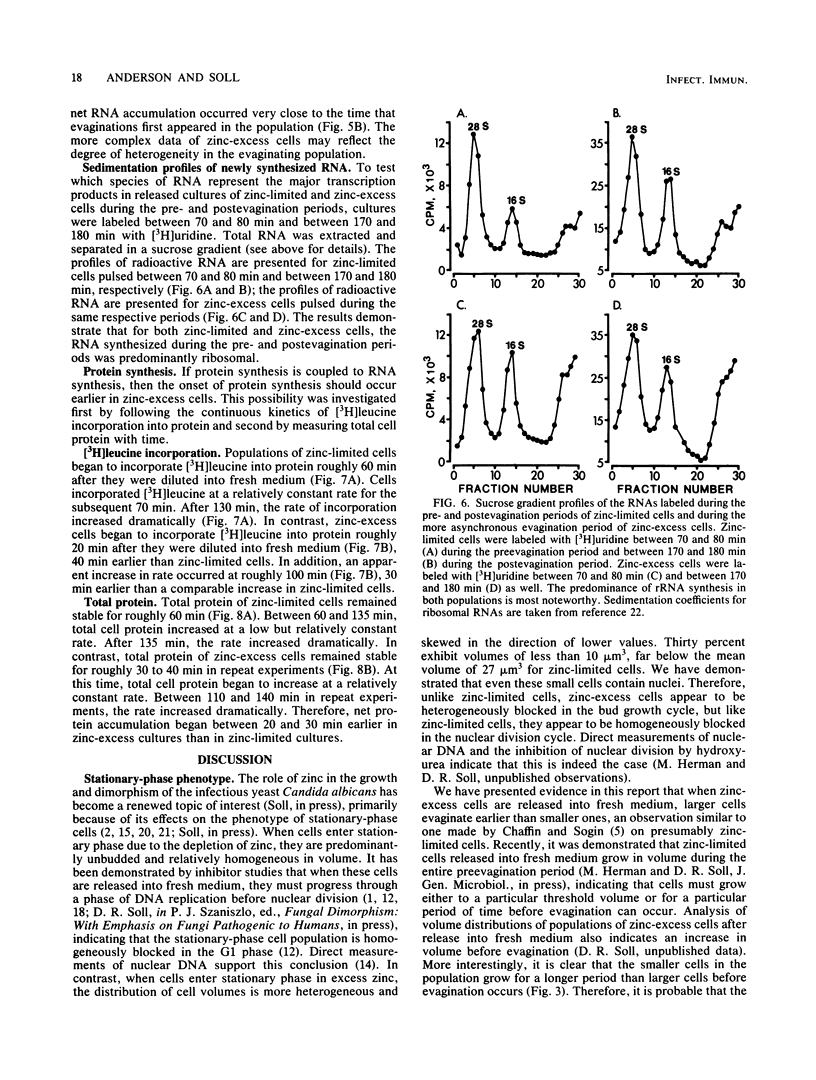
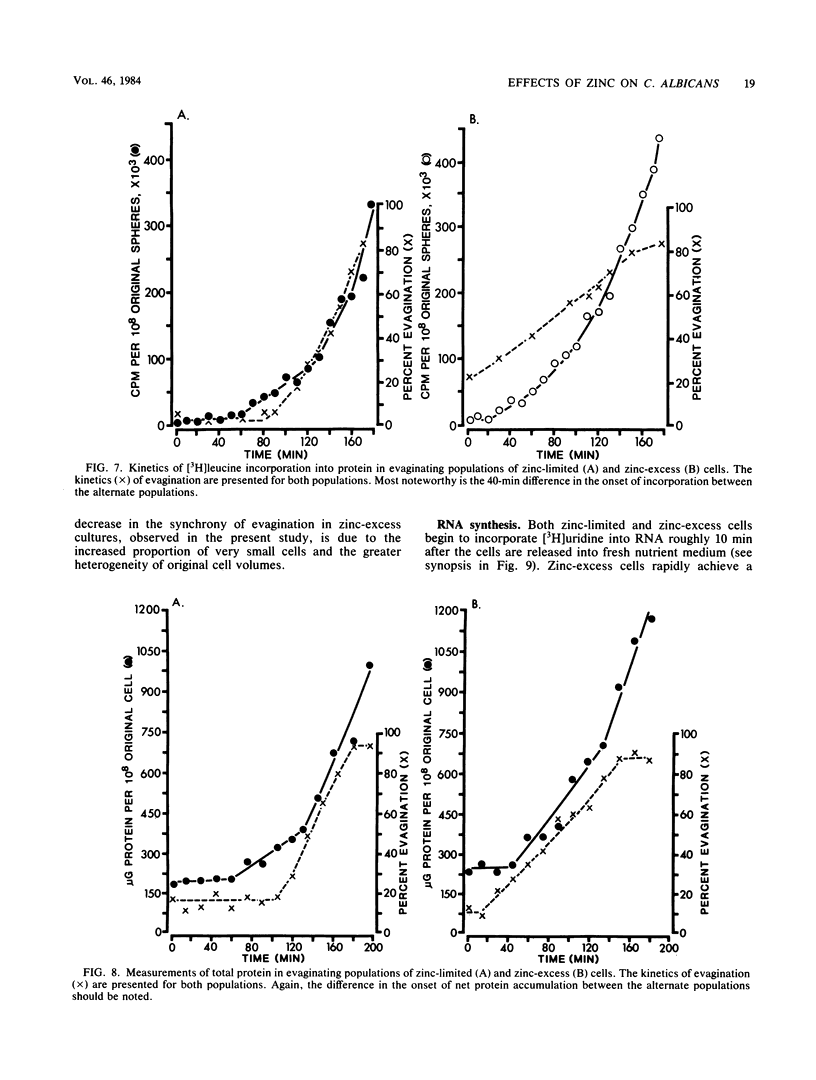
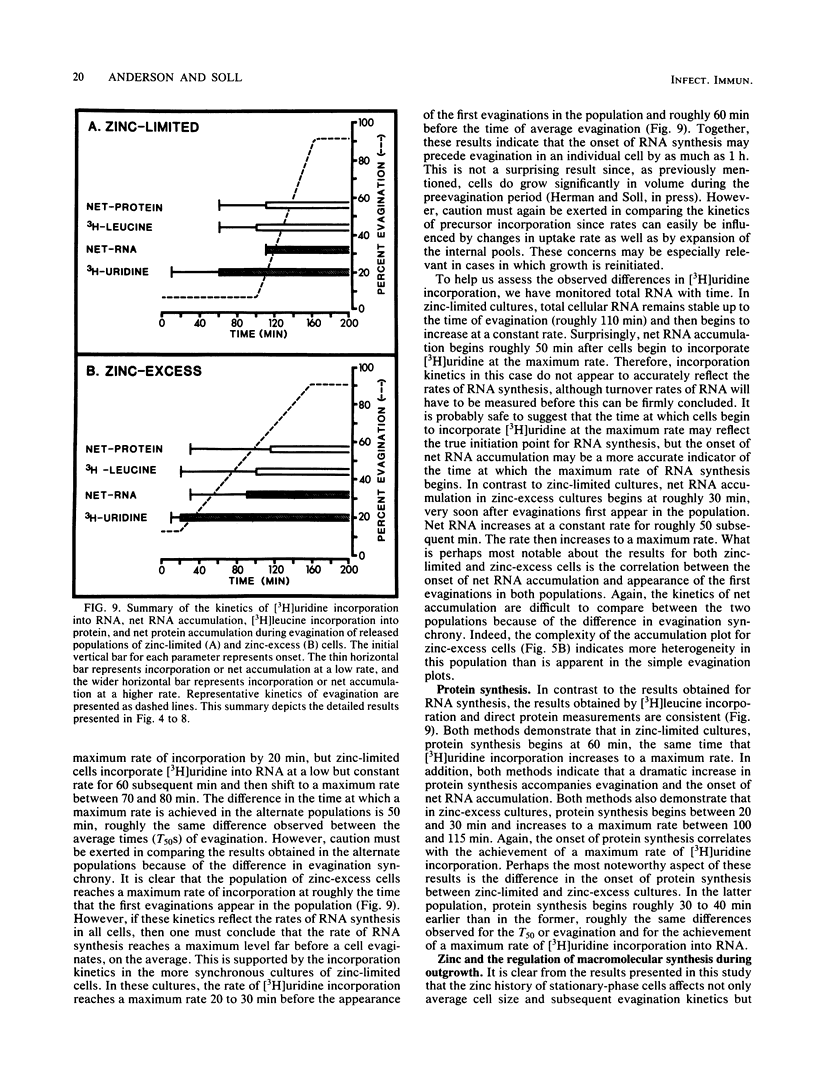
When cultures of Candida albicans which had entered stationary phase due to the depletion of zinc (zinc-limiting conditions) were compared with cultures which had entered stationary phase due to the depletion of another growth-limiting component (zinc-excess conditions), at least two cellular characteristics were found to differ: (i) zinc-limited cells appeared more homogeneous and larger on the average than zinc-excess cells, and (ii) zinc-limited cells evaginated on the average of 40 min later than zinc-excess cells. In the present study, it is demonstrated that the distribution of volumes for a stationary-phase culture of zinc-excess cells is skewed towards very small volumes, but even the smallest cells contain nuclei; in contrast, the volumes of zinc-limited cells are evenly distributed around a much larger mean value; the evagination kinetics of zinc-excess cells released into fresh medium are far less synchronous than are those of zinc-limited cells, and the smaller cells in the population take much longer to evaginate than do the larger cells; the onset of net RNA accumulation and achievement of a maximum rate of [3H]uridine incorporation occur significantly earlier in zinc-excess cells than in zinc-limited cells released into fresh medium; and the onset of net protein accumulation and [3H]leucine incorporation occur significantly earlier in zinc-excess cells than in zinc-limited cells released into fresh medium. These results indicate that although zinc-excess cells are extremely heterogeneous in volume, they may still be homogeneously blocked in the nuclear division cycle, and that the later average evagination time of released zinc-limited cells may be due to a delay in the onset of protein synthesis, which in turn may be due to the time necessary to reaccumulate zinc to levels sufficient for the reinitiation of RNA synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell G. W., Werth A., Soll D. R. The regulation of nuclear migration and division during synchronous bud formation in released stationary phase cultures of the yeast Candida albicans. Exp Cell Res. 1980 May;127(1):103–113. doi: 10.1016/0014-4827(80)90418-8. [DOI] [PubMed] [Google Scholar]
- Brummel M., Soll D. R. The temporal regulation of protein synthesis during synchronous bud or mycelium formation in the dimorphic yeast Candida albicans. Dev Biol. 1982 Jan;89(1):211–224. doi: 10.1016/0012-1606(82)90308-6. [DOI] [PubMed] [Google Scholar]
- Buffo J., Herman M. A., Soll D. R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984 Mar 15;85(1-2):21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Sogin S. J. Germ tube formation from zonal rotor fractions of Candida albicans. J Bacteriol. 1976 May;126(2):771–776. doi: 10.1128/jb.126.2.771-776.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Mattia E., Cassone A. Inducibility of germ-tube formation in Candida albicans at different phases of yeast growth. J Gen Microbiol. 1979 Aug;113(2):439–442. doi: 10.1099/00221287-113-2-439. [DOI] [PubMed] [Google Scholar]
- Mitchell L. H., Soll D. R. Commitment to germ tube or bud formation during release from stationary phase in Candida albicans. Exp Cell Res. 1979 Apr;120(1):167–179. doi: 10.1016/0014-4827(79)90547-0. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Bedell G. W., Brummel M. Zinc and regulation of growth and phenotype in the infectious yeast Candida albicans. Infect Immun. 1981 Jun;32(3):1139–1147. doi: 10.1128/iai.32.3.1139-1147.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Bedell G., Thiel J., Brummel M. The dependency of nuclear division on volume in the dimorphic yeast Candida albicans. Exp Cell Res. 1981 May;133(1):55–62. doi: 10.1016/0014-4827(81)90356-6. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Herman M. A. Growth and the inducibility of mycelium formation in Candida albicans: a single-cell analysis using a perfusion chamber. J Gen Microbiol. 1983 Sep;129(9):2809–2824. doi: 10.1099/00221287-129-9-2809. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Mitchell L. H. Filament ring formation in the dimorphic yeast Candida albicans. J Cell Biol. 1983 Feb;96(2):486–493. doi: 10.1083/jcb.96.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Stasi M., Bedell G. The regulation of nuclear migration and division during pseudo-mycelium outgrowth in the dimorphic yeast Candida albicans. Exp Cell Res. 1978 Oct 1;116(1):207–215. doi: 10.1016/0014-4827(78)90077-0. [DOI] [PubMed] [Google Scholar]
- WIDRA A. PHOSPHATE DIRECTED Y-M VARIATION IN CANDIDA ALBICANS. Mycopathol Mycol Appl. 1964 Sep 30;23:197–202. doi: 10.1007/BF02068455. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H. Control of dimorphism in Candida albicans by zinc: effect on cell morphology and composition. J Gen Microbiol. 1975 Feb;86(2):370–372. doi: 10.1099/00221287-86-2-370. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Iwata K. In vitro an in vivo protein synthesis in Candida albicans. I. Isolation and protein synthesis of polysomes. Sabouraudia. 1970 Nov;8(3):177–188. [PubMed] [Google Scholar]


