Abstract
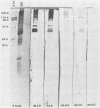
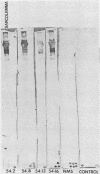
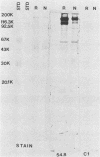
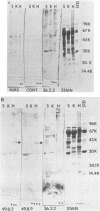
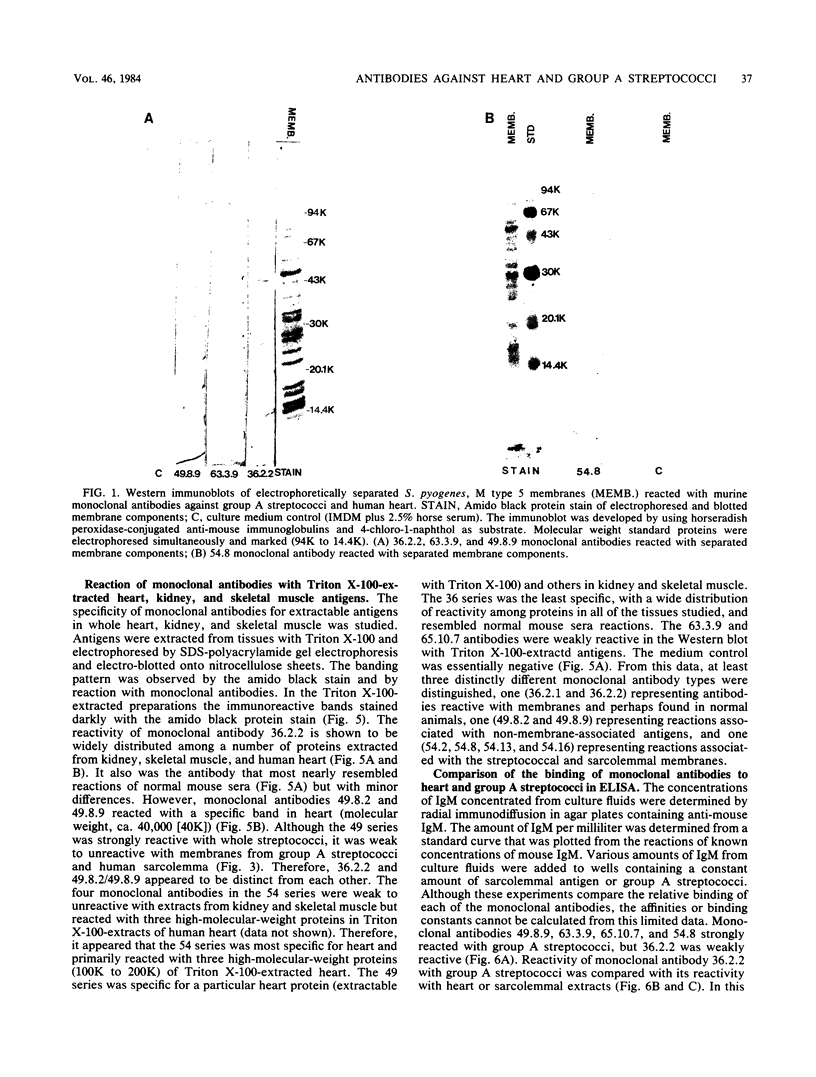
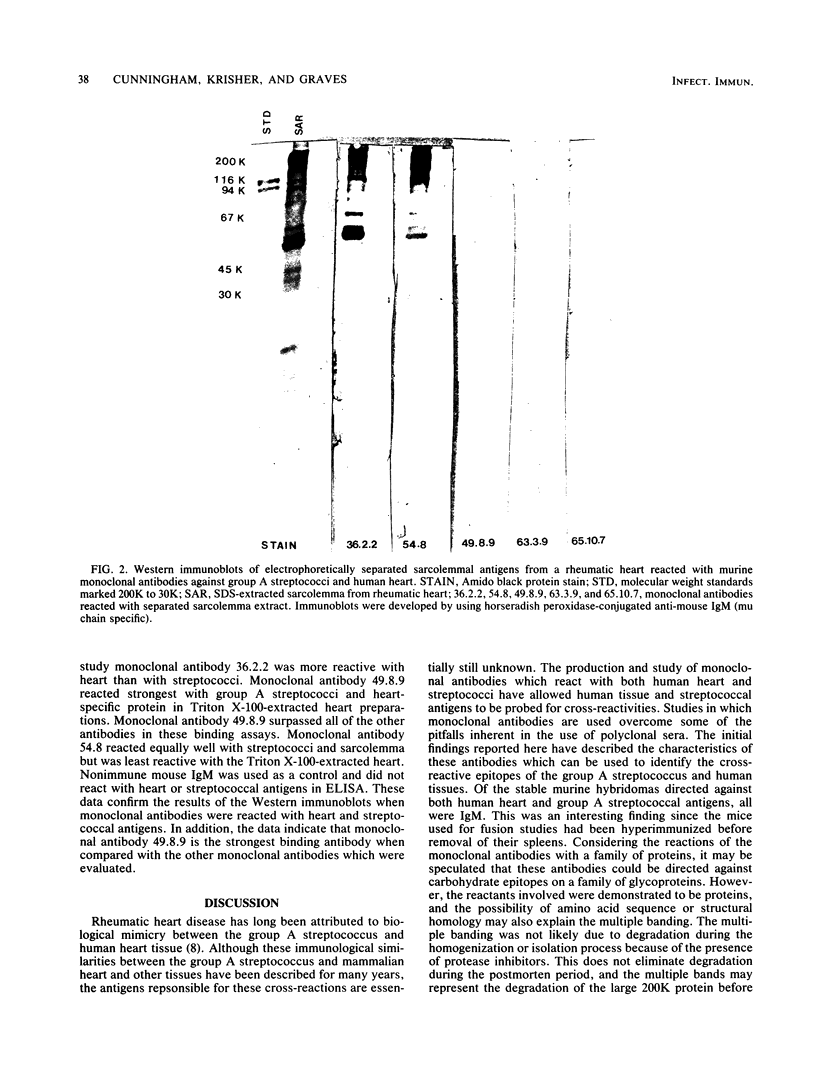
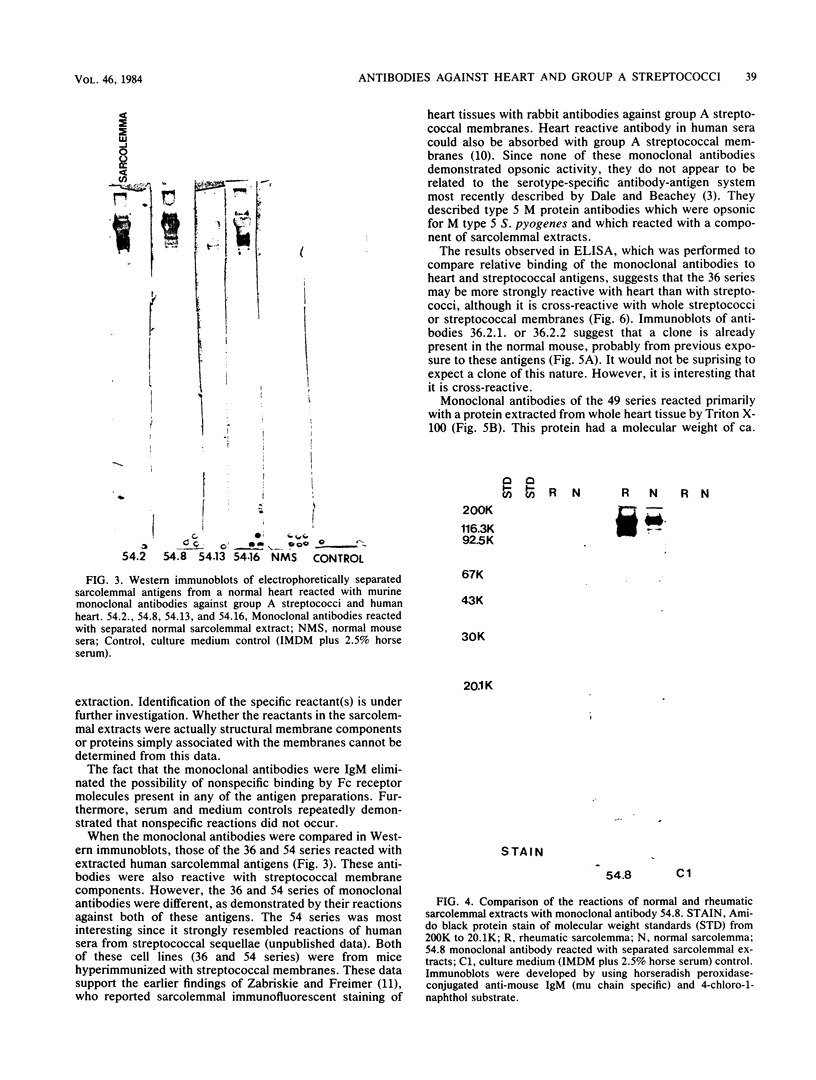
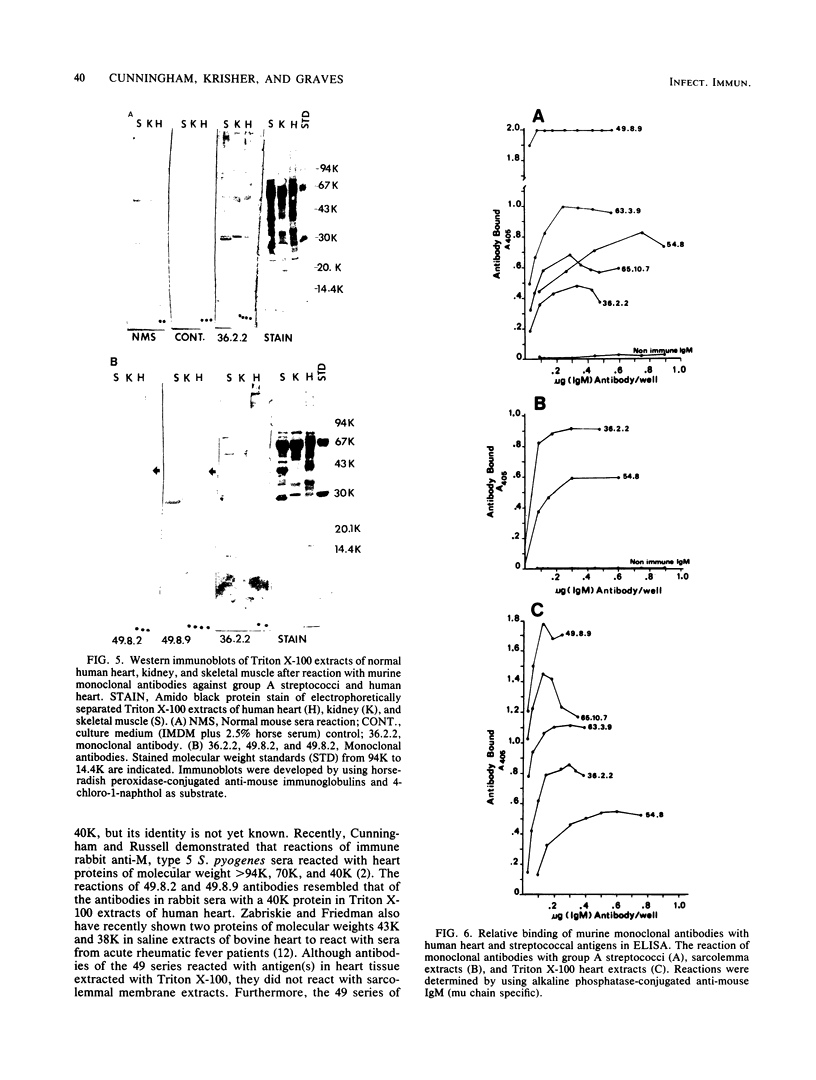
Ten selected murine hybridoma cell lines that produce monoclonal antibodies against M type 5 Streptococcus pyogenes and human heart antigen were isolated. All of the monoclonal antibodies studied were determined to be the immunoglobulin M isotype. The antibodies were characterized on the basis of their reactions with Triton X-100-extracted whole human heart antigens, sodium dodecyl sulfate-extracted sarcolemmal antigens, and whole streptococci or their membranes. Enzyme-linked immunosorbent assays and Western immunoblotting techniques were used to compare the reactivity of the monoclonal antibodies. All 10 of the antibodies were first selected for their reactivity with Triton X-100-extracted heart antigens and whole group A, M type 5 streptococci. These antibodies were then divided into two categories: strong reactors or weak reactors with human sarcolemmal and streptococcal membranes. Among the strong reactors, two different types of monoclonal antibodies were observed on the basis of their immunobanding patterns with sarcolemmal and streptococcal membranes on Western blots. Monoclonal antibodies that were strong reactors with sarcolemmal and group A streptococcal membrane antigen were directed against a determinant on a family of proteins. The major reactants of sarcolemmal extracts were high-molecular-weight proteins near 200,000. Some monoclonal antibodies demonstrated more specificity for the heart than did others when reacted with separated Triton X-100-extracted tissue antigens from the heart, kidney, and skeletal muscle. One of the monoclonal antibodies that reacted with group A streptococci reacted with a Triton X-100-extracted heart antigen ca. 40,000 daltons in size. None of these monoclonal antibodies opsonized type 5 Streptococcus pyogenes, and in enzyme-linked immunosorbent assays most of the antibodies were found to react to a lesser degree with other groups of streptococci. Monoclonal antibody was used to probe normal and rheumatic sarcolemma for differences in reactivity. Although the rheumatic heart reacted more intensely, no major differences between the immunobanding patterns of normal and rheumatic hearts were observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Cunningham M. Type-specific inhibition of preopsonization versus immunoprecipitation by Streptococcal M proteins. Infect Immun. 1973 Jul;8(1):19–24. doi: 10.1128/iai.8.1.19-24.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Russell S. M. Study of heart-reactive antibody in antisera and hybridoma culture fluids against group A streptococci. Infect Immun. 1983 Nov;42(2):531–538. doi: 10.1128/iai.42.2.531-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Protective antigenic determinant of streptococcal M protein shared with sarcolemmal membrane protein of human heart. J Exp Med. 1982 Oct 1;156(4):1165–1176. doi: 10.1084/jem.156.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Goldstein I., Rebeyrotte P., Parlebas J., Halpern B. Isolation from heart valves of glycopeptides which share immunological properties with Streptococcus haemolyticus group A polysaccharides. Nature. 1968 Aug 24;219(5156):866–868. doi: 10.1038/219866a0. [DOI] [PubMed] [Google Scholar]
- KAPLAN M. H., BOLANDE R., RAKITA L., BLAIR J. PRESENCE OF BOUND IMMUNOGLOBULINS AND COMPLEMENT IN THE MYOCARDIUM IN ACUTE RHEUMATIC FEVER. ASSOCIATION WITH CARDIAC FAILURE. N Engl J Med. 1964 Sep 24;271:637–645. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- KAPLAN M. H., SUCHY M. L. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. II. CROSS-REACTION OF ANTISERA TO MAMMALIAN HEART TISSUE WITH A CELL WALL CONSTITUENT OF CERTAIN STRAINS OF GROUP A STREPTOCOCCI. J Exp Med. 1964 Apr 1;119:643–650. doi: 10.1084/jem.119.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unny S. K., Middlebrooks B. L. Streptococcal rheumatic carditis. Microbiol Rev. 1983 Mar;47(1):97–120. doi: 10.1128/mr.47.1.97-120.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Rijn I., Bleiweis A. S., Zabriskie J. B. Antigens in Streptococcus mutans cross reactive with human heart muscle. J Dent Res. 1976 Apr;55(Spec No):C59–C64. doi: 10.1177/002203457605500326011. [DOI] [PubMed] [Google Scholar]
- Zabriskie J. B., Friedman J. E. The role of heart binding antibodies in rheumatic fever. Adv Exp Med Biol. 1983;161:457–470. doi: 10.1007/978-1-4684-4472-8_26. [DOI] [PubMed] [Google Scholar]
- Zabriskie J. B., Hsu K. C., Seegal B. C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970 Aug;7(2):147–159. [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]