Abstract
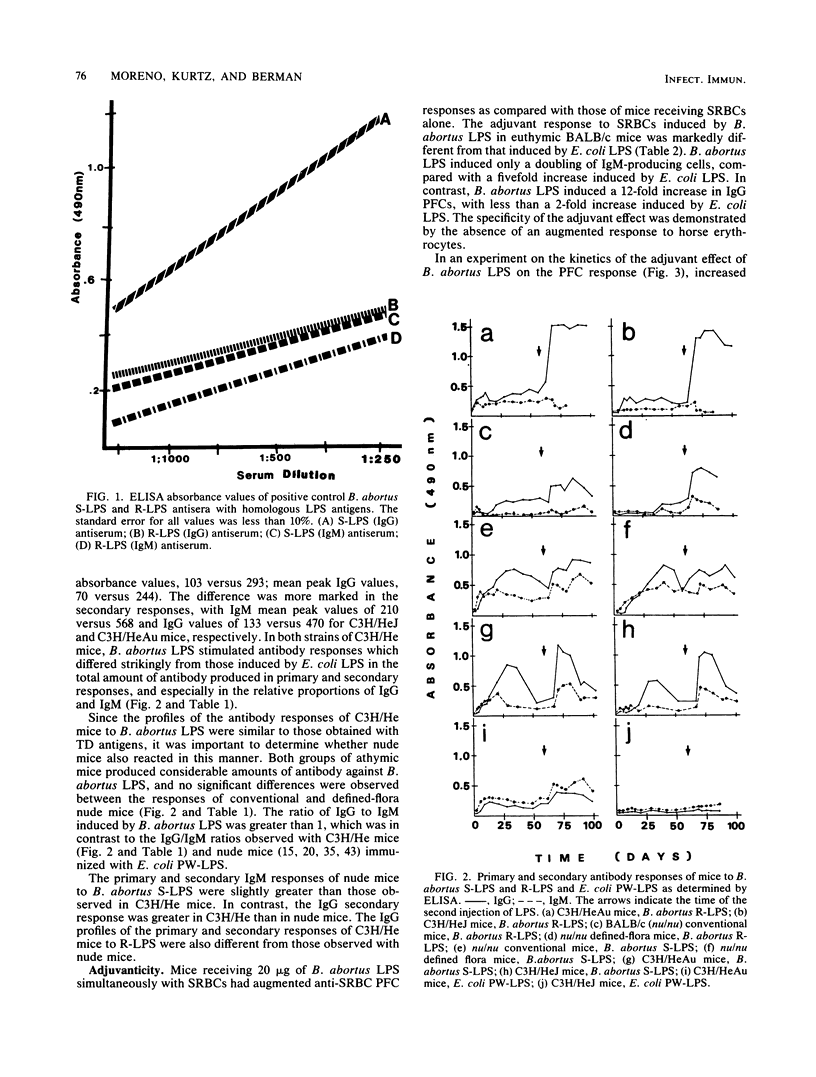
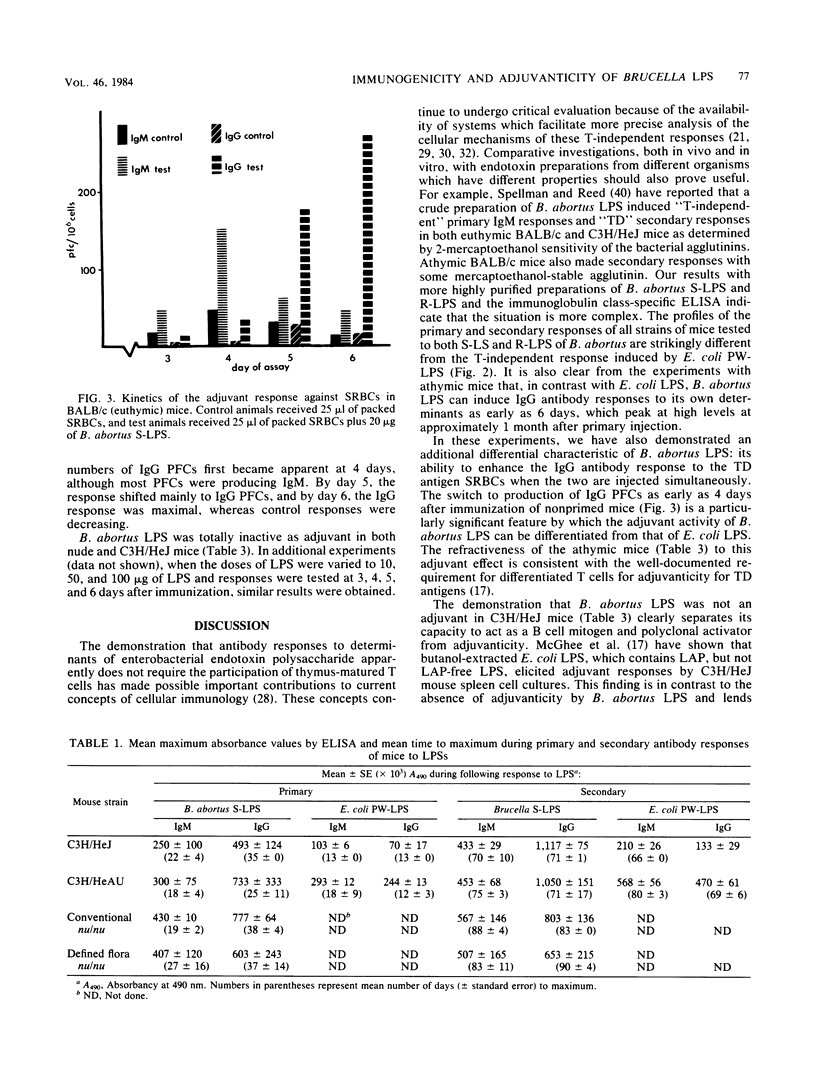
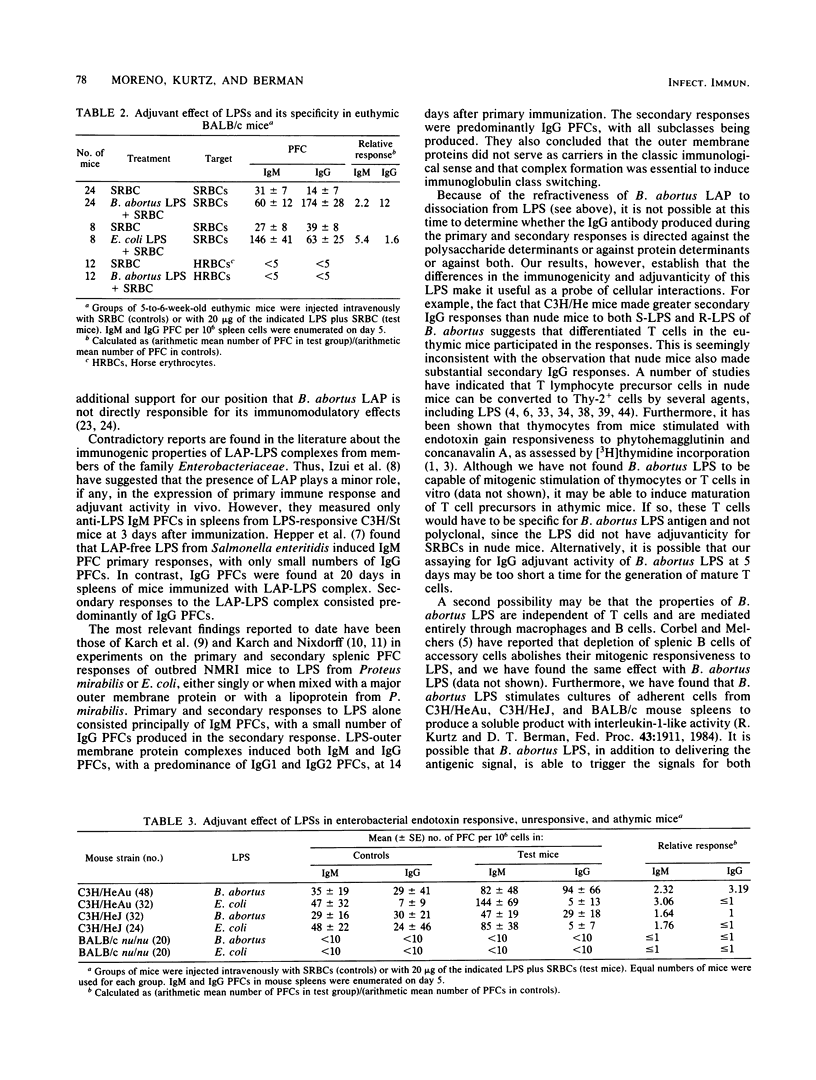
The immunogenic and adjuvant properties of Brucella abortus and Escherichia coli lipopolysaccharides (LPSs) were studied in endotoxin-responsive, athymic, and euthymic BALB/c mice and in responsive C3H/HeAu mice and congenic nonresponsive C3H/HeJ mice. Consistent with previous reports, E. coli LPS did not stimulate significant primary or secondary antibody responses in C3H/HeJ mice and induced the production of immunoglobulin M (IgM) and low levels of IgG in C3H/HeAu mice. In contrast, B. abortus smooth and rough LPS stimulated primary and secondary antibody responses and induced the production of IgM and high levels of IgG in both responsive and nonresponsive strains of C3H/He mice and in nude mice. When used as adjuvant, B. abortus LPS augmented the IgG plaque-forming-cell response of C3H/HeAu and BALB/c euthymic mice to the T-dependent antigen sheep erythrocytes. E. coli LPS augmented only the IgM plaque-forming-cell response in the same mouse strains. Neither B. abortus nor E. coli LPS was adjuvant for C3H/HeJ or nude mice. The dichotomy between the antibody and adjuvant responses of both C3H/HeJ mice and athymic mice to B. abortus LPS may be a function of the true thymus independence and dependence of these responses. In addition, the refractiveness of C3H/HeJ and nude mice to B. abortus LPS as adjuvant, but not as mitogen or polyclonal B cell activator, clearly dissociates these phenomena.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Ruco L., Uccini S., De Franceschi G. S., Baroni C. D., Doria G. Biological effects of Escherichia coli lipopolysaccharide (LPS) in vivo. II. Selection in the mouse thymus of PHA- and con A-responsive cells. Immunology. 1976 Aug;31(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Baroni C. D., De Franceschi G. S., Uccini S., Adorini L., Cnen G. D., Ruco L. Biological effects of Escherichia coli lipopolysaccharide (LPS) in vivo. I. Selection in the mouse thymus of killer and helper cells. Immunology. 1976 Aug;31(2):217–224. [PMC free article] [PubMed] [Google Scholar]
- Chen B. P., Splitter G. A. Suppressor T cells in thymus-reconstituted nude mice: regulation of mitogen-induced transformation. Cell Immunol. 1980 Apr;51(1):129–140. doi: 10.1016/0008-8749(80)90243-9. [DOI] [PubMed] [Google Scholar]
- Corbel C., Melchers F. Requirement for macrophages or for macrophage- or T cell-derived factors in the mitogenic stimulation of murine B lymphocytes by lipopolysaccharides. Eur J Immunol. 1983 Jul;13(7):528–533. doi: 10.1002/eji.1830130703. [DOI] [PubMed] [Google Scholar]
- Hammerling U., Chin A. F., Abbott J., Scheid M. P. The ontogeny of murine B lymphocytes. I. Induction of phenotypic conversion of Ia-to Ia+ lymphocytes. J Immunol. 1975 Nov;115(5):1425–1431. [PubMed] [Google Scholar]
- Hepper K. P., Garman R. D., Lyons M. F., Teresa G. W. Plaque-forming cell response in BALB/c mice to two preparations of LPS extracted from Salmonella enteritidis. J Immunol. 1979 Apr;122(4):1290–1293. [PubMed] [Google Scholar]
- Izui S., Morrison D. C., Curry B., Dixon F. J. Effect of lipid A-associated protein and lipid A on the expression of lipopolysaccharide activity. I. Immunological activity. Immunology. 1980 Jul;40(3):473–482. [PMC free article] [PubMed] [Google Scholar]
- Karch H., Gmeiner J., Nixdorff K. Alteration of the immunoglobulin G subclass responses in mice to lipopolysaccharide: effects of nonbacterial proteins and bacterial membrane phospholipids or outer membrane proteins of Proteus mirabilis. Infect Immun. 1983 Apr;40(1):157–165. doi: 10.1128/iai.40.1.157-165.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Modulation of the IgG subclass responses to lipopolysaccharide by bacterial membrane components: differential adjuvant effects produced by primary and secondary stimulation. J Immunol. 1983 Jul;131(1):6–8. [PubMed] [Google Scholar]
- Lamb V. L., Jones L. M., Schurig G. G., Berman D. T. Enzyme-linked immunosorbent assay for bovine immunoglobulin subclass-specific response to Brucella abortus lipopolysaccharides. Infect Immun. 1979 Oct;26(1):240–247. doi: 10.1128/iai.26.1.240-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen O. P., Eerola E. The effect of different antibody affinities on ELISA absorbance and titer. J Immunol Methods. 1982 Oct 29;54(2):233–240. doi: 10.1016/0022-1759(82)90064-3. [DOI] [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx A., Ionescu J., Pop A. Immunochemical studies on Brucella abortus lipopolysaccharides. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Feb;253(4):544–553. [PubMed] [Google Scholar]
- McGhee J. R., Farrar J. J., Michalek S. M., Mergenhagen S. E., Rosenstreich D. L. Cellular requirements for lipopolysaccharide adjuvanticity. A role for both T lymphocytes and macrophages for in vitro responses to particulate antigens. J Exp Med. 1979 Apr 1;149(4):793–807. doi: 10.1084/jem.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner E. C., Rudbach J. A., Voneschen K. B. Cellular responses to bacterial lipopolysaccharide: T cells recognize LPS determinants. Scand J Immunol. 1983 Jul;18(1):21–28. doi: 10.1111/j.1365-3083.1983.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Farrar J., Paul W. E., Fuller-Farrar J., Schaefer M., Howard M. T cell dependence and factor reconstitution of in vitro antibody responses to TNP-B. Abortus and TNP-Ficoll: restoration of depleted responses with chromatographed fractions of a T cell-derived factor. J Immunol. 1983 Aug;131(2):633–637. [PubMed] [Google Scholar]
- Moreno E., Berman D. T., Boettcher L. A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981 Jan;31(1):362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T. Brucella abortus lipopolysaccharide is mitogenic for spleen cells of endotoxin-resistant C3H/HeJ mice. J Immunol. 1979 Dec;123(6):2915–2919. [PubMed] [Google Scholar]
- Moreno E., Jones L. M., Berman D. T. Immunochemical characterization of rough Brucella lipopolysaccharides. Infect Immun. 1984 Mar;43(3):779–782. doi: 10.1128/iai.43.3.779-782.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Speth S. L., Jones L. M., Berman D. T. Immunochemical characterization of Brucella lipopolysaccharides and polysaccharides. Infect Immun. 1981 Jan;31(1):214–222. doi: 10.1128/iai.31.1.214-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. A reappraisal of "T-independent" antigens. II. Studies on single, hapten-specific B cells from neonatal CBA/H or CBA/N mice fail to support classification into TI-1 and TI-2 categories. J Immunol. 1984 Apr;132(4):1696–1701. [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A reappraisal of "T-independent" antigens. I. Effect of lymphokines on the response of single adult hapten-specific B lymphocytes. J Immunol. 1984 Apr;132(4):1687–1695. [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Haemopoietic stem cells and progenitors of functional T-lymphocytes in the bone marrow of 'nude' mice. Clin Exp Immunol. 1973 Aug;14(4):597–607. [PMC free article] [PubMed] [Google Scholar]
- Pritchard H., Riddaway J., Micklem H. S. Immune responses in congenitally thymus-less mice. II. Quantitative studies of serum immunoglobulins, the antibody response to sheep erythrocytes, and the effect of thymus allografting. Clin Exp Immunol. 1973 Jan;13(1):125–138. [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Akiya F. I., Elin R. J., Hochstein H. D., Luoma M. K., Milner E. C., Milner K. C., Thomas K. R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976 Jan;3(1):21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Reed N. D. Immunological responses of mice to lipopolysaccharide: lack of secondary responsiveness by C3H/HeJ mice. Infect Immun. 1977 May;16(2):513–517. doi: 10.1128/iai.16.2.513-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato V. L., Waksal S. D., Herzenberg L. A. Identification and separation of pre T-cells from nu/nu mice: differentiation by preculture with thymic reticuloepithelial cells. Cell Immunol. 1976 Jun 1;24(1):173–185. doi: 10.1016/0008-8749(76)90142-8. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman J. M., Reed N. D. Immune and mitogenic responses by BALB/c, C3H/HeJ, and nude mice to Brucella abortus bacterin and lipopolysaccharide. Infect Immun. 1979 May;24(2):371–378. doi: 10.1128/iai.24.2.371-378.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Moore R. N., Rosenstreich D. L. Correction of defective macrophage differentiation in C3H/HeJ mice by an interferon-like molecule. J Immunol. 1982 Jan;128(1):380–387. [PubMed] [Google Scholar]
- Von Eschen K. B., Rudbach J. A. Dissociation of the anti-hapten and anti-carrier responses of mice injected with dinitrophenylated lipopolysaccharide. Infect Immun. 1981 Jan;31(1):327–333. doi: 10.1128/iai.31.1.327-333.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H. Immunological responses of 'nude' mice. Clin Exp Immunol. 1971 Feb;8(2):305–317. [PMC free article] [PubMed] [Google Scholar]


