Abstract
Clinical and epidemiological studies have shown that HDLs, a class of plasma lipoproteins, heterogeneous in size and density, have an atheroprotective role attributed, for years, to their capacity to promote the efflux of cholesterol from activated cholesterol-loaded arterial macrophages. Recent studies, however, have recognized that the physical heterogeneity of HDLs is associated with multiple functions that involve both the protein and the lipid components of these particles. ApoA-I, quantitatively the major protein constituent, has an amphipathic structure suited for transport of lipids. It readily interacts with the ATP-binding cassette transporter ABCA1, the SR-B1 scavenger receptor; activates the enzyme lecithin-cholesterol acyl transferase (LCAT), which is critical for HDL maturation. It also has antioxidant and antiinflammatory properties, along with the HDL-associated enzymes paraoxonase, platelet activating factor acetylhydrolase (PAF), and glutathione peroxidase. Regarding the lipid moiety, an atheroprotective role has been recognized for lysosphingolipids, particularly sphingosine-1-phosphate (S1P). All of these atheroprotective functions are lost in the post-translational dependent dysfunctional plasma HDLs of subjects with systemic inflammation, coronary heart disease, diabetes, and chronic renal disease. The emerging notion that particle quality has more predictive power than quantity has stimulated further exploration of the HDL proteome, already revealing unsuspected pro- or antiatherogenic proteins/peptides associated with HDL.—Scanu, A. M., Edelstein, C. HDL: bridging past and present with a look at the future.
Keywords: apoA-I, apoA-II, ATP-binding cassette transporter, lecithin-cholesterol acyl transferase, paraoxonase
The high-density lipoproteins (HDLs) represent a class of lipid- and protein-containing particles heterogeneous in size and density. The term HDL originates from the classic studies of Gofman and colleagues (1), who made use of the analytical ultracentrifuge in a flotational mode to separate plasma lipoproteins according to their hydrated density, a function of the ratio between the protein and lipid components. Those seminal studies also recognized the presence in plasma of very-low-density lipoproteins (VLDLs) and low-density lipoproteins (LDLs), each exhibiting size and density heterogeneity. VLDLs, LDLs, and HDLs were also found to migrate in the pre-β, β, and α-1 position, respectively, in an electrophoretic field. This review centers around human HDLs, having as a goal the bridging of old and current findings with an emphasis on apoA-I and apoA-II, the two main constitutive apolipoproteins of this class of lipoproteins in health and in some disease states.
STRUCTURAL STUDIES ON THE STEP FORMATION OF HDL IN VITRO
The role of delipidation techniques
Ultracentrifugation opened the way for studies on the physicochemical properties of HDL, including its lipid composition. However, it left unanswered the question regarding the protein moiety. Lipids posed an obstacle to protein analyses and initially a perception prevailed that organic solvents used for removing the lipids cause solubility problems in the delipidated proteins and irreversible structural changes. In the early 1950s a technique was developed by which the lipids from human serum could be extracted at –20°C using mixtures of ethanol and ethyl ether using a modified Soxhlet apparatus (2) (Fig. 1). The resulting product proved to be soluble in aqueous buffers. Paper electropherograms stained with Sudan IV revealed the total absence of the α-1 migrating band and a residual faint band migrating in the β position, indicating that LDL and HDL were the target of the delipidation procedure. Subsequently, when the same delipidation procedure was applied to LDL and HDL, following their ultracentrifugal isolation from plasma (3), the resulting apoHDL, but not apoLDL, was totally water-soluble and exhibited a single peak by free boundary electrophoresis (Fig. 2) and a single sedimenting band in the analytical ultracentrifuge. Moreover, by optical rotatory dispersion (4), circular dichroism, and infrared spectroscopy (5, 6), the high α helical conformation in apoHDL was comparable to that in parent HDL. In addition, apoHDL, labeled by radioiodination, reassociated with the mother lipoprotein when incubated in vitro with either whole human serum or the HDL from which apoHDL was derived (7). The studies that followed established that apoHDL is made of two proteins, now called apoA-I and apo-AII, and that apoA-II is a homodimer of two identical polypeptide chains linked together by a single disulfide bond in position 6 from the N terminus (for review, see ref. 8).
Figure 1.
Modified Soxhlet apparatus for the continuous cold ether extraction of the ethanol-diethyl ether HDL precipitate. Reprinted with permission (2).
Figure 2.
Moving boundary electrophoresis of serum and delipidated HDL using a modified Tiselius method showing a single peak migrating in the α1-globulin position of the original serum. Reprinted with permission (2).
Relipidation studies
When either holo apoHDL or the isolated apoA-I and apoA-II were subjected to relipidation in systems where each product was sonicated in the presence of either purified phospholipid dispersions or mixtures of lipids extracted from HDL (9), studies of the products obtained led to the notions that both apoA-I and apoA-II can readily undergo delipidation and relipidation, that apoA-I and apo A-II differ in their affinity for the HDL surface, and that apoA-II can displace apoA-I from the HDL surface (10, 11).
STRUCTURAL STUDIES ON HUMAN APOA-I
Lipid-free apoA-I
ApoA-I is synthesized as a preproprotein that undergoes proteolytic processing to form mature ApoA-I, which is essentially the only form present in plasma (12) (Table 1). It is a single-polypeptide chain of 243 amino acids that represents ∼70% of the apoHDL by weight. From predictions of its secondary structure based on the knowledge of the amino acid sequence (13, 14), apo A-I consists of amphipathic helices of repeating 11 or 22 amino acids separated by proline residues (15). Experimental data at the air-water interface (16,17,18,19) have shown unambiguously the high surface activity of apoA-I and its dependence on its amphipathic properties (20) also documented by spectroscopic studies (21). Denaturation and ultracentrifugal studies indicated that apoA-I is asymmetric (22) and flexible with a tendency to self-associate into dimers, tetramers, and octamers (23). The crystal structure of lipid-free apoA-I has been determined (24), showing two main helical domains that consist of a four-helix antiparallel bundle in the first 75% of the N-terminal region, followed by a two-helix bundle in the C-terminal region.
TABLE 1.
Solution properties of apoA-I in lipid-free form and after lipidation
| Condition | References |
|---|---|
| ApoA-I | |
| Primary structure | |
| Mature form is a single polypeptide chain, 28 kDa, containing 243 amino acids lacking glycosylation and disulfide linkages | (12,13,14) |
| Secondary structure | |
| α-helical content: 50–57% by circular dichroic measurements | (20) |
| 11-mer repeats evolving into 22-mer amphipathic helical repeats | (15) |
| Monomer with a high surface activity at the air-water, solid (bead)-water and lipid-water interfaces | (16,17,18,19) |
| Tertiary structure | |
| Asymmetric protein highly flexible in aqueous solution | (22, 96) |
| Crystal structure at 2.4 Å resolution: two main helical domains consisting of a four-helix antiparallel bundle in the first 75% of the N-terminus, followed by a two-helix bundle in the C-terminal domain | (24) |
| Quaternary structure | |
| Self associates in solution as a function of concentration; preferred mode: monomer-dimer-tetramer-octamer equilibrium | (23) |
| ApoA-I in discoidal particles, proposed models | |
| Belt model (favored): helices wrapped around a phospholipid bilayer with the long helical axis perpendicular to the fatty acyl chains | (27, 28, 97) |
| Hairpin model: two molecules of apoA-I form an open-ended structure | (27, 30) |
| ApoA-I in spherical particles, reconstitution studies | |
| Sonication approach: mixtures of apoA-I and lipids from whole HDL become incorporated into spherical reconstitute HDL where apoA-I is the determinant of particle size and structure | (9, 31) |
| Enzymatic (LCAT) approach: upon incubation with LCAT, discs become spherical HDL where the newly formed cholesteryl esters in the core increase the thermodynamic stability of the particle | (33) |
ApoA-I in discoidal particles
ApoA-I can be partially relipidated in the presence of phospholipids either with or without cholesterol (25). Most studies have dealt with particles containing two molecules of apoA-I and ∼150 molecules of phospholipids having a hydrated diameter of 96 Å. Models of apoA-I in these discs have been proposed (for recent review, see ref. 26). The more widely accepted is the belt model described by Segrest et al. (27) where the amphipathic helices of apoA-I are wrapped around the circumference of the discoidal phospholipid bilayer with the helical axis perpendicular to the lipid acyl chains. Evidence for this model has come from structural analyses using polarized internal reflection infrared spectroscopy (28) and solid-state NMR (29). The double-belt model and variations thereof appear to best apply when the discs contain two molecules of apoA-I (20, 27, 30).
ApoA-I in spherical particles
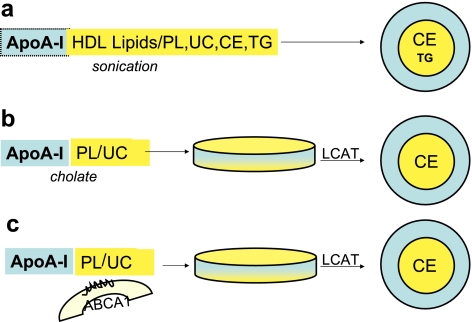
Early reconstitution studies of apoA-I with HDL lipids showed that the process is kinetically controlled and requires the presence of apoA-I either as a monomer or as a dimer. In those studies the number of apoA-I molecules incorporated into reconstituted HDL was found to determine the size and structure of the reassembled particle (9, 31). Moreover, reconstitution could be accomplished when discoidal ApoA-I was incubated in the presence of LCAT and LDL. The resulting product was a spherical particle with a diameter of 93 Å containing 3 molecules of apoA-I (32). This enzymatically based reassembly method has seen wide use, although contrary to native HDL that has a lipid core containing both cholesteryl esters and triglycerides, the lipid core of these reconstituted particles contains only cholesteryl esters. Sparks et al. (33) prepared spherical particles by sonicating mixtures containing two molecules of apoA-I, phospholipids and varying ratios of cholesteryl esters and triglycerides. In that system, the triglyceride to cholesteryl esters ratio influenced the thermodynamic stability of the HDL particle. Moreover, the structure of apoA-I in spherical particles was found to differ from that in discs. Li et al. (34) using fluorescence resonance energy transfer (FRET) found that apoA-I in spherical particles, prepared with the LCAT method, had reduced intermolecular interactions and a more variable conformation. In contrast, other studies using circular dichroism and fluorescence spectroscopy (35) have shown that the apoA-I conformation is similar in both discs and spheres. Thus, it is apparent that understanding of the structural entities influencing the registry and conformational properties of apoA-I in spherical HDL still require more work in order to gain a better insight into the stabilizing role deriving from the interaction between core neutral lipids and the acyl chains of the phospholipids occupying the surface monolayer. A schematic representation of the relipidation steps leading to the formation of discoidal and spherical particles is shown in Fig. 3. In Fig. 4 we show examples of discoidal and spherical HDL isolated from human subjects.
Figure 3.
In vitro and in vivo steps for the formation of spherical HDL. a) In this in vitro model, apoA-I is either isolated from apoHDL or prepared by recombinant technology. It is then mixed with lipids extracted from whole HDL, and the mixture is sonicated above the transition temperature, permitting cholesterol ester (CE) solubilization. This procedure generates spherical particles with characteristics similar to native HDL. b) ApoA-I, prepared as in a, is incubated with synthetic phospholipids (PL) and unesterified cholesterol (UC) in the presence of cholate, producing discoidal particles. Addition of LCAT in the presence of LDL (not shown) results in the generation of CEs that occupy the core of a spherical particle having PLs and UC in the surface monolayer. c) This simplified in vivo model depicts a somatic cell that cannot produce apoA-I but contains the membrane ABCA1 transporter. ApoA-I is derived from synthesis and secretion in liver and intestine and also during the HDL remodeling processes occurring in the plasma. The ABCA1 transporter facilitates the cellular efflux of cholesterol and phospholipids that, with apoA-I, jointly form discoidal pre-β HDL particles that, by the action of LCAT, are subsequently transformed into a mature spherical HDL. TG, triglycerides.
Figure 4.
Electron micrographs of discoidal and spherical HDLs. Left: nascent HDL particles isolated from the plasma of a subject that cannot form spherical HDLs because of a deficiency in LCAT. The particles are disc shaped, 15–20 nm in diameter and 4.5 nm thick. Right: spherical HDLs (7.5–12 nm in diameter) isolated from the plasma of a normal subject. Scale bars = 100 nm. (Photos kindly provided by Dr. Trudy Forte, Children’s Hospital Oakland Research Institute, Oakland, CA).
STRUCTURAL STUDIES ON HUMAN APOA-II
Lipid-free apoA-II
Synthesized in a prepro form, the mature circulating apoA-II is the second main constitutive component of HDL, representing ∼20% by weight of the HDL protein (12) (Table 2). Each homodimer has a molecular weight of 17,400, consisting of two identical polypeptide chains of 77 amino acid residues covalently linked by a single disulfide bond located at position 6 from the amino acid terminus (12, 36). The apoA-II homodimer has been shown to undergo self-association in solution in a monomer-dimer-trimer mode (37). On reduction, the resulting single chain self-associates according to a monomer-dimer-tetramer motif (38) also exhibited by the naturally occurring apoA-II monomer present in the plasma of rhesus monkeys (39). Human apo A-II has a low free energy of unfolding, 0.82 kcal/mol, as measured by CD (40). Crystallographic studies by Kumar et al. (41) conducted on dimers of human apoAII have shown amphipathic helices that aggregate into tetramers held together by the hydrophobic patches within the helices. Of note, ApoA-II is also dimeric in the chimpanzee (42) but monomeric in the rhesus monkey genus Macaca due to the replacement of cysteine by serine in position 6 (43) and in the baboon (another species of Old World monkey). ApoA-II is also monomeric in New World monkeys such as the squirrel monkey and the cebus as well as in intermediate species such as the gibbon. However, pigs, dogs, and chickens contain negligible or no apoA-II in their plasma (44). Thus, apoA-II does not appear to be essential in all animal species. Moreover, in humans it is unclear whether dimers and monomers have an equivalent modulating effect on the structure and biology of HDL and on lipoprotein metabolism in general.
TABLE 2.
Solution properties of apoA-II in lipid-free form and after lipidation
| Condition | References |
|---|---|
| ApoA-II | |
| Primary structure | |
| Mature form: homodimer, 17.4 kDa, of two identical monomers, 77 amino acid residues linked by a single disulfide bond | (12, 36) |
| Secondary structure | |
| 33% α-helix; repeats of 11-mer amphipathic units | (15) |
| High surface activity | (17,18,19) |
| Tertiary structure | |
| Low free energy of unfolding, 0.82 kcal/mol | (40) |
| Crystal structure: amphipathic helices aggregating into tetramers | (41) |
| Quaternary structure | |
| Self-associates to a monomer-dimer-trimer equilibrium | (37, 38) |
| ApoA-II in discoidal particles | |
| Increase in α-helical content from 33% to 69% upon interacting with phospholipids and cholesterol in aqueous suspensions | (45) |
| Total incorporation occurs at all initial lipid:protein ratios; lipid association preferred over self-association | (48) |
| Crystallographic and cross-linking studies of complexes hypothesize a belt-like orientation of apoA-II around a lipid bilayer | (41, 47) |
| ApoA-II in spherical particles, reconstitution studies | |
| Sonication with HDL-derived lipids generates particles of a broad density and size distribution | (48) |
| Apo A-II containing spherical particles obtained by displacing apoA-I | (10, 98, 99) |
ApoA-II in discoidal particles
Studies on the binding of apo A-II to lipids have received comparatively less attention than those on apo A-I. The high surface activity of apoA-II accounts for its high affinity for phospholipids to form discrete discoidal complexes. On lipid binding, the α-helical content of apoA-II increased from 33 to 69% (45). Moreover, at the various lipid-to-protein ratios examined, all of the apoA-II was incorporated into the reconstituted particle due to the high free energy of stabilization of the protein secondary structure when associated with phospholipids (46). Structural information on apoA-II in discoidal particles is limited to two independent investigations (41, 47). Kumar et al. (41) crystallized apoA-II complexed with octyl glucoside, a lipid surrogate. The product retained the tetrameric stucture of lipid-free apoA-II, but the helices rather than the side-by-side packing were complexed with octyl glucoside resulting in a head-to-tail oligomerization into an irregular double-stranded array. The results of these crystallographic studies along with recent ones obtained from cross-linking experiments in systems containing apoA-II and POPC (47) have favored a model of a belt-like orientation of apoA-II around the lipid bilayer.
ApoA-II in spherical particles
Early work by Ritter and Scanu (48) showed that sonication of apoA-II with lipids extracted from HDL resulted in the formation of complexes having a broad density and size distribution in contrast to apoA-I that, under similar conditions, generated particles of a defined density and size. Of interest, although the lipid composition in the reassembled HDLs varied, the moles of apoA-II remained constant, 4/particle. This ratio has also been reported by Durbin and Jonas (49) in their studies dealing with 103 Å discoidal structures containing apoA-II, POPC, and cholesterol. Lagocki and Scanu (10) have shown that discrete spherical particles containing only apoA-II could be generated in a system in which apoA-II displaced the apoA-I present in canine HDL. The displacement of apoA-I by apoA-II resulted in the formation of lipoprotein particles containing 6 mol of apoA-II.
STRUCTURAL STUDIES ON APOA-I AND APOA-II IN NATIVE AND RECONSTITUTED HDL
The protein moiety of the HDL isolated in the density range of 1.063–1.21 g/ml contains ∼75% apoA-I and 25% apoA-II by weight. However, the protein of the pre-β discoidal particles characteristic of the early steps of the reverse cholesterol transport contains only apoA-I. ApoA-II is also absent from the large buoyant apoE-containing HDLs. These intriguing structural gaps in apoA-II particle affiliation have not been explained and raise questions about mechanisms controlling apoA-I and apoA-II distribution as a function of HDL particle size. In reconstitution studies, the mixing in vitro of two discoidal complexes, one containing apoA-I and the other apoA-II, in the presence of LCAT generated, via a fusion process, a spherical HDL in which the apoA-I/apoA-II molar ratio was 0.7:1, a range consistent with that seen in native HDL (50). Early studies from this laboratory (9) have shown that sonication of apoHDL in the presence of turbid aqueous suspensions of lipids extracted from HDL, caused the formation of particles that resembled native HDL based on chemical, immunological, ultracentrifugal, circular dichroism, and electron microscopy criteria. In that study, when the two main apoHDL components apoA-I and apoA-II, isolated by Sepharose column chromatography (51, 52), were individually sonicated in the presence of whole HDL lipid extracts, in each case the particles formed floated in the density 1.063–1.21 g/ml range but differed in protein:lipid ratio and lipid core composition. Moreover, in the analytical ultracentrifuge, the reconstituted complex containing apoA-I exhibited a single symmetrical peak whereas the one containing apoA-II was broad and heterogeneous, a finding corroborated later by the reassembly experiments conducted by Ritter and Scanu (31, 48). Overall, those results were taken to support the concept that, in the case of a spherical HDL, apoA-II by itself has no size determining capacity contrary to apoA-I, which retains its dominant structural role even in the presence of apoAII, at least within the range of the apoA-I/apoA-II molar ratios in native HDL. This result indicates that apoA-I and apoA-II can coexist at the HDL surface. In model systems, a salt bridge between these two apolipoproteins has been reported (53), but much more information is needed, particularly regarding the surface of naturally occurring HDL particles.
FUNCTIONAL CONSIDERATIONS ON THE ATHEROPROTECTIVE EFFECT OF APOA-I
ApoA-I is recognized to be an essential player in the early steps of macrophage reverse cholesterol transport (RCT), a process involved in the atheroprotective role of HDL. This notion has been supported by studies of apoA-I deficient mice, which exhibited more atherosclerosis than their controls (54) and the opposite protective effect observed in transgenic mice overexpressing apoA-I (55). Moreover, in human subjects with acute coronary syndrome, a short-term infusion of a recombinant apoA-I Milano (a dimeric mutant) complexed with phospholipids caused a significant reduction in coronary atheroma volume (56). Further support comes from the studies of mimetic peptides representing analogs of the phospholipid binding class A amphipathic α helical motif that is present in naturally occurring human apoA-I (57). The mimetic approach is not new. In 1979 a synthetic amphipathic helical docosapeptide was shown to have similar surface properties to that of apoA-I (58) and to activate LCAT (59). Currently, the most extensively studied among the apoA-I mimetics has been peptide D-4F composed of 18 d-amino acids designed to resist degradation by the intestinal peptidases and also permitting administration by the oral route (60). D-4F has the capacity to bind fatty acid hydroperoxides and proinflammatory oxidized phospholipids, processes that play a role in the antiinflammatory and atheroprotective function of HDL. In this respect, mice and monkey models given D-4F orally have been shown to undergo a marked decrease in the experimentally induced atherosclerotic lesions (61). From the mechanistic viewpoint, in the apo E-null mouse model, D-4F was found to cause a redistribution of apoA-I from α migrating to small size (7 to 8 nm) pre-β migrating HDL enriched in active paraoxonase (62), probably via combined displacement and remodeling processes. These newly formed particles exhibited antiinflammatory properties, reduced lipoprotein lipid peroxides, and also increased cholesterol efflux from cholesterol-loaded macrophages, all antiatherogenic mechanisms. D-4F has also been found to be favorably operative in inflammatory disorders other than atherosclerosis. Moreover, the studies have moved from the preclinical to the clinical phase where a single oral dose of the peptide was proven to be safe in patients with a high risk for cardiovascular disease and has encouraged long-term studies in order to assess antiatherogenic efficiency with an emphasis on monitoring the inflammatory index based on the ability of the peptide to inhibit LDL-induced monocyte chemotactic activity, which is related to the production of monocyte chemoattractant-1 (MCP-1) (60).
Irrespective of the long-term clinical outcomes that must be viewed with uncertainty at this time, the results obtained thus far with peptide D-4F, or others of this kind, are relevant to the understanding of lipoprotein metabolism in general. First, administered peptides designed to be antiinflammatory can reach the circulation, associate with mature HDL particles, and retain antiinflammatory properties. Second, this association causes HDL remodeling and the formation of small pre-β migrating particles containing apoA-I, but not apoA-II, enriched in paraoxonase, thus having antiinflammatory and antiatherogenic properties. This indicates that pre-β particles are not exclusive of the early steps of the RCT process. Also, as a consequence of remodeling, the mature HDL, by losing some of its apoA-I component becomes enriched in apoA-II and impoverished in paraoxonase given the antagonistic action of apoA-II for this enzyme. This change in apoA-I to apoA-II ratio would be proportional to the degree of apoA-I displacement and it would be expected to be associated with changes in the biological properties of the modified HDL either in a pro- or antiatherogenic mode. Studies in this direction ought to be forthcoming given our current inadequate knowledge about the role of apoA-II in lipoprotein metabolism and atherogenesis (see below).
FUNCTIONAL PROPERTIES OF APOA-II RELATED TO LIPOPROTEIN METABOLISM AND ATHEROGENESIS
Much of the current information on this subject has been derived from the study of genetically modified mice as summarized in a recent review (63). The early work by Weng et al. (64) conducted in apoA-II knockout mice showed a dramatic decrease in plasma HDL cholesterol associated with a rapid clearance of remnant particles, suggesting an important role of apoA-II in triglyceride metabolism. In keeping with these results were subsequent studies showing that transgenic mice overexpressing mouse apoA-II have elevated plasma levels of triglycerides and free fatty acids in addition to a decrease in triglyceride hydrolysis (65). This view is further corroborated by recent observations in mice in which an increased expression of apoA-II caused an acute inhibitory effect on the hydrolysis of VLDL and chylomicron triglycerides, which suggests that in acquiring apoA-II from circulating HDL that acts as a reservoir, triglyceride-rich particles become a poor substrate for lipoprotein lipase (66). This conclusion has received further support from in vitro studies showing that apoA-II can transfer from HDL to VLDL. All of these observations have been related to the pathogenesis of insulin resistance, adiposity, diabetes, and metabolic syndrome, which are all conditions associated with cardiovascular pathology. While these findings are important from the viewpoint of mouse pathology, they may not necessarily apply to humans given the important differences in lipoprotein metabolism between these two species. For instance, LDL is the main cholesterol transporter in humans, whereas in mice, HDL serves this function. Moreover, CETP is present in humans but not in mice, and the susceptibility to atherogenesis is also markedly different between these two species. In mouse models expressing either monomeric mouse apoA-II or dimeric human apoA-II, marked differences were found in the in vivo effect between these two apolipoprotein species, attributed not to monomer-dimer considerations, but to sequence differences between the two (67). Nonetheless, like mouse models, studies in humans have implicated the apoA-II gene in visceral fat accumulation and metabolism of triglyceride-rich particles and related to the expression of a −265T/C polymorphism in the promoter region (68). The presence of the −265C allele has also been associated with decreased plasma levels of apoA-II, in association with a decreased waist circumference particularly in middle-aged healthy subjects, to an enhancement in the postprandial metabolism of large VLDLs and more recently, to anomalies in food consumption as in obesity (69).
PROTEOMICS OF HDL
Proteomic studies that have been conducted recently in various laboratories (see review in ref. 70) exemplify the growing interest originating from clinical observations on the atheroprotective properties of HDL. What is emerging is that the HDL proteome contains, in addition to the previously recognized quantitatively “minor” apolipoproteins, enzymes, and transport proteins relevant to lipid metabolism and many others that are not in the lipid area but are involved in the processes of acute-phase response, proteinase inhibition, and complement regulation. The presence of small peptides and fragments of yet unknown function has also been reported (71). This unsuspected array of HDL-associated proteins with a marked diversity in biological function has opened new avenues of investigations that may provide a better understanding of the role played by HDL in atherogenesis and inflammatory processes in general and help identify novel biomarkers of cardiovascular disease. It must be stressed, however, that proteomic studies are still in their infancy and generalizations suffer from the heterogeneity of subjects studied, standardization of the methods of lipoprotein isolation from the plasma, species of HDL analyzed, and mass spectroscopic methods used. Verification of the reported HDL-associated elements by other procedures is in order and so is their mode of association to the particle surface. Functionality issues also need to be resolved as well as how many of these proteins are specifically associated with HDL and how many with apo B100-containing lipoproteins. A concern must also be raised about using procedures for lipoprotein isolation based on density gradient ultracentrifugation in high-salt media containing KBr or NaBr since these salts may cause either a total or a partial dissociation of some associated elements from the HDL surface. In this respect, a recent report has shown that for raising plasma density, D2O is to be preferred over salts (72). From the above, it is apparent that studies should not be confined to ultracentrifugally isolated HDL but run in parallel with samples separated by alternative procedures such as column chromatography. Despite these shortcomings, proteomics have added a new dimension to the studies of HDL by uncovering a host of bioactive proteins and peptides that may be using the HDL surface as a platform and hypotheses deserving exploration. Moreover, proteomics should find a major application in the study of the proatherogenic post-translational modifications of apolipoproteins like apoA-I and apoA-II by studying the HDL particles isolated from the plasma of subjects with chronic inflammatory condition, metabolic syndrome, diabetes, or chronic renal disease (see below).
HDL AND BIOACTIVE LIPIDS
For a number of years, phosphatidylcholine and sphingomyelin have been recognized as the major components of the HDL surface along with minor amounts of phosphoglycerols, phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol. Later, several other classes of glycosphingolipids have been described, namely glucosylceramides, galactosylceramides, and lactosylceramides, sulfatides, gangliosides, and globosides (73, 74). The discovery in HDL of a soluble phospholipase A2 was followed by the identification of phospholipid lysoderivatives (75,76,77). Subsequently, another lysosphingolipid, sphingosine 1 phosphate (S1P), was discovered and found to be mostly carried by HDL and also positively correlated with HDL-C, apoA-I, and apoA-II levels (78). Consequently, HDL-associated S1P is now considered as the primary determinant of S1P concentration in the plasma.
Until recently, the source of S1P in the blood was uncertain. Sphingosine kinase, the enzyme that phosphorylates sphingosine to produce S1P, is expressed in platelets (79) as well as in a variety of peripheral blood cells, including erythrocytes, neutrophils, and mononuclear cells (80). Platelets store and release large amounts of S1P on stimulation by thrombin or calcium (79, 81, 82), but several recent studies have established that erythrocytes are the principal source of plasma S1P (83,84,85). Of note, erythrocytes cannot produce sphingosine and also display relatively low levels of sphingosine kinase activity (84). In addition, they efficiently convert exogenous sphingosine to S1P. The fact that erythrocytes lack S1P-degrading enzymes (e.g., sphingosine lyase and S1P phosphatase) permits them to accumulate large amounts of S1P (84, 85). The mechanism underlying SIP efflux from erythrocytes to HDL has not been clearly established, but it may involve the ATP-binding cassette transporters that are expressed in these cells. While the association with HDL appears to be dependent for the atheroprotective effect of SIP, it is still unclear whether this effect is also dependent on HDL speciation and by the apoA-I to apoA-II ratio. In this respect a recent report has shown that S1P is enriched in the small, dense HDL3 particles containing apoA-I that are viewed as having the most atheroprotective potential. Regarding signaling events, several have been proposed acting on endothelial cells, macrophages, vascular smooth muscle cells, and T-cells (see review, ref. 86). While recognizing the importance of the reported functional studies, it must be noted that on the structural level much remains to be determined regarding elements in HDL that are required for S1P bioactivity.
Oxidized phospholipids (ox-PL) also contribute to the HDL pathobiology. An excellent review on this subject has recently appeared (87). Phospholipids contain polyunsaturated fatty acids that undergo ready peroxidation. Ox-PL are involved in cellular signaling, implicated in a variety of inflammatory processes and also implicated in atherogenesis. Plasma levels of ox-PC have been found to be associated with an increased risk for cardiovascular disease. Ox-PL in the plasma are mainly associated with apoB100-containing lipoproteins inclusive of Lp(a), an LDL variant. The role that HDL plays in ox-PC metabolism is unclear but it is expected to be favorable given the antiinflammatory potential of HDL. Along these lines, Gharavi et al. (88) have reported that HDL inhibits the proinflammatory pathway induced in human endothelial cells by ox-PL signaling, perhaps by decreasing the generation of superoxides. This would represent an additional mechanism in favor of the atheroprotective action of HDL.
HDL IN PATHOLOGICAL STATES: FROM ATHEROPROTECTIVE TO PROATHEROGENIC. EMPHASIS ON POST-TRANSLATIONAL MODIFICATIONS
A number of studies have provided evidence that HDL undergoes post-translational changes that can affect its atheroprotective profile; for review see (89). Hypochlorite (HOCl), either by itself or generated from the myeloperoxide (MPO)-H2O2-chloride system, has been shown to target apoA-I and to preferentially chlorinate tyrosine 192 and also nitrate the same residue. Moreover, HOCl converts each of the three methionine residues 86, 112, and 148 to methionine sulfoxide. An HOCl-generated conversion of tryptophan to hydroxy- and dihydroxytryptophan was also observed along with a nitrosylation of this amino acid residue induced by nitrogen species.
The most important consequence of these structural modifications has been the inability of the modified apoA-I species to interact with the ATP-binding cassette transporter ABCA1 (90), thus impairing the reverse cholesterol transport system. Moreover, the HOCl-induced modifications affect the ability of apoA-I to bind lipids and impairs the formation of stable discoidal and spherical HDL in vitro (91). As anticipated, the extent of the observed structural modifications has been dependent on the degree of oxidative damage. These observations in vitro and in cell culture systems have relevance to human pathology since the modified forms of apoA-I have been shown to be present in circulating HDL in subjects with atherosclerotic cardiovascular disease as well as in the plaques of the artery wall (92, 93).
Another clinical situation leading to a dysfunctional apoA-I is diabetes mellitus. In this case the lysine residues of apoA-I are involved in the process of nonenzymatic glycation, the first step by which absorbed sugars go through a series of very slow reactions leading to the formation of advanced glycation end products (AGE). AGE-modified-apoA-I was found to significantly impair cholesterol efflux from human macrophages in culture, a process that was reversed by agents inhibiting AGE forms (94).
While all of the above studies strengthen the notion that apoA-I is the key molecule involved in many of the physiological functions of HDL, observations must be extended to ApoA-II, based on the evidence that it may have a modulatory action on apoA-I function and that apoA-II can undergo oxidative changes of two of the three methionine residues (95). Thus, it is apparent that in abnormal situations the pathobiological potential of HDL cannot be based just on plasma concentrations but requires the definition of particle quality, in which mass spectroscopy technology will play an increasingly important role.
CONCLUSIONS
For a number of years, HDL has been identified as serving an atheroprotective role by promoting reverse cholesterol transport, a process facilitating the efflux of cholesterol from cholesterol-loaded macrophages in the artery wall. This notion has been in keeping with the information derived from epidemiological studies indicating an inverse relationship between low plasma HDL cholesterol levels and coronary disease. Subsequent structural-functional studies have drawn attention to the critical role played by the unique helical make up of the two constitutive protein components of HDL, ApoA-I and ApoA-II. In particular, ApoA-I has been the most abundant and most extensively studied of the two, having besides its ready tendency to undergo delipidation/relipidation both in vitro and in vivo, the ability to interact with the ATP-binding cassette transporter ABC1 and the SR-B1 hepatic receptor as well as activate LCAT. Besides these lipid-related functions, apoA-I has gained increasing recognition for its antioxidant and antiinflammatory properties and even as a modulator of innate immunity. With the introduction of advanced MS techniques, quantitatively ∼100 “minor” proteins, some related and some unrelated to lipoprotein metabolism, have been identified in addition to special HDL associated lysosphingolipids with an atheroprotective role. We have now come to view the various subclasses of HDL as “dynamic platforms” harboring at their surface, proteins/peptides, mobilized from tissues likely via the ubiquitous ATP-binding cassette transporters Fig. 5. We have also come to recognize that in the presence of systemic inflammatory settings, apolipoproteins and lipids may lose their atheroprotective role so that, for instance, high plasma levels of HDL rather than protective may be proatherogenic. Conversely, low plasma levels such as in familial hypoalphalipoproteinemia, the loss of protectiveness will result in a proatherogenic profile. From the therapeutic viewpoint, we have seen the promising entry into the clinical arena of ApoA-I mimetic peptides properly designed to favor the redistribution of HDL into atheroprotective prebeta migrating species. Clearly, the HDL field is ripe for systems biology studies in which interdisciplinary approaches lead to a clarification of the many unknowns that still surround this complex lipoprotein class.
Figure 5.
HDL viewed as a platform for accepting proteins and peptides either related or unrelated to lipoprotein metabolism from various cells. Recent proteomic studies have uncovered the unsuspected presence in mature HDL of several quantitatively minor components that are involved in inflammation, complement regulation, innate immunity, and proteinase inhibition along with peptides and fragments, some with an unknown function. These new findings raise a number of questions regarding the pathobiology of HDL, the mode of association of these elements to the HDL surface, and the dependence of this association on their function.
FUTURE DIRECTIONS
As expected, the wealth of new findings on HDL has opened new areas of exploration. One of them ought to be directed at gaining a better insight into apoA-II, a mysterious apolipoprotein still surrounded by uncertainties regarding its function either atheroprotective or proatherogenic. Moreover, better standardization of the techniques for HDL isolation and purification must be established to avoid confounders in the interpretation of MS data and also to permit meaningful comparisons among laboratories both in normal and pathological situations. It would also be critical to determine the nature of the interactions of the proteins/peptides associated with the HDL surface and the dependence that this association plays on their function. Of equal importance would be to compare in vivo samples from the same subject, the HDL proteome both in the plasma and the atheromatous plaque, in order to assess the effect that a different environmental condition may play on the stability and function of the chosen parameter. The promising results obtained with apoA-I mimetics should be expanded in order to generate additional ones patterned not only to amphipathic apoA-I but also to other apolipoproteins and nonapolipoprotein amphiphiles. Since therapy will be the ultimate target of these studies, the safety of the mimetic peptides must be assessed in suitable animal models. Finally, the lipid signaling field should benefit by the application of lipidomic techniques directed at the identification of new molecules isolated from HDL from physiological and pathological situations.
Acknowledgments
We thank Dr. Trudy Forte for providing the electron micrographs. We also acknowledge the support from the National Heart, Lung, and Blood Institute, National Institutes of Health grant HL 63209.
References
- Gofman J W, Lindgren F T, Elliott H. Ultracentrifugal studies of lipoproteins of human serum. J Biol Chem. 1949;179:973–979. [PubMed] [Google Scholar]
- Scanu A, Schiano N. Method of continuous ether extraction of lipids applied to the study of serum lipoproteins. I. Normal human sera. Riv Ist Sieroter Ital. 1954;29:276. [PubMed] [Google Scholar]
- Scanu A, Lewis L A, Bumpus F M. Separation and characterization of the protein moiety of human alpha-lipoprotein. Arch Biochem Biophys. 1958;74:390–397. doi: 10.1016/0003-9861(58)90009-2. [DOI] [PubMed] [Google Scholar]
- Scanu A. Studies on the conformation of human serum high-density lipoproteins HDL2 and HDL3. Proc Natl Acad Sci. 1965;54:1699–1705. doi: 10.1073/pnas.54.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A, Hirz R. On the structure of human serum high-density lipoprotein: studies by the technique of circular dichroism. Proc Natl Acad Sci. 1968;59:890–894. doi: 10.1073/pnas.59.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A, Granda J L. Comparative optical properties of human serum low- and high-density lipoproteins before and after delipidation. Progr Biochem Pharmacol. 1968;4:153. [Google Scholar]
- Scanu A, Hughes W L. Recombining capacity toward lipids of the protein moiety of human serum α1-lipoprotein. J Biol Chem. 1960;235:2876–2883. [PubMed] [Google Scholar]
- Scanu A M, Wisdom C. Serum lipoproteins structure and function. Annu Rev Biochem. 1972;41:703–730. doi: 10.1146/annurev.bi.41.070172.003415. [DOI] [PubMed] [Google Scholar]
- Scanu A, Cump E, Toth J, Koga S, Stiller E, Albers L. Degradation and reassembly of a human serum high-density lipoprotein. Evidence for differences in lipid affinity among three classes of polypeptide chains. Biochemistry. 1970;9:1327–1335. doi: 10.1021/bi00808a005. [DOI] [PubMed] [Google Scholar]
- Lagocki P A, Scanu A M. In vitro modulation of the apolipoprotein composition of high density lipoprotein. Displacement of apolipoprotein A-I from high density lipoprotein by apolipoprotein A-II. J Biol Chem. 1980;255:3701–3706. [PubMed] [Google Scholar]
- Edelstein C, Halari M, Scanu A M. On the mechanism of the displacement of apolipoprotein A-I by apolipoprotein A-II from the high density lipoprotein surface. Effect of concentration and molecular forms of apolipoprotein A-II. J Biol Chem. 1982;257:7189–7195. [PubMed] [Google Scholar]
- Scanu A M, Edelstein C, Gordon J I. Apolipoproteins of human plasma high density lipoproteins. Biology, biochemistry and clinical significance. Clin Physiol Biochem. 1984;2:111–122. [PubMed] [Google Scholar]
- Brewer H B, Fairwell T, LaRue A, Ronan R, Houser A, Bronzert T J. The amino acid sequence of human apoA-I, an apolipoprotein isolated from high density lipoproteins. Biochem Biophys Res Commun. 1978;80:623–630. doi: 10.1016/0006-291x(78)91614-5. [DOI] [PubMed] [Google Scholar]
- Shoulders C C, Kornblihtt A R, Munro B S, Baralle F E. Gene structure of human apolipoprotein Al. Nucleic Acids Res. 1983;11:2827–2837. doi: 10.1093/nar/11.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J P, Jones M K, De Loof H, Brouillette C G, Venkatachalapathi Y V, Anantharamaiah G M. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- Shen B W, Scanu A M. Properties of human apolipoprotein A-I at the air-water interface. Biochemistry. 1980;19:3643–3650. doi: 10.1021/bi00557a001. [DOI] [PubMed] [Google Scholar]
- Shen B W. Lipid-protein interaction at solid-water interface. Adsorption of human apo-high density lipoprotein to amphiphilic interfaces. J Biol Chem. 1985;260:1032–1039. [PubMed] [Google Scholar]
- Krebs K E, Ibdah J A, Phillips M C. A comparison of the surface activities of human apolipoproteins A-I and A-II at the air/water interface. Biochim Biophys Acta. 1988;959:229–237. doi: 10.1016/0005-2760(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Ibdah J A, Krebs K E, Phillips M C. The surface properties of apolipoproteins A-I and A-II at the lipid/water interface. Biochim Biophys Acta. 1989;1004:300–308. doi: 10.1016/0005-2760(89)90077-5. [DOI] [PubMed] [Google Scholar]
- Brouillette C G, Anantharamaiah G M, Engler J A, Borhani D W. Structural models of human apolipoprotein A-I: a critical analysis and review. Biochim Biophys Acta. 2001;1531:4–46. doi: 10.1016/s1388-1981(01)00081-6. [DOI] [PubMed] [Google Scholar]
- Saito H, Lund-Katz S, Phillips M C. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Progr Lipid Res. 2004;43:350–380. doi: 10.1016/j.plipres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Edelstein C, Scanu A M. Effect of guanidine hydrochloride on the hydrodynamic and thermodynamic properties of human apolipoprotein A-I in solution. J Biol Chem. 1980;255:5747–5754. [PubMed] [Google Scholar]
- Vitello L B, Scanu A M. Studies on human serum high density lipoproteins. Self-association of apolipoprotein A-I in aqueous solutions. J Biol Chem. 1976;251:1131–1136. [PubMed] [Google Scholar]
- Ajees A A, Anantharamaiah G M, Mishra V K, Hussain M M, Murthy H M K. Crystal structure of human apolipoprotein A-I: insights into its protective effect against cardiovascular diseases. Proc Natl Acad Sci. 2006;103:2126–2131. doi: 10.1073/pnas.0506877103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jonas A. Reconstitution of high density lipoproteins. Methods Enzymol. 1986;128:553–567. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- Davidson W S, Thompson T B. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- Segrest J P, Jones M K, Klon A E, Sheldahl C J, Hellinger M, De Loof H, Harvey S C. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J Biol Chem. 1999;274:31755–31758. doi: 10.1074/jbc.274.45.31755. [DOI] [PubMed] [Google Scholar]
- Koppaka V, Silvestro L, Engler J A, Brouillette C G, Axelsen P H. The structure of human lipoprotein A-I. Evidence for the “belt” model. J Biol Chem. 1999;274:14541–14544. doi: 10.1074/jbc.274.21.14541. [DOI] [PubMed] [Google Scholar]
- Li Y, Kijac A Z, Sligar S G, Rienstra C M. Structural analysis of nanoscale self-assembled discoidal lipid bilayers by solid-state NMR spectroscopy. Biophys J. 2006;91:3819–3828. doi: 10.1529/biophysj.106.087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S, Sorci-Thomas M G, Alexander E T, Samuel M P, Thomas M J. Intermolecular contact between globular N-terminal fold and C-terminal domain of apoA-I stabilizes its lipid-bound conformation: studies employing chemical cross-linking and mass spectrometry. J Biol Chem. 2005;280:33015–33025. doi: 10.1074/jbc.M505081200. [DOI] [PubMed] [Google Scholar]
- Ritter M C, Scanu A M. Role of apolipoprotein A-I in the structure of human serum high density lipoproteins. Reconstitution studies. J Biol Chem. 1977;252:1208–1216. [PubMed] [Google Scholar]
- Jonas A, Kezdy K E, Williams M I, Rye K A. Lipid transfers between reconstituted high density lipoprotein complexes and low density lipoproteins: effects of plasma protein factors. J Lipid Res. 1988;29:1349–1357. [PubMed] [Google Scholar]
- Sparks D L, Davidson W S, Lund-Katz S, Phillips M C. Effects of the neutral lipid content of high density lipoprotein on apolipoprotein A-I structure and particle stability. J Biol Chem. 1995;270:26910–26917. doi: 10.1074/jbc.270.45.26910. [DOI] [PubMed] [Google Scholar]
- Li H H, Lyles D S, Pan W, Alexander E, Thomas M J, Sorci-Thomas M G. ApoA-I structure on discs and spheres. Variable helix registry and conformational states. J Biol Chem. 2002;277:39093–39101. doi: 10.1074/jbc.M206770200. [DOI] [PubMed] [Google Scholar]
- Jonas A, Wald J H, Toohill K L, Krul E S, Kezdy K E. Apolipoprotein A-I structure and lipid properties in homogeneous, reconstituted spherical and discoidal high density lipoproteins. J Biol Chem. 1990;265:22123–22129. [PubMed] [Google Scholar]
- Brewer H B, Lux S E, Ronan R, John K M. Amino acid sequence of human apolp-gln-II (apoA-II), an apolipoprotein isolated from the high-density lipoprotein complex. Proc Natl Acad Sci. 1972;69:1304–1308. doi: 10.1073/pnas.69.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitello L B, Scanu A M. Studies on human serum high-density lipoproteins. Self-association of human serum apolipoprotein A-II in aqueous solutions. Biochemistry. 1976;15:1161–1165. doi: 10.1021/bi00650a031. [DOI] [PubMed] [Google Scholar]
- Teng T L, Barbeau D L, Scanu A M. Sedimentation behavior of native and reduced apolipoprotein A-II from human high density lipoproteins. Biochemistry. 1978;17:17–21. doi: 10.1021/bi00594a003. [DOI] [PubMed] [Google Scholar]
- Barbeau D L, Teng T L, Scanu M. The self-association of apolipoprotein A-II from plasma high density lipoproteins of rhesus monkey (Macaca mulatta) J Biol Chem. 1977;252:6745–6749. [PubMed] [Google Scholar]
- Gursky O, Atkinson D. High- and low-temperature unfolding of human high-density apolipoprotein A-2. Protein Sci. 1996;5:1874–1882. doi: 10.1002/pro.5560050913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M S, Carson M, Hussain M M, Murthy H M K. Structures of apolipoprotein A-II and a lipid-surrogate complex provide insights into apolipoprotein-lipid interactions. Biochemistry. 2002;41:11681–11691. doi: 10.1021/bi026069w. [DOI] [PubMed] [Google Scholar]
- Scanu A M, Edelstein C, Wolf R H. Chimpanzee (pan troglodytes) serum high density lipoproteins: isolation and properties of their two major apolipoproteins. Biochim Biophys Acta. 1974;351:341–347. doi: 10.1016/0005-2795(74)90197-4. [DOI] [PubMed] [Google Scholar]
- Edelstein C, Lim C T, Scanu A M. The serum high density lipoproteins of Macacus rhesus. II. Isolation, purification, and characterization of their two major polypeptides. J Biol Chem. 1973;248:7653–7660. [PubMed] [Google Scholar]
- Scanu A M. Polypeptide A-II of serum high density lipoproteins: a lipophilic protein of phylogenetic interest. PAABS Revista. 1975;4:1–5. [Google Scholar]
- Massey J B, Gotto A M, Pownall H J. Thermodynamics of lipid-protein association: enthalpy of association of apolipoprotein A-II with dimyristoylphosphatidylcholine-cholesterol mixtures. Biochim Biophys Acta. 1984;794:137–141. doi: 10.1016/0005-2760(84)90306-0. [DOI] [PubMed] [Google Scholar]
- Massey J B, Gotto A M, Jr, Pownall H J. Dynamics of lipid-protein interactions. Interaction of apolipoprotein A-II from human plasma high density lipoproteins with dimyristoylphosphatidylcholine. J Biol Chem. 1980;255:10167–10173. [PubMed] [Google Scholar]
- Silva R A G D, Schneeweis L A, Krishnan S C, Zhang X, Axelsen P H, Davidson W S. The structure of apolipoprotein A-II in discoidal high density lipoproteins. J Biol Chem. 2007;282:9713–9721. doi: 10.1074/jbc.M610380200. [DOI] [PubMed] [Google Scholar]
- Ritter M C, Scanu A M. Apolipoprotein A-II and structure of human serum high density lipoproteins. An approach by reassembly techniques. J Biol Chem. 1979;254:2517–2525. [PubMed] [Google Scholar]
- Durbin D M, Jonas A. Lipid-free apolipoproteins A-I and A-II promote remodeling of reconstituted high density lipoproteins and alter their reactivity with lecithin:cholesterol acyltransferase. J Lipid Res. 1999;40:2293–2302. [PubMed] [Google Scholar]
- Clay M A, Pyle D H, Rye K A, Barter P J. Formation of spherical, reconstituted high density lipoproteins containing both apolipoproteins A-I and A-II is mediated by lecithin:cholesterol acyltransferase. J Biol Chem. 2000;275:9019–9025. doi: 10.1074/jbc.275.12.9019. [DOI] [PubMed] [Google Scholar]
- Edelstein C, Lim C T, Scanu A M. On the subunit structure of the protein of human serum high density lipoprotein. I. A study of its major polypeptide component (Sephadex, fraction III) J Biol Chem. 1972;247:5842–5849. [PubMed] [Google Scholar]
- Scanu A M, Lim C T, Edelstein C. On the subunit structure of the protein of human serum high density lipoprotein. II. A study of Sephadex fraction IV. J Biol Chem. 1972;247:5850–5855. [PubMed] [Google Scholar]
- Rye K A, Wee K, Curtiss L K, Bonnet D J, Barter P J. Apolipoprotein A-II inhibits high density lipoprotein remodeling and lipid-poor apolipoprotein A-I formation. J Biol Chem. 2003;278:22530–22536. doi: 10.1074/jbc.M213250200. [DOI] [PubMed] [Google Scholar]
- Moore R E, Kawashiri M A, Kitajima K, Secreto A, Millar J S, Pratico D, Rader D J. Apolipoprotein A-I deficiency results in markedly increased atherosclerosis in mice lacking the LDL receptor. Arterioscler Thromb Vasc Biol. 2003;23:1914–1920. doi: 10.1161/01.ATV.0000092328.66882.F5. [DOI] [PubMed] [Google Scholar]
- Benoit P P, Emmanuel F P, Caillaud J M M D, Bassinet L, Castro G P, Gallix P D V M, Fruchart J C P, Branellec D P, Denefle P P, Duverger N P. Somatic gene transfer of human apoA-I inhibits atherosclerosis progression in mouse models. Circulation. 1999;99:105–110. doi: 10.1161/01.cir.99.1.105. [DOI] [PubMed] [Google Scholar]
- Nissen S E, Tsunoda T, Tuzcu E M, Schoenhagen P, Cooper C J, Yasin M, Eaton G M, Lauer M A, Sheldon W S, Grines C L, Halpern S, Crowe T, Blankenship J C, Kerensky R. Effect of recombinant apoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- Anantharamaiah G M, Mishra V K, Garber D W, Datta G, Handattu S P, Palgunachari M N, Chaddha M, Navab M, Reddy S T, Segrest J P, Fogelman A M. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J Lipid Res. 2007;48:1915–1923. doi: 10.1194/jlr.R700010-JLR200. [DOI] [PubMed] [Google Scholar]
- Fukushima D, Kupferberg J P, Yokoyama S, Kroon D J, Kaiser E T, Kezdy F J. A synthetic amphiphilic helical docosapeptide with the surface properties of plasma apolipoprotein A-I. J Am Chem Soc. 1979;101:3703–3704. [Google Scholar]
- Yokoyama S, Fukushima D, Kupferberg J P, Kezdy F J, Kaiser E T. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J Biol Chem. 1980;255:7333–7339. [PubMed] [Google Scholar]
- Bloedon L T, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot B J, Movva R, Navab M, Fogelman A M, Rader D J. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah G M, Reddy S T, Fogelman A M. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat Clin Pract. 2006;3:540–547. doi: 10.1038/ncpcardio0661. [DOI] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah G M, Reddy S T, Hama S, Hough G, Grijalva V R, Wagner A C, Frank J S, Datta G, Garber D, Fogelman A M. Oral D-4F causes formation of pre-{beta} high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- Blanco-Vaca F, Escola-Gil J C, Martin-Campos J M, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J Lipid Res. 2001;42:1727–1739. [PubMed] [Google Scholar]
- Weng W, Breslow J L. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proc Natl Acad Sci. 1996;93:14788–14794. doi: 10.1073/pnas.93.25.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani L W, Goto A M, Lusis A J. Studies with apolipoprotein A-II transgenic mice indicate a role for HDLs in adiposity and insulin resistance. Diabetes. 2001;50:643–651. doi: 10.2337/diabetes.50.3.643. [DOI] [PubMed] [Google Scholar]
- Castellani L W, Nguyen C N, Charugundla S, Weinstein M M, Doan C X, Blaner W S, Wongsiriroj N, Lusis A J. Apolipoprotein AII is a regulator of very low density lipoprotein metabolism and insulin resistance. J Biol Chem. 2008;283:11633–11644. doi: 10.1074/jbc.M708995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong E L, Stoltzfus L J, Brion C M, Murugesh D, Rubin E M. Contrasting in vivo effects of murine and human apolipoprotein A-II. J Biol Chem. 1996;271:5984–5987. doi: 10.1074/jbc.271.11.5984. [DOI] [PubMed] [Google Scholar]
- Van 't Hooft F M M D P, Ruotolo G M D P, Boquist S M D P, de Faire U M D P, Eggertsen G M D P, Hamsten A M D P. Human evidence that the apolipoprotein A-II gene is implicated in visceral fat accumulation and metabolism of triglyceride-rich lipoproteins. Circulation. 2001;104:1223–1228. doi: 10.1161/hc3601.095709. [DOI] [PubMed] [Google Scholar]
- Corella D, Arnett D K, Tsai M Y, Kabagambe E K, Peacock J M, Hixson J E, Straka R J, Province M, Lai C-Q, Parnell L D, Borecki I, Ordovas J M. The −256t>c polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem. 2007;53:1144–1152. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- Reilly M P, Tall A R. HDL proteomics: pot of gold or Pandora’s box? J Clin Invest. 2007;117:595–598. doi: 10.1172/JCI31608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortin G L, Shen R-F, Martin B M, Remaley A T. Diverse range of small peptides associated with high-density lipoprotein. Biochem Biophys Res Commun. 2006;340:909–915. doi: 10.1016/j.bbrc.2005.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlman M, Davidsson P, Kanmert I, Rosengren B, Boren J, Fagerberg B, Camejo G. Proteomics and lipids of lipoproteins isolated at low salt concentrations in D2O/sucrose or in KBr. J Lipid Res. 2008;49:481–490. doi: 10.1194/jlr.D700025-JLR200. [DOI] [PubMed] [Google Scholar]
- Dawson G, Kruski A W, Scanu A M. Distribution of glycosphingolipids in the serum lipoproteins of normal human subjects and patients with hypo- and hyperlipidemias. J Lipid Res. 1976;17:125–131. [PubMed] [Google Scholar]
- Hara A, Taketomi T. Occurrence of sulfatide as a major glycosphingolipid in WHHL rabbit serum lipoproteins. J Biochem. 1987;102:83–92. doi: 10.1093/oxfordjournals.jbchem.a122044. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. [miscellaneous article] Arterioscler Thromb Vasc Biol. 2003;23:1283–1288. doi: 10.1161/01.ATV.0000079011.67194.5A. [DOI] [PubMed] [Google Scholar]
- Porn M I, Akerman K E, Slotte J P. High-density lipoproteins induce a rapid and transient release of Ca2+ in cultured fibroblasts. Biochem J. 1991;279:29–33. doi: 10.1042/bj2790029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Jackson S P, Newnham H H, Mitchell C A, Salem H H. An essential role for lysophosphatidylcholine in the inhibition of platelet aggregation by secretory phospholipase A2. Blood. 1995;86:4166–4174. [PubMed] [Google Scholar]
- Zhang B, Tomura H, Kuwabara A, Kimura T, Miura S I, Noda K, Okajima F, Saku K. Correlation of high density lipoprotein (HDL)-associated sphingosine 1-phosphate with serum levels of HDL-cholesterol and apolipoproteins. Atherosclerosis. 2005;178:199–205. doi: 10.1016/j.atherosclerosis.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- Yang L, Yatomi Y, Miura Y, Satoh K, Ozaki Y. Metabolism and functional effects of sphingolipids in blood cells. Br J Haematol. 1999;107:282–293. doi: 10.1046/j.1365-2141.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostagland Other Lipid Mediat. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- Hanel P, Andreani P, Graler M H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab S K, Cornelissen I, Pereira J O P, Regard J B, Ying X, Camerer E, Yao-Wu Z, Yong H, Cyster J G, Coughlin S R. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Argraves K M, Argraves W S. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–2333. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- Deigner H-P A, Hermetter A B. Oxidized phospholipids: emerging lipid mediators in pathophysiology. Curr Opin Lipidol. 2008;19:289–294. doi: 10.1097/MOL.0b013e3282fe1d0e. [DOI] [PubMed] [Google Scholar]
- Gharavi N M, Gargalovic P S, Chang I, Araujo J A, Clark M J, Szeto W L, Watson A D, Lusis A J, Berliner J A. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. [miscellaneous article] Arterioscler Thromb Vasc Biol. 2007;27:1346–1353. doi: 10.1161/ATVBAHA.107.141283. [DOI] [PubMed] [Google Scholar]
- Shao B, Heinecke J W, Enrique C, Lester P. Using tandem mass spectrometry to quantify site-specific chlorination and nitration of proteins: model system studies with high density lipoprotein oxidized by myeloperoxidase. Methods Enzymol. 2008;440:33–63. doi: 10.1016/S0076-6879(07)00803-8. [DOI] [PubMed] [Google Scholar]
- Zheng L, Settle M, Brubaker G, Schmitt D, Hazen S L, Smith J D, Kinter M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in abca1-dependent cholesterol efflux from macrophages. J Biol Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Gantz D L, Gursky O. Effects of protein oxidation on the structure and stability of model discoidal high-density lipoproteins. Biochemistry. 2008;47:3875–3882. doi: 10.1021/bi7023783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergt C, Pennathur S, Fu X, Byun J, O'Brien K, McDonald T O, Singh P, Anantharamaiah G M, Chait A, Brunzell J, Geary R L, Oram J F, Heinecke J W. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs abca1-dependent cholesterol transport. Proc Natl Acad Sci. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Nukuna B, Brennan M-L, Mingjiang S, Goormastic M, Settle M, Schmitt D, Xiaoming F, Thomson L, Fox P L, Ischiropoulos H, Smith J D, Kinter M, Hazen S L. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang A, Murphy A, Coughlan M, Thomas M, Forbes J, O'Brien R, Cooper M, Chin-Dusting J, Sviridov D. Advanced glycation of apolipoprotein A-I impairs its anti-atherogenic properties. Diabetologia. 2007;50:1770–1779. doi: 10.1007/s00125-007-0718-9. [DOI] [PubMed] [Google Scholar]
- Anantharamaiah G M, Hughes T A, Iqbal M, Gawish A, Neame P J, Medley M F, Segrest J P. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988;29:309–318. [PubMed] [Google Scholar]
- Gursky O, Atkinson D. Thermal unfolding of human high-density apolipoprotein A-1: implications for a lipid-free molten globular state. Proc Natl Acad Sci. 1996;93:2991–2995. doi: 10.1073/pnas.93.7.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagotopulos S E, Horace E M, Maiorano J N, Davidson W S. Apolipoprotein A-I adopts a belt-like orientation in reconstituted high density lipoproteins. J Biol Chem. 2001;276:42965–42970. doi: 10.1074/jbc.M106462200. [DOI] [PubMed] [Google Scholar]
- Rye K A, Hime N J, Barter P. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J Biol Chem. 1997;272:3953–3960. doi: 10.1074/jbc.272.7.3953. [DOI] [PubMed] [Google Scholar]
- Rye K A, Barter P J. The influence of apolipoproteins on the structure and function of spheroidal, reconstituted high density lipoproteins. J Biol Chem. 1994;269:10298–10303. [PubMed] [Google Scholar]