Abstract
The internal state of an individual—as it relates to thirst, hunger, fear, or reproductive drive—can be inferred by referencing points on its movement path to external environmental and sociological variables. Using time-series approaches to characterize autocorrelative properties of step-length movements collated every 3 h for seven free-ranging African elephants, we examined the influence of social rank, predation risk, and seasonal variation in resource abundance on periodic properties of movement. The frequency domain methods of Fourier and wavelet analyses provide compact summaries of temporal autocorrelation and show both strong diurnal and seasonal based periodicities in the step-length time series. This autocorrelation is weaker during the wet season, indicating random movements are more common when ecological conditions are good. Periodograms of socially dominant individuals are consistent across seasons, whereas subordinate individuals show distinct differences diverging from that of dominants during the dry season. We link temporally localized statistical properties of movement to landscape features and find that diurnal movement correlation is more common within protected wildlife areas, and multiday movement correlations found among lower ranked individuals are typically outside of protected areas where predation risks are greatest. A frequency-related spatial analysis of movement-step lengths reveal that rest cycles related to the spatial distribution of critical resources (i.e., forage and water) are responsible for creating the observed patterns. Our approach generates unique information regarding the spatial-temporal interplay between environmental and individual characteristics, providing an original approach for understanding the movement ecology of individual animals and the spatial organization of animal populations.
Keywords: behavior, foraging, GPS data, predation, movement ecology
Characteristics of an animal's movement path offer insights into the external influences and internal states of an individual across time and space (1, 2). Scaled across a population or ecosystem, such information can offer new understanding of the salient factors driving the spatiotemporal structure of populations (3, 4). The methods used to garner this information have been the focus of research in the field of ecology for some time (5–7). Perhaps the most common of these methods involves analyzing correlations among consecutive displacements (i.e., step length or approximate velocity) and turning angles in the context of random walk models (4, 7). The strength of first-order autocorrelations in the directionality of movement has been used to categorize foraging strategy (8–10), whereas potential information contained in the autocorrelative structure of step lengths has largely been ignored. Given the existence of such structure in our data, as revealed by the analyses presented here, simple random walk models are clearly inadequate for characterizing movement in some animals. Our approach provides unique insight into the relationship between environmental factors and movement as influenced by the internal states and movement and navigational capacities of an organism, the connections between which form the foundation of the movement ecology framework in this volume (2). In this article, we identify salient environmental factors influencing the spatial-temporal movement patterns of elephants on the landscape in and around Samburu National Reserve, Kenya.
The role of autocorrelation, at different temporal scales, in the movement pathways of animals is an important understudied phenomenon that is critical for predictive modeling of population spatial properties (10, 11). Treating cyclic (strongly repetitive) patterns of movement as behavioral signals can offer new insight into the factors impacting population organization and the spatial ecology of animals (12). Relating these movement cycles, detectable through autocorrelation in step lengths or other movement properties, to the ecological context in which they occur can provide insights into the internal states of individuals and serve to identify salient motivations of recognizable canonical activity modes (e.g., foraging vs. heading for water) (1, 2). Animal biology (e.g., gut retention times) or the organization of landscape features (e.g., the spatial relationship between water, forage, and refuges) may drive repetitive movement behaviors, such as drinking water during the midday heat or foraging before rest periods. However, movement modes as reflected by changes in the significant frequencies of the periodogram explaining movement variation or shifts between temporally independent and dependent periods may represent switches from one behavioral mode to another. As such, autocorrelation, or lack thereof, in movement properties along pathways can serve as a metric for the definition of canonical activity modes themselves (1, 2), enhancing our ability to understand the factors impacting spatial behavior.
Here, we explore the autocorrelative properties in GPS (global positioning system)-generated 3-hourly step-length sequences over a 6-month period for seven wild African elephants (comparable results using 1 h data for five of these seven elephants are presented in supporting information (SI) Text). We calculate estimates of Fourier spectrums (periodograms) to compactly characterize different scales of temporal dependence. Because the displacements in stepwise elephant movements are potentially nonstationary (i.e., the dominant frequencies explaining most of the movement may change or disappear), periodograms provide good but preliminary insight into autocorrelative properties of elephant movements. We build on these findings using wavelet analysis, which allows temporal identification (localization) of different movement signatures across all temporal scales within a dataset. Bootstrap significance tests are applied to define periods where the complexity of the autocorrelative signal exceeds a first-order autoregressive model fit to the data, a conservative model of temporal dependence. To determine the formative drivers of different movement modes identified from autocorrelative properties in 3 h step lengths, we (i) compare time-specific step-length distributions during periods of movement dominated by specific frequencies, (ii) test the ability of simulated movement behaviors based on a simple diurnal rule to replicate these frequency spectrums, and (iii) relate localized, time-specific movement properties with ecological and social properties to gain understanding of the context of these different behaviors. For the latter, we invoke a comparative framework to identify salient internal and external features impacting the type of temporal correlation defining behavioral movement modes, testing the following three hypotheses.
H1: Autocorrelation in Movement Will Be Greater During the Dry Season than the Wet Season.
Seasonal changes in the distribution of resources (both forage and water) impact spatial structure, demography, and movement properties in a variety of organisms (4), including elephants (13). Autocorrelation in step lengths is likely to vary in relation to the spatial arrangement of resources, where the dynamics of repetitive interpatch or forage to water movements common during the dry season will differ from those when resources are more homogenous or the distance between water and forage is reduced. Therefore, autocorrelation in movements should be more pronounced during the dry season when the distribution of resources is predicted to elicit repetitive movement.
H2: Autocorrelation in Movement Is More Common in the Movement Patterns of Socially Dominant Individuals.
Interference competition, by eliciting spatial avoidance or range shifts, is likely to disrupt the ability of individuals to maintain repetitive cycles of behaviors (14, 15), decreasing autocorrelation in movements. Social rank serves as a proxy for the degree of interference competition experienced by an individual, where higher-ranking individuals are less influenced by such competitive interactions. As a result, the occurrence of repetitive cycles in movement is predicted to be more common among high-ranked individuals.
H3: Autocorrelation in Movement Decreases in Regions with Greater Predation Risk.
In landscapes preferred by predators or where contact with predators is more frequent, individuals may alter their movement behavior in response to increased risk (16, 17). Decreasing the predictability of movements (i.e., less autocorrelation) serves as antipredator behavior in areas of increased risk (18). In the study system and across their range, humans are the primary predator of elephants. Protected areas, where human access is limited, serve largely as predator exclusion zones. Consequently, the proportion of time devoted to cyclical movements is likely to increase within protected areas relative to communal regions outside protected areas.
Results
For movements of the study elephants analyzed at a sampling interval of 1 or 3 h, turning angles did not show any temporal autocorrelation, mirroring results from a previous study of elephant movement (19) that showed no significant autocorrelation in this metric at lags >15 min. In contrast, analysis of step lengths using traditional time-domain autoregressive AR(p) model selection based on Akaike's Information Criterion chose p between 24 and 27 for the 3 h sampled data, depending on the individual, but offered little insight to differentiation in movements among individuals and across time. Analyses using the signal processing methods of Fourier and wavelet analysis revealed complex and nonstationary autocorrelation in the step lengths of these elephants.
Periodograms (representation of the relative contribution of different Fourier spectral frequencies to the temporal structure of the times series) revealed strong diurnal cycles (frequencies with one or more cycles per day) in the movement step lengths of all seven elephants that were clearly different from theoretical red-noise spectral functions (Figs. S1 and S2). Scalograms (approximations of the true wavelet spectrum) (20, 21) show that the type or existence of autocorrelation was not continuous across time in the movements of any of the seven elephants, with substantial heterogeneity in the type (frequency) of autocorrelation present in movement pathways (Fig. 1 and Figs. S3 and S4), thereby providing structure within and across individuals that can be linked to ecological and social factors. Isolating diurnal displacement patterns through analysis of the distribution of step lengths associated with the two dominant peaks in Fourier spectrums, at frequencies of one and two cycles per day, demonstrates the occurrence of such autocorrelation movement structure is a function of phases of resting and directional movement (Fig. S5). Movement differences between time periods, including significant frequencies in the scalogram and those lacking significant frequencies (i.e., those with no temporal autocorrelation), appear to be a function of the number and duration of discrete rest periods and the distances moved during morning and afternoon activity bouts. The number of rest periods as an explanatory hypothesis for these statistical signatures was further supported by a movement simulation study, the basic result of which shows that similar spectral signatures emerge for movement activity containing one or two rest periods (see SI Text and Figs. S6 and S7). Building on these insights, we test hypotheses regarding the environmental factors eliciting such movement behavior.
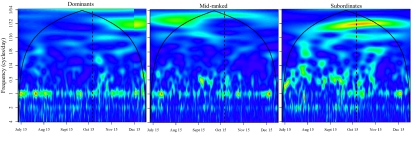
Fig. 1.
Averaged modulus values of wavelet scalograms of 3-hourly net displacement time series from three dominant, two midranked, and two subordinate elephants, normalized so that larger values (stronger fit) correspond to warmer colors (values of 1 are given a red color) and smaller values correspond to cooler colors (values of zero are purple) (see Figs. S3 and S4 for individual scalograms, the latter pertaining to 1-hourly data). The solid line denotes the cone of influence outside of which modulus values are affected by zero padding and should not be considered; the vertical dashed line gives the approximate date of transition from the dry to wet season as described in the text. The temporal heterogeneity in the frequency distribution of modulus values is greatest among subordinate elephants (warmer-colored regions are more common in lower-frequency sections of the scalogram), with midrank elephants showing greater heterogeneity than dominants. The heterogeneity dampens in the latter part of the study (wet season), when cyclical properties of movements across all individuals converge to the primarily diurnal patterns (cooler colors predominate in low-frequency areas of the scalogram) characteristic of dominants regardless of season (Fig. S1).
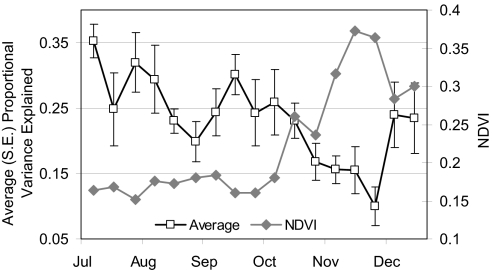
The relationship between ecosystem level processes and the timing of synchronized periods (across all individuals) with and without strong autocorrelation provides information on macroscale drivers of animal movement behaviors. To address H1, a time-specific metric of the relative strength of autocorrelation in the study population's movements was derived as the proportion of variance in movement explained by autocorrelation (see Methods) during time periods corresponding to Normalized Difference Vegetation Index (NDVI) sampling intervals (Table S1). Comparing the relative strength of autocorrelation, averaged across the seven elephants, against ecosystem variation in primary productivity (NDVI) demonstrated the relative strength of autocorrelation was significantly negatively correlated with ecosystem productivity (rs = −0.789, P < 0.001). Thus, in support of H1, movement autocorrelation was strongest in the studied population during the dry season, whereas random movement across all temporal scales tended to coincide with the NDVI peak reflecting the period when resources (water and forage) are abundant and more homogenously distributed across the ecosystem (Fig. 2).
Fig. 2.
The average (across all seven elephants) proportion (error bars represent standard errors) of variance in movements explained by autocorrelation (black squares) was significantly negatively correlated (rs = −0.789, P < 0.001) with NDVI (gray diamonds), showing that periods of the strongest cyclical movement occurred during the dry season when resource quality is relatively poor and water is limited. Periods when movements tended to be more random occurred during the wet season when resources are abundant.
Comparison of periodograms calculated separately on movements occurring during the wet and dry season (a likely break point in the statistical stationarity of movements), where the seasonal transition was defined using remotely sensed NDVI data, depicted further evidence of seasonal behavioral shifts in movements. All elephants demonstrated similar cyclical movement characteristics during the wet season, when the frequency with the most explanatory power occurred at one cycle per day in addition to significant periodogram peaks at frequencies of two and three cycles per day, although with decreasing power (Figs. S1 and S2). Significant frequencies and relative powers at each frequency, however, differed across individuals during the dry season (Table 1 and Figs. S1 and S2). The three dominant individuals showed the same temporal dependence in movement characteristics for both the dry and wet seasons, whereas the dominant frequency in movements of three of the four subordinates shifted across seasons from two cycles per day to one cycle per day.
Table 1.
Results from periodograms of movement data presented across seasons
| Rank | ID | Dry season cycle/day | Wet season cycle/day |
|---|---|---|---|
| High | M54 | 1 (2, 3) | 1 (2, 3) |
| High | M5 | 1 (2, 3) | 1 (2, 3) |
| High | R28 | 1 (2, 3) | 1 (2, 3) |
| Mid | R22 | 1 (2, 3) | 1 (2, 3) |
| Mid | M31 | 2 (1, 3) | 1 (2, 3) |
| Low | R37 | 2 | 1 (2, 3) |
| Low | M46 | 2 | 1 (2, 3) |
Findings show the dominant frequency (cycles per day) of autocorrelated signals with significant but less powerful frequencies in parentheses. The structure of cyclic movements of dominant individuals does not differ across season, showing the form of cyclical movements is not impacted by variation in resource abundance for this group of individuals. Periodograms of subordinate individuals differ from that of dominants in the dry season but converge to the same pattern as dominant individuals during the wet season (see Fig. S1).
Analyses of individual autocorrelative signals offer insights into the influence of individualistic social and location-specific properties on movements necessary for the evaluation of H2 and H3. Differences in the proportion of time spent conducting cyclical movements did not relate to rank as posited in H2; however, results indicate the frequency spectrum varied in relation to social rank. Wavelet analyses were performed to improve detection of autocorrelative differences across individuals. Specifically, low-frequency autocorrelation generally becomes more prevalent in individuals lower down the social rank hierarchy, whereas dominant individuals did not demonstrate low-frequency cyclical movements (Fig. 1 and Figs. S3 and S4). Diurnal cycling was most consistent among dominant individuals (Figs. S1 and S2). However, the autocorrelative patterns of the studied elephants converged to similar diurnal cyclic patterns during the wet season when cooler colors in the spectrogram are apparent in lower-frequency regions across all elephants (Fig. 1 and Figs. S3 and S4), analogous to the convergence demonstrated in seasonal periodograms (Fig. S1).
Overlaying wavelet results in a geographic information system (GIS) allowed evaluation of the relationships between the presence of autocorrelative movement properties and landscape features in the study ecosystem (Fig. 3). The strength and type of dominant frequencies varied in relation to the protected status (i.e., degree of risk) of an individual's location in support of H3 (Fig. 4). During the dry season, the proportion of time scalogram coefficients were significant at diurnal frequencies (one or more cycles per day) within protected areas was greater than the proportion of time outside protected areas (Table S2; Wilcoxon signed rank Z = −12, P = 0.023). In contrast, the proportion of time spent in cyclical movements in the low-frequency range (less than one cycle per day) was greater outside than inside protected areas, although not significantly (Z = 3, P = 0.125). However, only the three lowest-ranking individuals conduct multiday cycling, limiting the power of this analysis (Table S2). Movement property differences in relation to protected areas were not statistically supported during the wet season (Fig. 4). In general, diurnal cycles in movements were more common than multiday cycles across individuals, seasons, and geographic locations.
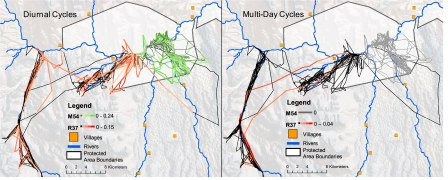
Fig. 3.
The frequencies and power of cyclical movements in elephants varied in relation to location during the dry season when interspecific conflict over resources is greatest. Movements outside protected areas in pastoralist lands are hypothesized to be disrupted by risk of conflict or predation by humans. The movements of a subordinate elephant, R37 (black equates to paths with no significant autocorrelation and red shades are color scaled by strength of wavelet fit), show her diurnal movement cycles (Left) were more common within protected areas, whereas her multiday cycles (Right) were more common outside protected areas (S2). A dominant elephant, M54 (gray equates to paths with no autocorrelation and green shades are scaled by strength of wavelet fit), rarely leaves the protected areas and demonstrates only diurnal cycling.
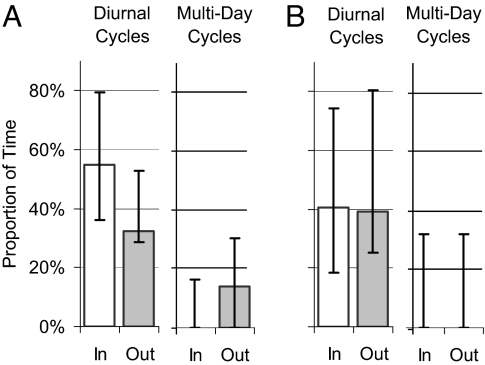
Fig. 4.
The median proportion of time (column with error bars representing minimum and maximum values) the study elephants conducted significant cyclical movements at different frequencies differed when within human-dominated communal areas or protected areas. (A) During the dry season when resources are limited, diurnal cyclical movements occurred predominantly within predator-free protected areas (Wilcoxon signed rank Z = −12, P = 0.023), whereas multiday cycles typically occurred in human-dominated regions outside protected areas, although not significantly (Z = 3, P = 0.125). (B) No differences were found during the wet season when resources are relatively abundant. Where columns are missing, median values were zero.
Discussion
Though classic movement modeling approaches incorporate first-order autocorrelative temporal dependence (4, 9), the quantification of more extensive temporal autocorrelation in patterns of movement has been neglected despite its significance for understanding and predicting the spatial use and behavior of animals. When ecological conditions are static, highly repetitive movements may offer the best utilization strategy where different requirements are fulfilled in a predictable manner (22, 23). Under such conditions, high degrees of autocorrelation in movements may reflect a risk reduction and energy conservation strategy. In contrast, when resources in an ecosystem vary dynamically or approach a uniform distribution, weak correlation in movement properties or a random search strategy may be optimal, as demonstrated for turning angles in simulation studies (24). In this analysis, we applied nonparametric time-series analysis methods (Fourier and wavelet analysis) to assess the relationships between autocorrelation in step lengths (i.e., cyclical movement behavior—not to be confused with looping behavior related to the geometry of pathways) and time- and context-specific sociological and ecological factors. Fourier and wavelet analysis provide statistical definition of autocorrelation in movement properties across all temporal scales in the data. Time series of elephant stepwise movements appear complex and strongly nonstationary, with dominant periods of autocorrelation occurring at one, two, and three cycles per day. Analysis of step-length distributions associated with significantly autocorrelated movements indicates that the relatively simple relationship between periods of rest and movement elicits these dominant frequency spectrum properties. This was verified by our ability to replicate periodogram and spectrogram characteristics using simulated movement data containing simplistic movement patterns constructed with once- or twice-a-day rest periods. The structure of movements is strongly influenced by seasonality in this study system and related to differences in individual social characteristics and landscape features associated with predation risk.
By testing for time-specific emergence of significant frequencies in periodograms, we identify autocorrelated versus random movement periods across all scales inherent in the movement data analyzed. Among the study elephants, the period with the least autocorrelation coincides with the wet season peak in NDVI, the period of highest primary productivity in the ecosystem (Fig. 2). This dampening of movement autocorrelation when resources are good indicates nonrandom movements are associated, at least partially, with foraging strategies when resources (both food and water) are limited or heterogeneity in their distribution is strongest, as predicted in models of optimal foraging strategies (24). Elephants visited permanent water a significantly greater proportion of days during the dry than the wet season (Table S3), probably on account of widely available, temporary wet-season pools that strongly alter the spatial proximity of water to forage. The relationship between the occurrence of midday rests and the distances traveled during morning and afternoon movement bouts are critical determinants of autocorrelative properties in the study system—behaviors most likely influenced by peak midday temperatures (impacting rest) and a function of the spatial distribution of water and forage.
Intraspecific competitive interactions were hypothesized to inhibit adherence to specific repetitive behavioral strategies, resulting in weaker autocorrelation among subordinate individuals relative to dominants. Surprisingly, all seven individuals were found to conduct significantly autocorrelated movements approximately half the time of the study (Table S4). Though our hypotheses were structured around the presence of cyclical movement behavior or the lack thereof, our results indicate the frequency spectrum varied in relation to social rank rather than the proportion of time spent conducting cyclical movements. We found dominant individuals maintained similar cyclical movement patterns across the study period, whereas subordinates demonstrated greater heterogeneity in the frequency and timing of such movements (Fig. 1). Seasonal variation in resource abundance impacted movement autocorrelation among the subordinate individuals, which demonstrated short twice-daily and long multiday autocorrelative signatures during the dry season, but converged to the primarily diurnal patterns matching those of dominants during the wet season when resource competition is weakest. Previous analyses on the relationship between daily travel distances and social rank (13) showed dominants travel approximately half the distance of subordinates during the dry season when rank-related differences in frequency spectrums are prevalent, but travel distances are comparable across ranks during the wet season when temporal dependence in movement is statistically similar. As such, energy expenditure and conservation in relation to forage quality and quantity appears to be a major factor driving shifts in cyclical movement strategies.
In addition to sociality and seasonal changes in resource distribution, we found that landscape properties influence autocorrelative properties of movements. Results from the wavelet analysis that were used to quantify the proportion of time movements were significantly autocorrelated in respect to the protected area status of their location (i.e., where protective status is assumed to be synonymous with predation risk). Scale-specific differences were found in relation to location, with significant multiday frequencies occurring predominantly outside protected areas. The spatial location of movement cycling was influenced by season, with diurnal autocorrelation occurring more frequently within protected areas during the dry season but lacking location specificity during the wet season (Fig. 4 and Table S2). The studied elephants use an open ecosystem in which areas outside parks are communally managed pastoralist lands. Though vegetative communities in protected and unprotected areas are similar, human-elephant conflicts in this region are greatest outside protected areas during the dry season when competition over limited resources is high. Therefore, dry season differentiation in movement autocorrelation related to protective status of locations, and the lack thereof during the wet season, most likely reflects conflict avoidance behavior. Anecdotal evidence indicates multiday movements are a result of elephants shifting between use of different areas, possibly reflecting an attempt to access resources across disconnected areas while limiting overlap with humans and livestock. Low-frequency (multiday temporal autocorrelation) movement properties found only among socially low-ranking individuals (those most susceptible to competition with conspecifics) and in locations of enhanced predation/conflict risk (outside protected areas) appear to arise in response to potentially disruptive forces.
Breeding cycles, migration, and other rhythmic behaviors in natural systems are often driven by predictable phenomena such as circadian light patterns or variation in temperature (25). Similarly, the heterogeneity in cyclical movement behavior among the study elephants relates to different strategies driven by environmental constraints (e.g., water availability and temperature) and biotic factors (e.g., predation risk and competition). Though the data used in this analysis covered a short period relative to an elephant's life span, datasets covering longer-term life-history events (like annual migratory or reproductive activity) could allow identification of low-frequency or higher-scale autocorrelation behavior. As has been shown for elk (10) and turkey vultures (26), the influence of different factors on movement autocorrelation are likely to vary across scales. The methods used here provide unique insight into cyclic properties of movement that standard methods fail to identify. This opens research directions on movement behaviors of evolutionary or management importance that have largely been overlooked (e.g., cyclical intermittence in movements driven by difficult-to-identify behaviors such as reorientation search strategies) (27).
Identification of patterns of periodicity allows us to draw inferences about regularly occurring canonical activity modes, the factors driving mode switches and their relative importance in structuring the spatial properties of populations. In the context of the movement ecology framework presented in this volume (2), the motion capacity (realized here as periods of movement interspersed with consistent periods of rest) and navigation capacity (knowledge of the location of forage, water, and other critical resources) elicit patterns of autocorrelation in the movement of elephants that are influenced by the elephant's internal state (aversion to risk and social competition), which are a manifestation of the external factors in the ecosystem (the presence of humans and socially dominant conspecifics relative to the distribution of resource). These unique insights into the relationship between social and ecological factors structuring an elephant community show that elephants make context-specific decisions regarding the scale and timing of cyclical movements as a function of their foraging strategy, sociospatial processes, and landscape properties.
Methods
Study Population.
The study was conducted between July and December 2001 in the area in and around the 220 km2 Samburu and Buffalo Springs National Reserves in northern Kenya (37.5° E 0.5° N). This semiarid region is dominated by Acacia-Comiphora savanna and scrub bush, and permanent water is limited to a few springs and the Ewaso N′giro River. Rainfall averages ≈350 mm per year and occurs during biannual rainy seasons generally taking place in April/May and November/December. For purposes of the analyses presented here, seasonal transitions were defined using spatially explicit remotely sensed NDVI data, a longitudinal metric of vegetative productivity (28, 29), as described elsewhere (30).
The elephant population using these reserves, numbering ≈900 individuals, has been closely monitored since 1997. Movement data were collected using GPS collars fitted on seven female elephants from previously defined distinct family groups (31). The rank of the matriarch of each family, defined as the most dominant individual in a family group, was calculated from individually recorded agonistic interactions following established techniques described elsewhere (32). Ranks of these families were not known at the time of collaring. Though collared families differed in respect to their rank status within the population, they were of similar sizes (range: 9–13 individuals) and all led by mature matriarchs estimated to be over the age of 35 years (31). Although home ranges of all individuals overlapped, individuals were assumed to be independent for purposes of this analysis as a function of differences in their social groups. Individuals were radio collared by a Kenya Wildlife Service (KWS) veterinarian following the protocol established by KWS.
Statistical Analysis.
The movement data from the seven focal individuals analyzed here are longitudinal records of GPS locations sampled at 3-hourly intervals between July 11 and December 31 of 2001 (with the exception of R28, which ends on December 15). GPS data for five of these individuals was collected hourly, but sampled at the 3 h interval for comparative purposes. However, analyses of data at both resolutions were conducted to ensure results were not a function of the sampling interval. Net displacements between successive locations were calculated using ESRI's ArcGIS 9.2 and log transformed to stabilize the variance. Missing values made up <4% of the data in total for all individuals (except M5, which was missing 8.76%, primarily from a 2-week collar failure). We estimated missing values using expected values from a Kalman Smoother with a first-order autoregressive state-space model assuming mean zero Gaussian process noise and mean zero Gaussian observation error (33). This imposed no artificial seasonal (in the parlance of time domain methods) structure in the data, which appeared at lags of >6 h in unreported preliminary data exploration.
Two approaches were used to identify the dominant frequencies in time series of movement step lengths, described extensively in the SI Text. First, the distribution of power (variance) across frequencies was estimated with the Fourier periodogram, in which dominant frequencies are identified by peaks in the periodogram as compared against theoretical white and best-fitting red-noise spectrums (SI Text). Though a Fourier periodogram provides a useful summary of dominant signals in a time series and has useful asymptotic properties (33), the method assumes that the process creating the data is stationary (i.e., any model of the process would have constant coefficients). A more general approach that does not assume stationarity of the process creating the data is wavelet analysis (21, 34), which calculates the modulus of the wavelet-transformed time series of step lengths as a function of both frequency and time, thereby allowing the identification of particular time and frequencies in which the moduli are significantly different to those expected from a red-noise fit of the data (see SI Text for technical details).
We determined the proportion of variance explained by autocorrelative processes more complex than a simple AR(1) model versus “noise” at each time step using a Plancherel formulation of the total time series variance to the modulus values of the wavelet transformation (21, 35) (SI Text). Averaging across blocks of time corresponding to NDVI sampling intervals offered a relative measure of the degree of autocorrelation in movements during that block for each individual. These time-specific metrics of overall autocorrelation were then averaged across all elephants for each NDVI sampling interval. Spearman's rank correlation was then calculated between NDVI and this average, representing the relative strength of autocorrelation in movements across all individuals.
The second use of temporal locations of significant levels of autocorrelation was to relate differences in the autocorrelative signals of movement behavior to spatial location, social rank, and season. We first calculated the proportions of time for which movement was autocorrelated at levels that significantly exceeded the best-fitting red-noise process of the data pertaining to each individual. These results were overlaid in a GIS to calculate the proportion of time cyclical movements that occurred within and outside protected areas. In addition, the proportion of time modulus values were significant for frequencies less than or equal to one cycle per day or greater than one cycle per day were compared within and outside parks.
Supplementary Material
Acknowledgments.
We thank the Kenyan Office of the President; the Kenya Wildlife Service (KWS); and the Samburu and Buffalo Springs National Reserve's County Council, wardens, and rangers for their support of our work. This work was supported by the National Science Foundation International Research Fellowship Program, Office of Science and Engineering Grant 0502340 (to G.W.) and a James S. McDonnell Foundation 21st Century Science Initiative Award (to W.M.G.). Field work was hosted by the Save the Elephants Research Centre in Samburu, and movement data came from the Save the Elephants Tracking Animals for Conservation Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801744105/DCSupplemental.
References
- 1.Getz WM, Saltz D. A framework for generating and analyzing movement paths on ecological landscapes. Proc Natl Acad Sci. 2008;105:19066–19071. doi: 10.1073/pnas.0801732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan R, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aars J, Johannesen E, Ims RA. Demographic consequences of movements in subdivided root vole populations. Oikos. 1999;85:204–216. [Google Scholar]
- 4.Turchin P. Quantitative Analysis of Movement: Measuring and Modeling Population Redistribution in Plants and Animals. Sunderland, MA: Sinauer Associates; 1998. pp. 33–176. [Google Scholar]
- 5.Edwards AM, et al. Revisiting Levy flight search patterns of wandering albatrosses, bumblebees and deer. Nature. 2007;449:1044–1048. doi: 10.1038/nature06199. [DOI] [PubMed] [Google Scholar]
- 6.Holyoak M, Casagrandi R, Nathan R, Revilla E, Spiegel O. Trends and missing parts in the study of movement ecology. Proc Natl Acad Sci. 2008;105:19060–19065. doi: 10.1073/pnas.0800483105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kareiva PM, Shigesada N. Analyzing insect movement as a correlated random-walk. Oecologia. 1983;56:234–238. doi: 10.1007/BF00379695. [DOI] [PubMed] [Google Scholar]
- 8.Bartumeus F, Da Luz MGE, Viswanathan GM, Catalan J. Animal search strategies: A quantitative random-walk analysis. Ecology. 2005;86:3078–3087. [Google Scholar]
- 9.Bergman CM, Schaefer JA, Luttich SN. Caribou movement as a correlated random walk. Oecologia. 2000;123:364–374. doi: 10.1007/s004420051023. [DOI] [PubMed] [Google Scholar]
- 10.Fryxell JM, et al. Multiple movement modes by large herbivores at multiple spatio-temporal scales. Proc Natl Acad Sci. 2008;105:19114–19119. doi: 10.1073/pnas.0801737105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiktorsson M, Ryden T, Nilsson E, Bengtsson G. Modelling the movement of a soil insect. J Theor Biol. 2004;231:497–513. doi: 10.1016/j.jtbi.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Cushman SA, Chase M, Griffin C. Elephants in space and time. Oikos. 2005;109:331–341. [Google Scholar]
- 13.Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I. Social dominance, seasonal movements, and spatial segregation in African elephants: A contribution to conservation behavior. Behav Ecol Sociobiol. 2007;61:1919–1931. [Google Scholar]
- 14.Eccard JA, Ylonen H. Costs of coexistence along a gradient of competitor densities: An experiment with arvicoline rodents. J Anim Ecol. 2007;76:65–71. doi: 10.1111/j.1365-2656.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 15.Focardi S, Pecchioli E. Social cohesion and foraging decrease with group size in fallow deer (Dama dama) Behav Ecol Sociobiol. 2005;59:84–91. [Google Scholar]
- 16.Trnka A, Prokop P. Do predators cause a change in passerine movement patterns as indicated by mist-net trapping rates? Ardea. 2006;94:71–76. [Google Scholar]
- 17.Frair JL, et al. Scales of movement by elk (Cervus elaphus) in response to heterogeneity in forage resources and predation risk. Landscape Ecol. 2005;20:273–287. [Google Scholar]
- 18.Roth TC, Vetter WE. The effect of feeder hotspots on the predictability and home range use of a small bird in winter. Ethology. 2008;114:398–404. [Google Scholar]
- 19.Dai XH, Shannon G, Slotow R, Page B, Duffy KJ. Short-duration daytime movements of a cow herd of African elephants. J Mammal. 2007;88:151–157. [Google Scholar]
- 20.Maraun D, Kurths J, Holschneider M. Nonstationary Gaussian processes in wavelet domain: Synthesis, estimation, and significance testing. Phys Rev E. 2007;75:1–14. doi: 10.1103/PhysRevE.75.016707. 016707. [DOI] [PubMed] [Google Scholar]
- 21.Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
- 22.Conradt L, Bodsworth EJ, Roper TJ, Thomas CD. Non-random dispersal in the butterfly. Maniola jurtina: Implications for metapopulation models. Proc R Soc London Ser B. 2000;267:1505–1510. doi: 10.1098/rspb.2000.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc R Soc London Ser B. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zollner PA, Lima SL. Search strategies for landscape-level interpatch movements. Ecology. 1999;80:1019–1030. [Google Scholar]
- 25.Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Elsevier Science; 2002. pp. 93–156. [Google Scholar]
- 26.Mandel JT, Bildstein KL, Bohrer G, Winkler DW. The movement ecology of migration in turkey vultures. Proc Natl Acad Sci. 2008;105:19102–19107. doi: 10.1073/pnas.0801789105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartumeus F, Levin SA. Fractal reorientation clocks: Linking animal behavior to statistical patterns of search. Proc Natl Acad Sci. 2008;105:19072–19077. doi: 10.1073/pnas.0801926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goward SN, Prince SD. Transient effects of climate on vegetation dynamics: Satellite observations. J Biogeogr. 1995;22:549–564. [Google Scholar]
- 29.Rasmussen MS. Developing simple, operational, consistent NDVI-vegetation models by applying environmental and climatic information: Part I. Assessment of net primary production. Int J Remote Sensing. 1998;19:97–117. [Google Scholar]
- 30.Wittemyer G, Rasmussen HB, Douglas-Hamilton I. Breeding phenology in relation to NDVI variability in free-ranging African elephant. Ecography. 2007;30:42–50. [Google Scholar]
- 31.Wittemyer G, Douglas-Hamilton I, Getz WM. The socio-ecology of elephants: Analysis of the processes creating multi-tiered social structures. Anim Behav. 2005;69:1357–1371. [Google Scholar]
- 32.Wittemyer G, Getz WM. Hierarchical dominance structure and social organization in African elephants. Anim Behav. 2007;73:671–681. [Google Scholar]
- 33.Shumway RH, Stoffer DS. Time Series Analysis and Its Applications. Harrisonburg, VA: Springer; 2000. pp. 213–333. [Google Scholar]
- 34.Maraun D, Kurths J. Cross wavelet analysis: Significance testing and pitfalls. Nonlin Processes Geophys. 2004;11:505–514. [Google Scholar]
- 35.Blatter C. Wavelets: A Primer. Natick, MA: A K Peters; 1998. pp. 69–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.