Abstract
The mammalian insulin-like growth factor 1 (IGF1), which is a member of a major growth-promoting signaling system, is produced by many tissues and functions throughout embryonic and postnatal development in an autocrine/paracrine fashion. In addition to this local action, IGF1 secreted by the liver and circulating in the plasma presumably acts systemically as a classical hormone. However, an endocrine role of IGF1 in growth control was disputed on the basis of the results of a conditional, liver-specific Igf1 gene knockout in mice, which reduced significantly the level of serum IGF1, but did not affect average body weight. Because alternate interpretations of these negative data were tenable, we addressed genetically the question of hormonal IGF1 action by using a positive experimental strategy based on the features of the cre/loxP recombination system. Thus, we generated bitransgenic mice carrying in an Igf1 null background a dormant Igf1 cDNA placed downstream of a transcriptional “stop” DNA sequence flanked by loxP sites (floxed) and also a cre transgene driven by a liver-specific promoter. The Igf1 cDNA, which was inserted by knock-in into the mutated and inactive Igf1 locus itself to ensure proper transcriptional regulation, was conditionally expressed from cognate promoters exclusively in the liver after Cre-mediated excision of the floxed block. Our genetic study demonstrated that the endocrine IGF1 plays a very significant role in mouse growth, as its action contributes approximately30% of the adult body size and sustains postnatal development, including the reproductive functions of both mouse sexes.
Keywords: gene targeting
The insulin-like growth factor (IGF) signaling system is the major determinant of mammalian organismal growth, as it provides the main common downstream conduit for the action of most growth-promoting gene products controlling body size (1, 2). IGF1 is one of the ligands of this family of effectors that is produced by many tissues and acts in an autocrine/paracrine fashion. In addition, it circulates in the plasma associated with binding proteins (IGFBPs). Classically, it was thought that the circulating IGF1, which postnatally is produced in the liver under growth hormone (GH) control, acts systemically as a hormone. In 1999, however, two groups using the same conditional Igf1 gene mutation in mice challenged the view of endocrine IGF1 effects (3, 4). Specifically, the apparent lack of effects on average body weight and length after liver-specific ablation of floxed Igf1 exon 4 using either an albumin-cre or an Mx1-cre transgene, which led to a markedly reduced level of circulating factor, was interpreted as demonstrating that liver-derived IGF1 is not required for postnatal growth. The albumin-cre mediated DNA excision appeared to be nearly complete in adult liver IGF1-deficient (LID) mice, but residual IGF1 (reportedly 25% of the normal level) thought to be produced by an unknown extrahepatic source was detected in serum.
In our view, however, the question about an endocrine IGF1 function remained open. Considering the inherent difficulty in drawing conclusions on the basis of negative results and the general lack of evidence for secretion of IGF1 into the circulation from any tissue other than the liver (see Discussion), we conjectured that incomplete and progressive action of albumin-cre becoming effective long after a critical postweaning period of growth-spurt could explain the observations (2). Consistent with this hypothesis, it was later shown that there is a stepwise decline in liver Igf1 mRNA in the LID model accompanied by a moderate reduction in serum IGF1 (40% of the normal level at an age of 50 days; ref. 5). In addition, the previous extreme view was revised and some endocrine function, especially in bone modeling, was assigned to IGF1 (6) based on results with bitransgenic mice in which LID was combined with global nullizygosity for the Igfals gene, which encodes the acid-labile subunit (ALS) of a ternary serum protein complex that also includes IGFBP3 and IGF1 (Igfals knockout mice; ALSKO). However, at 8–9 weeks of age, the LID/ALSKO double mutants did not differ in body weight (approximately 80% of normal) or length (approximately 90% of normal) from single ALSKO mice, despite slightly more severe deficits appearing transiently at earlier times.
Considering that Stat5b mediates most of the GH signaling in hepatocytes (7) and controls the transcription of both the Igf1 and Igfals genes, it is notable that liver-specific ablation of floxed Stat5a/b genes by cre driven by the albumin promoter linked to the albumin and α-fetoprotein enhancers resulted in growth retardation commencing at weaning (approximately 70% of the normal weight at 10 weeks; ref. 8). Still, the issue of the exact contribution of liver-derived circulating IGF1 was not settled, because an independent liver-specific deletion of Stat5a/b through the action of the particular albumin-cre transgene previously used in the LID model, did not show reduced body growth, although the serum IGF1 levels were approximately 50% of normal at 5 and 8 weeks of age (9). To address this unresolved question, we have used a genetic approach capable of producing conclusive results, which we report here.
Results
Experimental Design.
Previously, the role of circulating IGF1 was examined by ablating the expression of the cognate gene in the liver (3, 4), while leaving the locus intact in extrahepatic tissues (elimination of endocrine, but maintenance of autocrine/paracrine functions). Considering the pitfalls of this approach (see above), we decided to pursue the opposite strategy and assess genetically the hormonal role of IGF1 on somatic growth on the basis of positive, rather than negative results by attaining IGF1 production in the liver of mice lacking Igf1 gene expression in all other tissues.
An important consideration was that a standard transgenic approach using a liver-specific, but unrelated promoter to drive transcription of an Igf1 cDNA (see, e.g., ref. 10) was unsuitable for our purposes because expression of Igf1 in the liver of Igf1 nullizygous mice would be unregulated, making uncertain the physiological significance of the results. The mechanisms controlling mammalian Igf1 gene transcription are complex, as they involve, for example, two promoters and also dispersed regulatory elements that serve GH signaling acting through the Stat5b pathway (11). Thus, to ensure proper regulation, we took advantage of the features of the cre/loxP recombination system and inserted by knock-in a conditionally-activatable Igf1 transgene into the natural, but inoperative, Igf1 locus itself that was still associated with intact (known and unknown) regulatory sequences.
Using this strategy, we retargeted the null Igf1 allele (in which exon 4 had been replaced with a neo selectable marker) in the ES cells previously used to derive knockout mice (12) and inserted in cis at the beginning of exon 3 an Igf1 cDNA. The latter was positioned downstream from a floxed DNA segment consisting of a selectable marker (puromycin-resistance gene driven by the Pgk promoter) followed by a triple polyadenylation signal (pA) blocking the expression of the cDNA (Fig. 1A). For simplicity, we refer to this allele carrying inactive or expressed cDNA as Igf1flox and Igf1Δflox, before and after Cre-mediated recombination, respectively.
Fig. 1.
Targeting of Igf1 cDNA into the mouse Igf1 locus. (A) Diagrams of the genomic arrangements of exons comprising the predominant alternate mouse Igf1 mRNAs are shown on top. The allele used for knock-in was already targeted (12) by replacement of exon 4 with a neomycin-resistance gene (neo). The locations of a 9.1 kb diagnostic fragment produced by KpnI digestion of genomic DNA that was used for genotyping (panels C and D) and of a 9.6 kb BglII fragment containing exon 3 that was used to generate the arms of a replacement vector are indicated. The sequence in the region of an ATG codon in exon 3 was modified to generate unique XhoI and AscI cloning sites into which we inserted a DNA fragment (3 kb) consisting of a floxed segment that included, in order, the following elements: a loxP site (black triangle); a puromycin (puro) selectable marker (0.6 kb) driven by the Pgk gene promoter (0.5 kb); the Pgk polyadenylation sequence (0.3 kb); a “stop” sequence (SV40 3x-pA; triple polyA; 0.75 kb); a second loxP site; and an Igf1 cDNA (0.44 kb) endowed with the polyadenylation sequence of the bovine growth hormone gene (0.3 kb). (B) The DNA sequence remaining at the beginning of the modified Igf1 exon 3 (the first “ag” is the 3′ splice site of the upstream intron) after Cre-mediated excision of the floxed selectable marker is displayed. The leftover loxP site and the ATG codon are underlined. An asterisk marks a TAG terminator encountered upstream from the ATG (in frame), to indicate that the latter serves by default as a codon of the initiator Met for the peptide that can be translated from the transcript of the cDNA (the presumptive signal peptide is truncated). The WT sequence is shown at the bottom, for comparison (the modified sequence has 86 additional bases). (C) Genotyping by Southern analysis using tail DNA digested with KpnI indicates the sizes in kb of the WT (W) targeted (T) and recombined (R) alleles. (D) Southern analysis of DNA extracted from various tissues of a LIP animal (Liver, Li; Kidney, Ki; Heart, He; Testis, Te; Lung, Lu; Thymus, Th; Salivary gland, SG; Brain, Br; and Spleen, SP) demonstrates that Cre-mediated recombination has occurred only in liver, as expected. (E) Northern analysis of RNA extracted from various tissues of a LIP mouse (lanes 2–10) indicates that the knocked-in Igf1 cDNA is expressed exclusively in the liver (lane 2). The sizes (in kb) of the predominant Igf1 transcripts produced in WT liver are shown in lane 1 (Igf1 cDNA was used as a probe). The same membrane was hybridized to 18S RNA (loading control; bottom). (F) Northern analysis of liver RNA from WT (WT) and LIP mice before and after RNase H treatment (dots indicate the main digestion products; for details, see text).
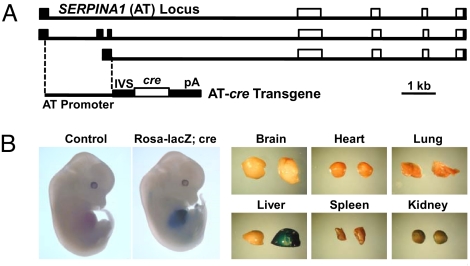
To remove the floxed block and allow liver-specific expression of Igf1, we generated a Cre-producing transgenic line in which cre was driven by a human SERPINA1 (alpha1 antitrypsin [AT]) gene promoter (AT-cre; Fig. 2A). Control experiments using a floxed lacZ reporter inserted into the Rosa26 locus and expressed conditionally after removal of a floxed block (13) indicated that Cre was expressed in embryonic and adult liver and not in other tissues (Fig. 2B). However, as we have previously shown (14), the efficiency of DNA excision depends in each case both on the target locus and the transgenic cre (different floxed loci respond variably to the same cre transgene, while the same floxed sequence is recombined to variable degrees by cre driven by different promoters). Thus, in contrast to the Rosa26-lacZ reporter, removal of the floxed DNA segment that blocks the expression of Igf1 cDNA in our construct was attained only to a level of 12.8 ± 0.3% in e18.5 embryos (n = 3) and reached a plateau at a level of 71.3 ± 1.3% in postnatal liver (the level was the same at p20 and p72; n = 4).
Fig. 2.
Liver-specific expression of an AT-cre transgene. (A) Diagrams of the genomic arrangements of exons comprising the predominant alternate mRNAs of the human SERPINA1 (AT) gene are shown on top. The position of the promoter fragment (also carrying one of the alternate exons) that was used to drive cre expression is indicated. In the transgenic construct, an intron (IVS) was provided upstream from the cre segment, which was linked to the SV40 polyadenylation sequence (for details, see SI Methods). (B) Comparison between WT (control) and bitransgenic e12.5 embryos carrying AT-cre and a Rosa-lacZ reporter shows that only the transgenic liver exhibits β-galactosidase staining. The same holds for the indicated tissues dissected from 20-day-old mice (in each pair of tissues, the control is on the left).
Mice carrying two doubly-targeted Igf1 alleles (with blocked Igf1 cDNA replacing exon 3 and deleted exon 4) were Igf1 nullizygotes (referred to here as “Null” mice; Igf1flox/flox), while animals with the same genotype but also carrying the AT-cre transgene were the desired experimental mice expressing Igf1 postnatally exclusively in the liver (referred to here as “liver IGF1 producers” or “LIP” mice; genotypically Igf1flox/flox;cre becoming eventually Igf1Δflox/Δflox in the liver).
In addition to LIP mice, we generated appropriate LIP controls, taking into account that WT mice were not directly comparable and could not serve for this purpose for two reasons. First, considering both the molecular manipulation of the locus and the incomplete activation of Igf1 cDNA expression by Cre-mediated excision of the floxed block, it was unknown to what extent the Igf1 transcripts in LIP liver could represent normal levels, despite normal transcriptional initiation (see below). Also unknown were the translatability, processing, and stability of prepro-IGF1 encoded by the cDNA. Although there is a single form of mature ligand (B-C-A-D domains), the isoforms of IGF1 precursor (signal peptide and domains B-C-A-D-E) differ in the sequences of the preregion and the E domain, which are removed by proteolytic processing (for a review see ref. 15). The particular cDNA sequence that we have used represents only one version of E domain and the encoded signal peptide is destined to be truncated (starting at an internal methionine codon; see Fig. 1B). This was inescapable because two alternate preregions are encoded by the mutually exclusive exons 1 and 2 transcribed by different promoters (Fig. 1A). Thus, an option of placing the floxed sequence in a position other than the beginning of exon 3 did not exist.
To provide controls, we used our Hs-cre1 transgenic line, in which Cre is constitutively expressed from a heat-shock gene promoter in 2-cell stage embryos (14), to remove the floxed block and allow ubiquitous expression of the Igf1 cDNA. Homozygous mice for the ubiquitously activated Igf1 cDNA (referred to here as “2X” mice; Igf1Δflox/Δflox) served as “surrogate wild-type” controls for the LIP mice. For further comparisons (see below), we also used “1X” mice (heterozygotes with a blocked Igf1 cDNA in one allele and an activated cDNA in the other allele; Igf1Δflox/flox) and “1XL” mice with one allele carrying a ubiquitously activated Igf1 cDNA and the other allele carrying a cDNA that can be activated only in the liver (Igf1Δflox/flox; cre). For easy reference, the genotypes of control and experimental mice are summarized in Table 1.
Table 1.
Igf1 expression in mice
| Genotype | cDNA expression status |
||
|---|---|---|---|
| Allele 1 | Allele 2 | ||
| 2X | Igf1Δflox/Δflox | ON | ON |
| 1X | Igf1Δflox/ | ON | OFF |
| 1XL | Igf1Δflox/flox; AT-cre | ON | OFF→ON |
| LIP | Igf1flox/flox; AT-cre | OFF→ON | OFF→ON |
| Null | Igf1flox/floxflox/flox | OFF | OFF |
Exon 4 of both alleles in all of these mice has been replaced with a neo cassette. An Igf1 cDNA connected to an upstream floxed block has been transcriptionally activated by Hs-cre and is ubiquitously expressed both embryonically and postnatally in 2X mice (both alleles) and in 1X and 1XL mice (1 allele). The second allele of 1XL mice and both alleles of LIP mice are activated postnatally by AT-cre only in the liver.
Genetic Crosses.
First, Igf1flox/+;cre bitransgenic progeny were derived from matings between Igf1flox/+ and AT-cre mice, which were then inter-crossed. Six genotypes (ignoring cre dosage), including LIP, Null and WT offspring, were obtained in expected Mendelian ratios (total of 443 animals from 60 litters). Unfortunately, very few LIP and Null neonates were able to survive (approximately 11% in each group; 6/56 and 4/37, respectively). The frequency of survival of Igf1 nullizygotes, which depends on genetic background, was as expected for 129xC57 hybrids (the reasons of neonatal death remain unknown; ref. 12). Control 2X, 1X, and 1XL mice were obtained by inter-crossing Igf1Δflox/flox; AT-cre animals.
Southern analysis of DNA extracted from various tissues confirmed that Cre-mediated recombination occurred exclusively in the liver (Fig. 1 C and D) which, as expected, was the only tissue expressing the Igf1 cDNA, as shown by Northern analysis (Fig. 1E). However, instead of three predominant transcripts (7.0 kb, 1.6 kb, and a broad zone of approximately 0.8 kb) that were detected in WT liver, the transgenic liver yielded a single zone of transcripts slightly larger than 0.8 kb (Fig. 1E). To ascertain whether the transgenic Igf1 cDNA was transcribed correctly, we hybridized liver RNA from WT and LIP animals with an antisense DNA oligonucleotide corresponding to Exon 4 sequence and then used RNase H, an enzyme degrading the RNA strands of DNA/RNA heteroduplexes, to truncate the Igf1 transcripts. Northern analysis indicated that three main products (truncated RNAs of 0.65, 0.55, and 0.4 kb) were produced from normal Igf1 transcripts (Fig. 1F; dots). Because of the molecular manipulations for cDNA insertion into the Igf1 locus, the prediction was that, if transgenic transcriptional initiation does not differ from normal, the sizes of the truncated species will be increased by 86 bp (see Fig. 1B). These were exactly the sizes of RNase H products derived from LIP liver RNA (the origin of a fourth truncated species of smaller size is unknown; Fig. 1F).
Growth Phenotypes.
To assess the growth process, we have used body weight as a coarse, but informative phenotypic criterion. Because of paucity of control females at the time of the analysis, we used only male mouse data throughout this study. Nevertheless, inspection of limited available data for female mice did not reveal unexpected differences from the pattern of male relative weights described below. Birthweights were monitored and then body weights were measured at regular intervals for a period of 10 weeks (by that time, the growth of mice with this mixed 129xC57 background is approaching a plateau phase). The mice were then killed to collect serum and tissues for analysis.
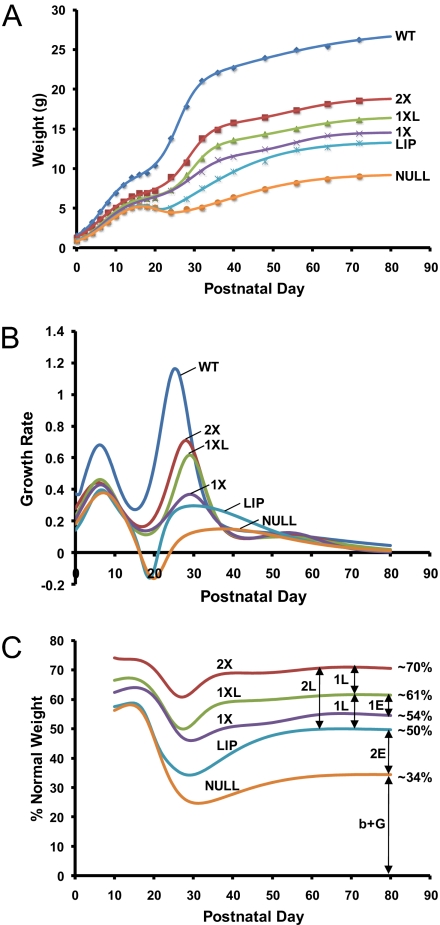
In the growth curves derived by appropriate regression analysis of the weight data, we first observed that the 2X control mice exhibited lower growth rates than WT animals and eventually attained approximately 70% of the normal weight (Fig. 3), indicating that our precaution in generating these controls was justified. The 1X mice were approximately 77% of their 2X counterparts in weight (54% of normal), as expected from the known haploinsufficiency of Igf1 heterozygotes (WT Igf1+/+ mice are approximately 15–20% heavier than Igf1+/− heterozygous siblings).
Fig. 3.
Growth analysis. (A) Growth curves derived by computer-aided regression analysis of average weights of WT (WT; n = 20), 2X (n = 4), 1XL (n = 6), 1X (n = 5), LIP (n = 3), and Null (n = 3) animals. For clarity, standard errors (all of small magnitude) are not displayed. (B) After growth curve fitting, the absolute growth rates (dW/dt) that are shown were calculated by subtracting the value of cumulative weight at a particular day (Wi) from that of the previous day (Wi-1), i.e., each curve point represents weight gain (g/day). (C) The growth curve regression data were also used to calculate relative weights (% of WT) for each genetically-modified mouse and to derive from the resulting steady-state values estimates of the contributions of endocrine (E) and local (L) IGF1 action to total body weight (for details, see Table 3). The distances between curves (arrows), corresponding to pairwise subtraction values between relative weights (Table 3), represent graphically the number of IGF1 “units” of endocrine and local action. Because the calculations were based on relative values, the final estimates of IGF1 contributions would be identical to those shown in Table 3 if, instead of the WT comparison standards, we had used weights relative to the 2X controls.
In terms of average daily weight gain, the postnatal mouse growth is a discontinuous process occurring in three phases (the third being less conspicuous than the others; see ref. 2). During the first phase, the growth rate peaks at approximately seven days and then declines, to be followed by a second growth spurt commencing at approximately two weeks, a time that coincides with the initiation of growth hormone action (2). During this second phase, the growth rate peaks at approximately 25 days and then declines. Although the timing of the first growth phase was approximately the same between WT and genetically-modified mice, the latter exhibited a growth rate delay during the second phase. Moreover, at the beginning of the second phase, the growth rate of Null and LIP animals became temporarily negative, as expected (2). A period of catch-up growth then followed, during which the LIP mice exhibited higher growth rate and eventually became significantly larger than their Null counterparts (Fig. 3 A and B). However, before weaning (first three postnatal weeks), the LIP and Null mice did not differ phenotypically in size (Fig. 3 A and B). Apparently, therefore, GH action did not commence exactly on time in LIP mice, an observation that we attribute to the delayed action of AT-cre (see above). In fact, when we measured the concentration of IGF1 in serum at embryonic day 18.5 (e18.5; Table 2), we observed that IGF1 was below detection limits in LIP embryos (apparently, approximately 13% excision of the floxed block at this stage was inadequate to produce measurable amounts of IGF1).
Table 2.
Serum IGF1
| Mouse | Concentration:e18.5, ng/ml | % normal | Concentration:p72, ng/ml | % normal |
|---|---|---|---|---|
| WT | 59.9 ± 3.4 (n = 7) | 100.0 | 253.1 ± 24.6 (n = 5) | 100.0 |
| 2X | 17.4 ± 1.4 (n = 4) | 29.0 | 129.2 ± 4.1 (n = 4) | 51.0 |
| 1X | 12.8 ± 1.1 (n = 3) | 21.4 | 66.0 ± 10.9 (n = 6) | 26.0 |
| 1XL | 14.6 ± 3.2 (n = 4) | 24.4 | 150.7 ± 17.1 (n = 4) | 59.5 |
| LIP | 0 | 0 | 112.1 ± 6.2 (n = 3) | 44.3 |
| Null | 0 (n = 3) | 0 |
The IGF1 concentrations in the serum of 2X, 1X, and 1XL embryos are not statistically different (Student's t test; P > 0.05). Postnatally, the IGF1 level in the serum of 1X mice is significantly different from the values observed in 2X, 1XL, and LIP mice (P < 0.05) which, however, do not differ between them.
The 1X and 1XL animals were also indistinguishable between them in weight until postnatal day 25 (p25) and then the latter grew with higher rate (Fig. 3 A and B). Therefore, under our experimental conditions, growth hormone-induced, liver-specific IGF1 apparently begins exerting postnatal hormonal action shortly after weaning. The issue of the GH-independent endocrine role of serum IGF1 produced in liver during the embryonic period remains unresolved (discussed in ref. 16) and cannot be addressed by our data. We simply note that significant differences between the levels of IGF1 in the serum of 2X, 1XL and 1X e18.5 embryos (approximately 21–29% of normal) were not detected (Table 2). On the other hand, a clear-cut dosage effect was observed postnatally (p72), as the level of circulating IGF1 was found to be 2-fold higher in 2X than in 1X mice (51% and 26% of the normal level, respectively). At the same time, a statistically significant difference in IGF1 levels between 2X, 1XL, and LIP mice was not detected (Table 2). This observation is consistent with a view that is largely compatible with information in the literature, which suggests that the liver is not just the main, but the exclusive source of circulating IGF1. However, a minor contribution of muscle to serum IGF1 cannot be unequivocally excluded at present (see Discussion).
We note that, to also assess linear growth, we measured body lengths and performed a limited allometric analysis, which showed that body weight is a good predictor of variation in body length [see supporting information (SI) Text and Fig. S1]. In addition, we examined the degree of growth plate maturation in the proximal tibia and showed that there is no significant difference between WT, 2X, and LIP mice (Fig. S1 and Table S1).
Relative Contributions of Endocrine and Local IGF1 Actions to Growth.
Previously, based on our comparative analysis of growth data from WT mice and mutants lacking IGF1 or the growth hormone receptor (Ghr) or both, we estimated (2) that the GH/IGF1 axis makes a major contribution to body weight at steady state (approximately 83%). This is the outcome of IGF1-mediated GH function (overlapping GH/I activity, O: approximately 34%), and also of independent functions of either GH (G: approximately 14%) or IGF1 (I: approximately 35%). Hence, the overall IGF1 contribution is 69% (34% O + 35% I). The remaining (“basal weight”; b: approximately 17%) was attributed to growth processes unrelated to GH and/or IGF1 signaling. As in the past, we again emphasize that the values of these estimates are not necessarily exact, but only indicative, and they can differ, albeit not dramatically, between data sets. For example, the relative weight of Igf1 Null mice (representing the sum b+G) was previously recorded as 31% (17%+14%) of normal, but is now calculated as 34% (because of lower than previously steady-state weight of WT animals; Fig. 3 A and C).
Within these limitations, we sought to provide, on the basis of the present growth analysis, a rough estimate of the relative contributions of endocrine (E) and local (L) IGF1 to mouse body weight (Table 3). Clearly, E + L = O + I (69% of body weight). However, O consists of two components: E, which is probably synthesized only in the liver (see above), and OL, which is the fraction of IGF1 synthesized in other tissues also in a GH-dependent fashion. OL and (the GH-independent) I act locally in an autocrine/paracrine fashion (L = OL+I). As shown in Fig. 3C and Table 3, we have estimated on the basis of the relative weights of 2X, 1XL, 1X, LIP, and NULL mice that, on average, 43% of the overall IGF1 action is endocrine and 57% is local. Accordingly, L is 39% of body weight (57% of 69%), of which 4% is OL (39–35%), while E is 30% (43% of 69%).
Table 3.
Contributions of endocrine and local IGF1 to growth
| Endocrine (E) activity, ″units″ | Local (L) activity, ″units″ | Relative weight, % of normal | |
|---|---|---|---|
| 2X | 2 | 2 | 70 |
| 1XL | 2 | 1 | 61 |
| 1X | 1 | 1 | 54 |
| LIP | 2 | 0 | 50 |
| NULL | 0 | 0 | 34 |
| Calculations: | |||
| E=(LIP-NULL)/2=(50−34)/2 = 8 | L = 1XL−LIP = 61−50 = 11 | ||
| E = 1XL−1X = 61−54 = 7 | L=(2X−LIP)/2=(70−50)/2 = 10 | ||
| L = 2X−1XL = 70−61 = 9 | |||
| Average E = 7.5 (43%) | Average L = 10 (57%) | ||
The relative contributions of endocrine and local IGF1 activities were calculated on the basis of relative weight data (see Fig. 3C), considering the dosage (relative ″units″) and the sites of expression of functional alleles in the mice that are compared.
Endocrine IGF1 and Fertility.
We have previously shown that Igf1 nullizygotes of both sexes are infertile and possess hypoplastic reproductive organs (17). Males exhibit a severe delay in Leydig cell development and very low levels of serum testosterone (approximately 18% of normal), and apparently lack mating behavior. This could explain their infertility, although sperm production at reduced numbers may be a contributing factor (in vitro fertilization tests have shown that this sperm is functional). The female mutants are infertile because they fail to ovulate, even after administration of gonadotropins.
We observed that, in contrast to the Null mice, LIP animals were fertile. At an age of 9.0–9.5 weeks, all three tested LIP males were able to impregnate normal females, which produced litters of normal size. It is not unlikely that the sexual maturation of these animals was slightly delayed, but a firm conclusion cannot be reached because of the small number of observations. Three LIP females, which were tested after completion of body weight determinations (10 weeks), were mated with normal males and became pregnant, two of them twice and the third 5 times, before being killed. They delivered 3–6 pups per litter. This seems to reflect mild subfertility, but we did not address this issue in any detail.
Discussion
We have presented direct and conclusive evidence that serum IGF1 postnatally supplied by the liver plays an endocrine role that is nearly as significant for organismal growth as the autocrine/paracrine action of IGF1 produced locally in various tissues.
Although extrahepatic contributions to serum IGF1 have been repeatedly suggested in the past by several authors, supporting evidence is still largely lacking. Previously, independent assays showed that serum IGF1 in two different strains of Ghr−/− mice was below detection limits and, if present at all, it could be as low as approximately 0.2% of the normal level (2, 18). Thus, if some extrahepatic tissue could make a contribution to serum IGF1, expression of the Igf1 gene in this source should be GH-dependent. This realization excludes as contributors all tissues in which Igf1 expression is GH-independent, such as the skeletal system (a sizable component of mouse body weight; approximately 8%) and also the brain, lung, heart, spleen, testis, and uterus (2, 19, 20). Igf1 gene transcription is GH-dependent in skeletal muscles, adipose tissue, ovary, and kidney (2, 20–22), although partially, in contrast to the complete dependence of the liver. Of these tissues, however, only the musculature (approximately 35% of body weight) remains a candidate for an auxiliary source of serum IGF1 (see below). Adipose tissue (14–17% of body weight) contains on a weight basis more IGF1 than any other tissue except the liver, but it does not appear to secrete this peptide (23). It is not surprising, therefore, that despite an increase of Igf1 mRNA in the adipose tissue of LID mice after GH administration, the level of circulating IGF1 remained unchanged (22). Moreover, neither the ovaries nor the kidneys appear to make a contribution to serum IGF1, as there is no gender difference in circulating IGF1 levels (see, e.g., ref. 24), which also remain unchanged after a 50% reduction of kidney IGF1 resulting from conditional ablation of a floxed Igf1 gene in the kidney (and also in some other tissues, including skeletal muscles), using a cre transgene driven by the promoter/enhancer of the gene encoding the α2 chain of collagen type 1 (Col1a2; ref. 25). The observation that, in the same conditional mutants, IGF1 was reduced to 25% of the normal level in muscle, while serum IGF1 was not affected, could be interpreted at face value as indicating that liver is the only source of circulating IGF1. However, this conclusion conflicts with results obtained by conditional ablation of Stat5a/b specifically in skeletal muscles, which indicated that this tissue may make a minor contribution to serum IGF1 (21). These mouse mutants, which exhibited mild growth retardation (85% of normal weight), did not show a difference from controls in serum IGF1 concentration at four weeks of age, but at eight weeks there was a slight reduction of the IGF1 level by approximately 15%, while muscle IGF1 was reduced to 40% of the control value and liver IGF1 remained at normal level. At present, the mechanism and significance of the timing of the Stat5a/b mutational impact on endocrine IGF1 are unclear and the discrepancy with other data (25) remains unresolved. We note that in two reports describing muscle-specific transgenic overexpression of Igf1 (26, 27) there was no change in serum IGF1, despite a 47-fold higher than normal level of the peptide in muscle extracts in one of the cases (26). In a third analogous case, IGF1 product did enter the circulation, but this probably occurred because of the presence of a somatostatin signal peptide supplied by the transgenic construct (28).
An interesting aspect of the endocrine role of IGF1 in LIP mice is its ability to support reproductive functions in a background lacking local IGF1. However, a series of separate studies would be clearly required to address questions on reproduction in some depth, including mechanistic details. Thus, considering that our analysis was mainly focused on growth, we will comment only briefly about the issues raised by our observations. A comparison between the infertile Igf1 null mice of both sexes on one hand and Ghr null mutants lacking endocrine IGF1 on the other, in which fertility, although somewhat impaired, is sustained (for a review, see ref. 29), tended to suggest that reproductive functions are mainly served by locally-acting IGF1. Nevertheless, our data show that endocrine IGF1 is capable of supporting reproductive performance all by itself. Aside of mechanistic relationships, which will undoubtedly turn out to be very complex, our results in regard to female LIP reproduction are consistent with observations on female Ghr null mice (30). Although fertile, these mutants exhibit delayed sexual maturation and produce small-size litters, presumably because of reduced ovulation rates. Their ovaries contain increased numbers of primordial and atretic follicles, while the numbers of healthy growing antral follicles is reduced. This defect was rescued, and the respective numbers of follicles were reversed, following IGF1 administration for two weeks (30).
To reconcile the apparent discrepancy between the observations with LIP and Ghr mutant mice, in which the endocrine and local IGF1 activities are reciprocal, we propose that these functions are largely interchangeable, so that mice maintaining either type do not deviate from normalcy to a great extent. Although this view provides an explanation for all of the available evidence, it would be very difficult, if at all feasible, to test experimentally whether the putative functional reciprocity reflects normal physiology, rather than opportunistic compensation of function by one component (endocrine or local) when the other is missing.
In various versions of the widely and repeatedly discussed “somatomedin hypothesis” (recently reviewed in ref. 31), it was posited that GH (somatotropin) exerts its effects indirectly through IGF1 (somatomedin; mediator of the actions of somatotropin). On the basis of our previous work (2) and other contributions, it is now widely accepted that, in addition to this overlapping relationship, GH and IGF1 also act independently of each other. In view of our data presented here, challenges to the endocrine role of IGF1, which is an important facet of the GH/IGF1 functional overlap, are no longer tenable. In fact, it has now become unquestionable that, in regard to its hormonal function, IGF1 is a bona fide somatomedin.
Materials and Methods
Mice.
Our Hs-cre1 deleter mouse strain has been described previously (14). The Rosa26-lacZ reporter strain (13) was obtained from the Jackson Labs. To generate mice expressing, upon Cre-mediated recombination, an Igf1 cDNA placed in exon 3 of the Igf1 locus, we constructed a replacement vector (Fig. 1A; for details, see SI Methods) and re-targeted in ES cells a null Igf1 allele (carrying neo in exon 4; ref. 12). From the progeny of transmitting chimeras mated with WT C57BL/6J females, we kept for subsequent crosses heterozygotes carrying in cis the Igf1 modifications in exons 3 and 4 of one allele. For liver-specific Cre-mediated recombination, we generated AT-cre transgenic mice (see SI Methods).
Molecular and Other Analyses.
The probe used for Northern analysis of total cell RNA (10 μg per lane) was the Igf1 cDNA component of the targeting vector. Analysis using RNase H (New England BioLabs) was performed after hybridization of RNA (20 μg per reaction) with 200 pmol of an oligodeoxynucleotide (5′-GATAGAGCGGGCTGCTTTTGTAG-3′) complementary to the sequence of nucleotides 121–143 of Igf1 exon 4 (total of 182 nucleotides). The digestion products were detected by Northern blotting using as a probe a 295 bp PCR product synthesized with the primers 5′-GATACACATCATGTCGTCTTCACAC-3′ (forward; representing nucleotides 6–30 of exon 3; total of 157 nucleotides) and the oligonucleotide used for RNase H digestion (reverse). The serum IGF1 concentration of ten-week-old mice was determined by ELISA of high-sensitivity (2.8 ng/ml; working range 10–500 ng/ml; Diagnostics Systems Laboratories Inc.). A 4-parameter logistic fit was used to analyze the data. Regression analysis of body weights to derive growth curves and calculate growth rates was performed by simultaneous computer-aided fitting of three logistic functions as described (2). Body lengths (from the tip of the nose to the base of the tail) were measured on anesthetized animals stretched on top of a ruler. Bone histology and morphometry of tibiae was performed as described (2).
Supplementary Material
Acknowledgments.
We thank Monica Mendelsohn for help in generating transgenic mice; Shouhong Xuan for clones; and Michael Gold, Thomas Kolar and Xi Sun for expert technical assistance. This work was supported by NCI grant 1 P01 CA97403 (Project 2) to A.E. and by a gift from the Berrie Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809223105/DCSupplemental.
References
- 1.Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- 2.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 3.Yakar S, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjögren K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Z, Yu R, Lu Y, Parlow AF, Liu JL. Age-dependent onset of liver-specific IGF-I gene deficiency and its persistence in old age: Implications for postnatal growth and insulin resistance in LID mice. Am J Physiol Endocrinol Metab. 2005;289:E288–E295. doi: 10.1152/ajpendo.00494.2004. [DOI] [PubMed] [Google Scholar]
- 6.Yakar S, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosui A, Hennighausen L. Genomic dissection of the cytokine-controlled STAT5 signaling network in liver. Physiol Genomics. 2008;34:135–143. doi: 10.1152/physiolgenomics.00048.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engblom D, et al. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev. 2007;21:1157–1162. doi: 10.1101/gad.426007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- 10.Liao L, et al. Liver-specific overexpression of the insulin-like growth factor-I enhances somatic growth and partially prevents the effects of growth hormone deficiency. Endocrinology. 2006;147:3877–3888. doi: 10.1210/en.2005-1537. [DOI] [PubMed] [Google Scholar]
- 11.Eleswarapu S, Gu Z, Jiang H. Growth hormone regulation of insulin-like growth factor-I gene expression may be mediated by multiple distal signal transducer and activator of transcription 5 binding sites. Endocrinology. 2008;149:2230–2240. doi: 10.1210/en.2007-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 13.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome. 2000;11:196–205. doi: 10.1007/s003350010037. [DOI] [PubMed] [Google Scholar]
- 15.Barton ER. The ABCs of IGF-I isoforms: Impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, et al. PERK eIF2alpha kinase regulates neonatal growth by controlling the expression of circulating insulin-like growth factor-I derived from the liver. Endocrinology. 2003;144:3505–3513. doi: 10.1210/en.2003-0236. [DOI] [PubMed] [Google Scholar]
- 17.Baker J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- 18.List EO, Coschigano KT, Kopchick JJ. Growth hormone receptor/binding protein (GHR/BP) knockout mice: A 3-year update. Mol Genet Metab. 2001;73:1–10. doi: 10.1006/mgme.2001.3164. [DOI] [PubMed] [Google Scholar]
- 19.Mathews LS, Norstedt G, Palmiter RD. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci USA. 1986;83:9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venken K, et al. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J Bone Miner Res. 2007;22:72–82. doi: 10.1359/jbmr.060911. [DOI] [PubMed] [Google Scholar]
- 21.Klover P, Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: A role for autocrine/paracrine insulin-like growth factor I. Endocrinology. 2007;148:1489–1497. doi: 10.1210/en.2006-1431. [DOI] [PubMed] [Google Scholar]
- 22.Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141:4436–4441. doi: 10.1210/endo.141.12.7825. [DOI] [PubMed] [Google Scholar]
- 23.Peter MA, Winterhalter KH, Boni-Schnetzler M, Froesch ER, Zapf J. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by growth hormone in rat white adipose tissue. Endocrinology. 1993;133:2624–2631. doi: 10.1210/endo.133.6.7694843. [DOI] [PubMed] [Google Scholar]
- 24.Bouxsein ML, et al. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res. 2002;17:570–579. doi: 10.1359/jbmr.2002.17.4.570. [DOI] [PubMed] [Google Scholar]
- 25.Govoni KE, et al. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007;148:5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman ME, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 27.Musaro A, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 28.Shavlakadze T, et al. Rskalpha-actin/hIGF-1 transgenic mice with increased IGF-I in skeletal muscle and blood: impact on regeneration, denervation and muscular dystrophy. Growth Horm IGF Res. 2006;16:157–173. doi: 10.1016/j.ghir.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol Reprod. 2004;71:17–27. doi: 10.1095/biolreprod.103.027060. [DOI] [PubMed] [Google Scholar]
- 30.Slot KA, et al. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006;131:525–532. doi: 10.1530/rep.1.00946. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan SA, Cohen P. The somatomedin hypothesis: 50 years later. (2007) J Clin Endocrinol Metab. 2007;92:4529–4535. doi: 10.1210/jc.2007-0526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.